Abstract
Purpose
To determine the efficacy of treatment of lateral elbow tendinopathy (LET) with platelet-rich plasma (PRP) injection and ultrasonic tenotomy and debridement (USTD) as well as risk factors for treatment failure.
Methods
This was a retrospective study including patients treated for LET with PRP or USTD between January 2018 and December 2021. The efficacy of both procedures was assessed using pain-related patient-reported outcome measures at the 12-week follow-up. Baseline subject characteristics and diagnostic ultrasound findings were analyzed as risk factors for failure of treatment. Failure was classified as a surgical indication for LET within a year of the PRP or USTD.
Results
Ultrasonic tenotomy and debridement and PRP both led to significant improvement in patient pain within the 12-week follow-up period. There was no significant difference in efficacy between the two procedures. Common extensor tendon tearing on ultrasound and Worker’s Compensation cases were found to be risk factors for failure of USTD. Lateral collateral ligament complex involvement and injection were found to be risk factors for failure of PRP.
Conclusions
Platelet-rich plasma and USTD are both effective interventions for LET. They have separate risk factors for failure that should be taken in consideration while deciding the treatment approach. These procedures are minimally invasive alternatives to some of the more invasive surgical options to treat LET.
Type of study/level of evidence
Therapeutic III.
Key words: Lateral elbow tendinopathy, Lateral epicondylitis, Platelet-rich plasma, Tennis elbow, Ultrasonic tenotomy and debridement
Lateral elbow tendinopathy (LET), also known as lateral epicondylitis or tennis elbow, is a common cause of pain in the adult population, with an estimated annual incidence of 3.3–3.5 per 1,000.1 It is a notable cause of pain and loss of function. Sixteen percent of patients report work restrictions and 4% miss at least 1 week of work over their condition course.2 The majority of cases are thought to self-resolve in 1–1.5 years.3 However, conservative treatments such as nonsteroidal anti-inflammatory drugs and bracing may not be the most effective option for all patients. In the subset of patients who do not improve with a 6-month course of conservative treatment, LET has a median course of 844 days.1 More direct interventions could be used after failure of conservative treatment to avoid such long disease courses, but there is no preferred modality.4
Although interventions such as bracing, corticosteroid injections, nonsteroidal anti-inflammatory drugs, and physical therapy have historically been used for LET, there is growing interest and use of platelet-rich plasma (PRP) injections and ultrasonic tenotomy and debridement (USTD) using the Tenex device from Tenex Health.4,5 A traditional surgical intervention, open surgical debridement of the lateral epicondyle, has higher risk profile, level of invasiveness, and cost, with current patient costs reaching over $10,000. Average costs for PRP injections and USTD at the institution where this study took place were $1,300 and $5,900, respectively. Ultrasonic tenotomy and debridement is typically covered by insurance, whereas insurance coverage for PRP is variable. With minimally invasive techniques such as PRP injections and USTD, it may be possible to avoid these more invasive surgical interventions.
Platelet-rich plasma is defined as a supraphysiologic concentration of platelets, obtained by centrifugation of a patient’s blood draw. When introduced into a region of damaged tissue, platelets release alpha granules that contain a variety of cytokines, growth factors, and various other immunomodulators that are theorized to stimulate, augment, and regulate the healing response.6 This method has been shown as an efficacious option for treating LET.7, 8, 9
An USTD device emits high-frequency sound waves to debride and remove tendinopathic tissue, without damaging healthy tissue.10 After obtaining local anesthesia, a small 2 mm stab incision was made to advance the device to the region of the pathologic tissue under ultrasound (US) guidance. Current studies demonstrate the effectiveness of USTD in treating LET with sustained decreases in patient pain.7,11, 12, 13
Although both procedures have been shown to be efficacious, very little is known about possible patient characteristic risk factors for outcomes. Postprocedure physical therapy has been positively associated with USTD outcomes for LET, and body mass index, bodyweight, and severity have been found to be risk factors for PRP outcomes for other conditions.12,14,15 There is a need for information on additional risk factors specific to outcomes in LET to assist in patient and physician decision making regarding the condition. The purpose of this study was to assess the efficacy of PRP and USTD while also studying patient characteristics as risk factors for treatment failure.
Methods
The inclusion criteria were as follows: (1) patients 18 years of age or older, (2) US-confirmed diagnosis of LET based on tendon thickening and hypoechoic heterogeneity with or without neovascularity on Doppler, and (3) treatment for LET with either PRP injection or UTSD. The inclusion timeframe was January 1, 2018, to December 7, 2021. Exclusion criteria were as follows: (1) pregnant patients and prisoners and (2) repeat procedures before completing the 12-week patient-reported outcome (PRO) follow-up period. Diagnostic USs and procedures were performed by two fellowship-trained sports medicine physicians (R.K.) at the University of Iowa. Procedure choice was based on physician and patient preference, with PRP more often desired for diffuse tendinopathy and lateral collateral ligament (LCL) complex pathology. Standard follow-up appointments were at 2, 6, and 12 weeks after the procedure.
This study was approved by the institutional review board at the institution where the study took place. As this was a retrospective review, this study was eligible for and used a waiver of consent.
PRP protocol
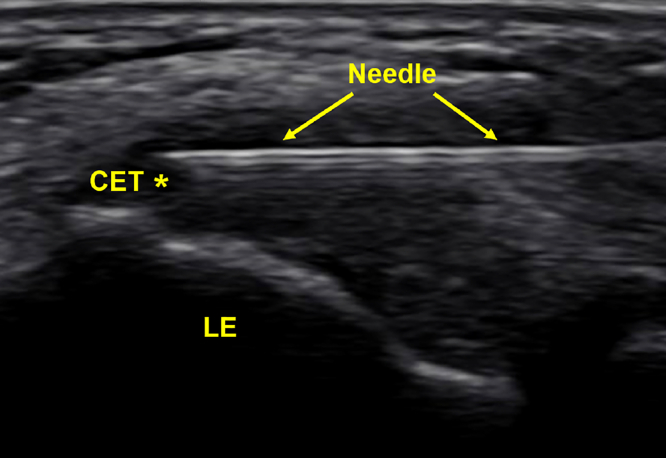
Sixty milliliters of blood was obtained from the contralateral antecubital fossa, then processed using a GPS III Platelet Concentration System from Zimmer Biomet or Arthrex Angel 2% PRP preparation, which have been found to produce statistically similar platelet concentrations of 1,343 ± 670 and 2,064 ± 526, respectively.16 Procedures were performed in a sterile manner with patients supine. Local anesthetic was used in all patients for procedural pain. One to six milliliters of PRP was introduced via a needle with US guidance at the common extensor tendon (CET), as well as the LCL complex if indicated. A US image of this procedure is shown in Figure 1.
Figure 1.
Labeled US image of a CET PRP injection at the lateral epicondyle (LE). Note hypoechogenicity at CET. Asterisk (∗) denotes area of pathology.
Ultrasonic tenotomy protocol
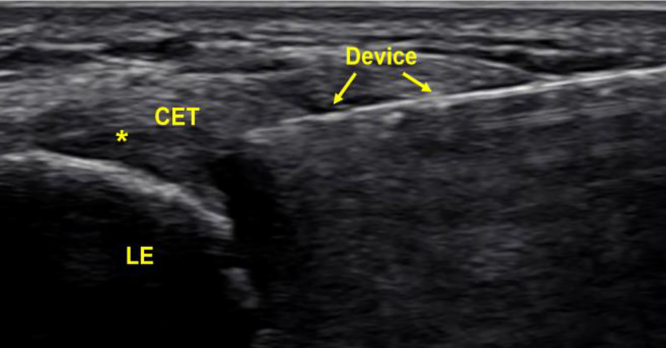
Ultrasonic tenotomy and debridement was performed in a sterile manner in the clinic with patients supine. Every patient received local anesthesia for the procedure. The device microtip was guided by the US to the region of tendinosis through a small stab incision with subsequent device debridement. The incision was closed with steri-strips and bandaged. A US image of this procedure is shown in Figure 2.
Figure 2.
Labeled US image of a CET USTD procedure at the lateral epicondyle (LE). As in Figure 2, the CET is hypoechoic indicating tendinosis. Asterisk (∗) denotes area of pathology.
PRO measures
Patient-reported outcome measures that were used in this study include Ortho Pain 4 and Percentage Improvement in Pain (PIP). Ortho Pain 4 is a four-question visual analog scale, asking for rating of average, worst, least, and current pain. Percentage Improvement in Pain is the percentage that patients feel their condition has improved since the procedure, with 0% being baseline and 100% being completely healthy. This has been validated for use in pain relief.17
Data collection
Patient data were recorded from the electronic medical record. Basic patient characteristics included age, sex, body mass index, diabetes status, occupation, procedure laterality, diagnosis, tobacco history, date of symptom onset, use of nonsteroidal anti-inflammatory drugs or acetaminophen medication before the procedure, and prior corticosteroid injections. Baseline PRO data were collected immediately prior to the procedure.
Diagnostic US findings recorded included the presence of partial-thickness CET tearing as well as associated LCL complex partial-thickness tearing and calcification. When the LCL complex was concomitantly treated with PRP, that information was recorded. Through a 12-week follow-up period, data collected included PROs, continued tobacco use, complications, and repeat procedures for symptom recurrence after the 12-week PRO follow-up. Complications were defined as any adverse reaction to the procedure.
Outcomes
The primary outcomes were as follows: (1) subject characteristics associated with failure of treatment and (2) efficacy of PRP and USTD for LET measured using PROs through a 12-week follow-up. The current approach at this study’s institution is to treat with minimally invasive options first, with subsequent surgical intervention upon failure of these treatments. If treatments relieve symptoms, patients do not follow-up with an orthopedic surgeon for surgical evaluation. So, failure of treatment was defined as a lack of improvement and subsequent surgical indication for LET within 12 months after PRP or USTD. Success was defined as a lack of surgical indication within that timeframe.
Statistical analysis
Participant demographic and tendinopathy characteristics were compared between the PRP and USTD groups using chi-square tests for categorical variables and independent t tests or Wilcoxon Rank Sum Tests for continuous variables, depending on the underlying distribution. The relationships between treatment, subject characteristics, and risk for failure were evaluated with logistic regression. Potential changes in PROs from baseline throughout the 12-week follow-up were evaluated using repeated-measures generalized linear regression. Analyses were completed using SAS software version 9.4 (SAS Institute Inc). Because of the retrospective nature of the study, power analysis was not performed.
Results
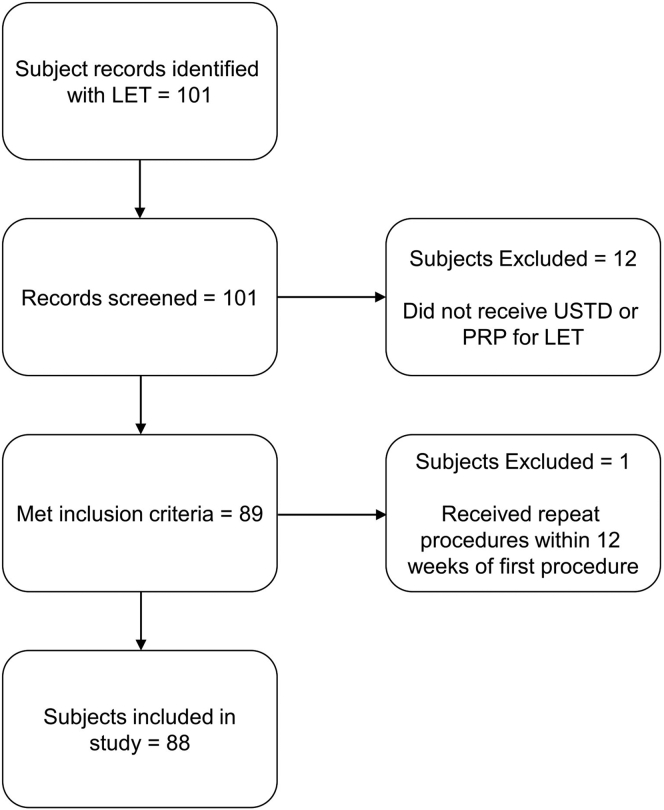
During the inclusion timeframe, 101 subjects were identified. Thirteen subjects did not meet inclusion criteria and were excluded (Fig. 3). Of the 88 subjects included, 37 were in the PRP group and 52 were in the USTD group. One subject was included in both groups as they received USTD in the right elbow and PRP in the left elbow. After accounting for patients who received bilateral procedures, 39 PRP injections and 57 USTD procedures were performed, totaling 96 procedures overall. Subject demographics and variables at baseline are listed in Table 1.
Figure 3.
Outline of the inclusion and exclusion process.
Table 1.
Baseline Demographic and Tendinopathy Characteristics. Characteristics of LET Include CET Tear, LCL Complex Involvement, and LCL Complex Injection
| Variable | USTD (n = 57) |
PRP (n = 39) |
P value | ||
|---|---|---|---|---|---|
| N | Mean ± SD (range) or n (%) | N | Mean ± SD (range) or n (%) | ||
| Age (y) | 57 | 46.9 ± 8.5 (26–72) | 39 | 48.8 ± 8.9 (31–65) | .29 |
| BMI (kg/m2) | 56 | 31.6 ± 5.5 (21–47.2) | 39 | 31.9 ± 7.1 (21.9–53) | .81 |
| Female sex | 57 | 29 (51) | 39 | 23 (59) | .43 |
| Tobacco use | 56 | 15 (27) | 39 | 6 (15) | .19 |
| Preprocedure corticosteroid injection | 57 | 29 (51) | 39 | 16 (41) | .34 |
| CET tear | 56 | 17 (30) | 39 | 5 (13) | .05 |
| LCL complex involvement | 56 | 9 (16) | 39 | 8 (21) | .58 |
| LCL complex injection | 38 | 9 (38) | — | ||
| Fibromyalgia | 56 | 7 (13) | 38 | 2 (5) | .30 |
| Preprocedure pain medication | 57 | 10 (18) | 39 | 4 (10) | .32 |
| Heavy work | 57 | 23 (40) | 39 | 22 (56) | .12 |
| Work comp | 57 | 14 (25) | 39 | 20 (51) | .01 |
| Time to procedure | 57 | 742.1 ± 1,069.6 (76–4,930) | 39 | 594.3 ± 823.1 (31–3,450) | .27 |
BMI, body mass index; comp, compensation.
Bolded P values indicate statistical significance.
The mean time from symptom onset to procedure was 742.1 days (range, 76–4,930) for USTD and 594.3 (range, 31–3,450) for PRP. With 3.5% of USTD subjects and 15.4% of PRP subjects underwent repeat procedures. No complications were reported for any patient.
Further surgical interventions were not required for 87.7% of USTD and 84.6% of PRP procedures. The two procedures had a combined surgical avoidance rate of 86.5%. The mean time from procedure to surgical indication in failed outcomes was 185.5 days (range, 111–309) for PRP and 164.7 (range, 92–271) for USTD.
Of the variables analyzed, CET tearing and Worker’s Compensation cases were risk factors for failure in the USTD group (Table 2). In the PRP group, LCL complex involvement and LCL complex injection were risk factors for failure of treatment (Table 3).
Table 2.
Relationship Between Subject Characteristics and Failure of the USTD Procedure
| Variable | N | OR (95% CI) | P value |
|---|---|---|---|
| Work comp (n vs y) | 57 | 31.50 (3.33–298.26) | .003 |
| CET tear (n vs y) | 56 | 7.71 (1.32–45.00) | .02 |
| Age (increase in age) | 57 | 0.99 (0.90–1.09) | .89 |
| BMI group, (increase in BMI group) | 56 | 1.12 (0.39–3.20) | .84 |
| Tobacco use (n vs y) | 56 | 1.11 (0.19–6.43) | .91 |
| Preprocedure corticosteroid injection (n vs y) | 57 | 2.71 (0.48–15.29) | .26 |
| LCL complex involvement (n vs y) | 56 | 0.28 (0.01–6.31) | .43 |
| Fibromyalgia (n vs y) | 56 | 0.38 (0.02–8.90) | .55 |
| Heavy work (n vs y) | 57 | 2.18 (0.44–10.80) | .34 |
| Preprocedure pain medication (n vs y) | 57 | 0.76 (0.08–7.11) | .81 |
| Subsequent procedure (n vs y) | 57 | 8.17 (0.45–148.20) | .16 |
| Time to procedure from onset | 57 | 1.60 (0.43–5.91) | .48 |
BMI, body mass index; comp, compensation; n, no; OR, odds ratio; y, yes.
Bolded P values indicate statistical significance.
Table 3.
Relationship Between Subject Characteristics and Failure of PRP Procedure
| Variable | N | OR (95% CI) | P value |
|---|---|---|---|
| LCL complex injection | 38 | 35.00 (3.20–381.51) | .003 |
| LCL complex involvement (n vs y) | 39 | 11.80 (1.75–79.54) | .01 |
| Age (increase in age) | 39 | 0.99 (0.90–1.10) | .90 |
| BMI Group, (increase in BMI group) | 39 | 1.49 (0.48–4.66) | .49 |
| Tobacco use (n vs y) | 39 | 3.63 (0.49–26.61) | .21 |
| Preprocedure corticosteroid injection (n vs y) | 39 | 1.54 (0.27–8.82) | .63 |
| CET tear (n vs y) | 39 | 1.45 (0.13–15.80) | .76 |
| Fibromyalgia (n vs y) | 39 | 0.94 (0.02–42.66) | .97 |
| Heavy work (n vs y) | 39 | 13.79 (0.66–287.59) | .09 |
| Preprocedure pain medication (n vs y) | 39 | 2.00 (0.17–23.25) | .58 |
| Work comp (n vs y) | 39 | 4.38 (0.61–31.60) | .14 |
| Subsequent procedure (n vs y) | 39 | 3.63 (0.49–26.61) | .21 |
| Time to procedure from onset | 39 | 0.69 (0.21–2.32) | .55 |
BMI, body mass index; comp, compensation; n, no; OR, odds ratio; y, yes.
Bolded P values indicate statistical significance.
Response rates of Ortho Pain 4 data for the USTD group were 49.1% at baseline, 59.6% at 2- and 6-week follow-up, and 45.6% at a 12-week follow-up. For the PRP group, Ortho Pain 4 data were available for 41.0% at baseline, 46.2% at 2-week follow-up, 53.8% at 6-week follow-up, and 59.0% at a 12-week follow-up. The availability of PIP for the USTD group was 70.2%, 71.9%, and 59.6% at 2-, 6-, and 12-week follow-up, respectively. For PRP, the availability of this measure was 59.0%, 84.6%, and 71.8% through the three follow-up points.
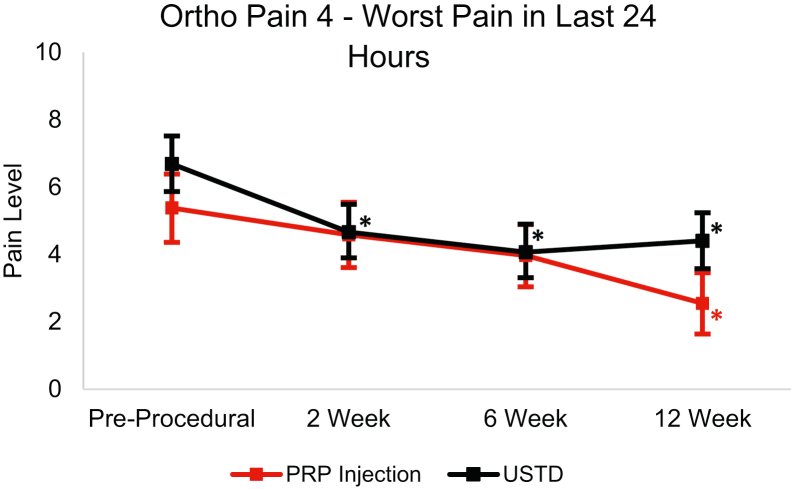
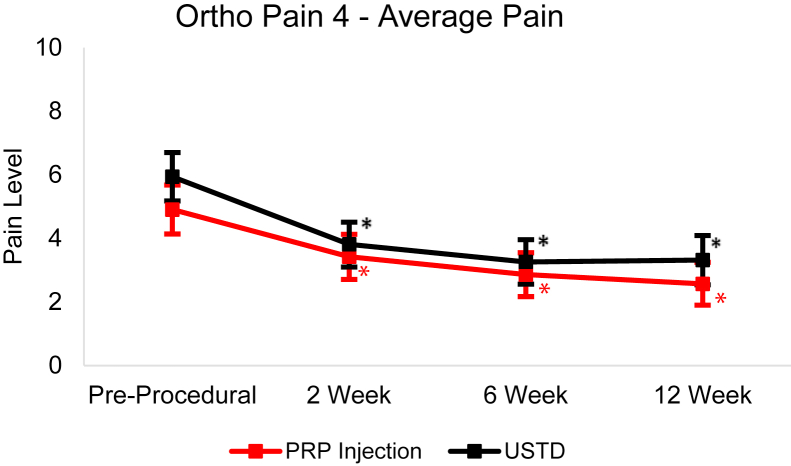
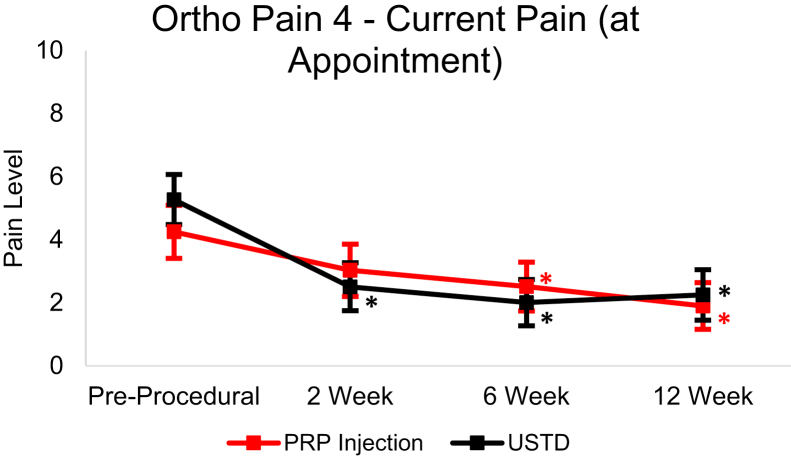
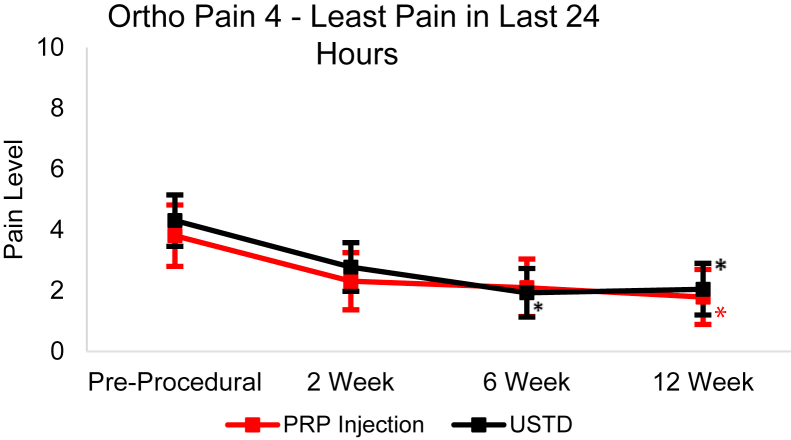
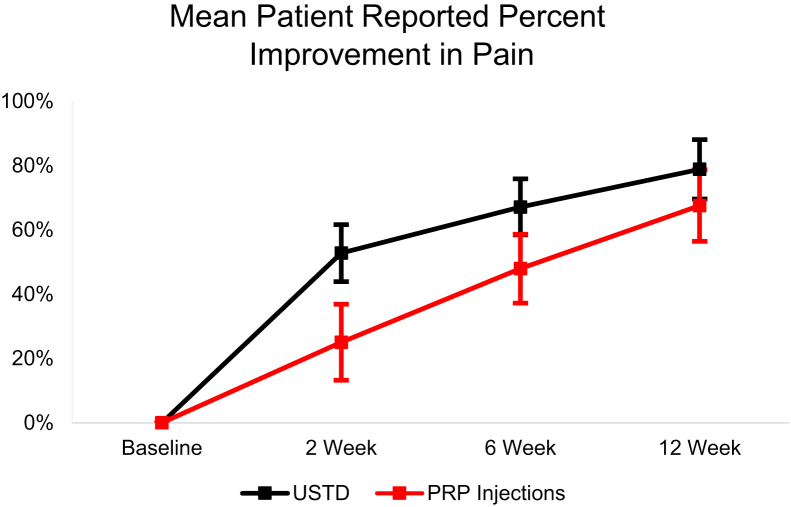
From baseline to the 12-week follow-up, the USTD group’s Ortho Pain 4 least squares mean significantly decreased (P < .0001) in all four questions (Figure 4, Figure 5, Figure 6, Figure 7). The least squares mean PIP was 78.81% at 12 weeks (Fig. 8). At 12 weeks, 21.9% of subjects reported complete resolution of pain, with 40.6% reporting at least 90% improvement in pain.
Figure 4.
Least squares mean Ortho Pain 4 worst pain in the last 24 Hours responses over 12-week follow-up with 95% CIs. Asterisk (∗) indicates statistically significant difference from baseline (P < .05).
Figure 5.
Least squares mean Ortho Pain 4 average pain responses over 12-week follow-up with 95% CIs. Asterisk (∗) indicates statistically significant difference from baseline (P < .05).
Figure 6.
Least squares mean Ortho Pain 4 current pain responses over 12-week follow-up with 95% CIs. Asterisk (∗) indicates statistically significant difference from baseline (P < .05).
Figure 7.
Least squares mean Ortho Pain 4 least pain in last 24 hours responses over 12-week follow-up with 95% CIs. Asterisk (∗) indicates statistically significant difference from baseline (P < .05).
Figure 8.
Least squares mean patient-reported PIP over the 12-week follow-up period with 95% CIs.
In the PRP group, the Ortho Pain 4 least squares mean significantly decreased in all four questions (Figure 4, Figure 5, Figure 6, Figure 7). P < .0001 for the worst pain in the last 24 hours, average pain, and current pain. For the least pain in the last 24 hours, the P = .0027. The least square mean PIP was 67.53% at 12 weeks (Fig. 8). At 12 weeks, 18.5% of subjects reported complete resolution of pain, and 37.0% reported at least 90% improvement in pain.
There were significant (P < .05) differences in successful and unsuccessful outcomes in PIP at 6- and 12-week follow-up in the USTD group. Only the 12-week follow-up had significant (P < .05) differences in PIP between successful and unsuccessful outcomes in the PRP group. There were no differences between the outcomes in the Ortho Pain 4 responses at any point.
Discussion
With the prevalence and prolonged course of recovery of LET, it would be beneficial to find efficacious treatment modalities for this condition. Some studies have found PRP to have little advantage over other treatments or even saline, whereas others have found PRP to be an effective treatment for LET.7,8,18,19 Ultrasonic tenotomy and debridement has also been shown as an effective treatment for LET.7,11,12 This study provides further evidence for the benefit patients may have from either PRP injections or USTD. Additionally, risk factors that could be used in the counseling and decision making of treatment options would be beneficial for patients and physicians. Although there have been some studies showing risk factors for PRP efficacy with body mass index or condition severity in knee osteoarthritis or bodyweight in carpal tunnel syndrome, there have been no studies on outcome risk factors for the use of PRP for LET. Due to its novelty, there is also little evidence on factors associated with failure of USTD.14,15
This study provides risk factors that may benefit both physicians and patients when deciding on treatment approaches. Common extensor tendon tearing and Worker’s Compensation are associated with USTD failure. While treating LET with presence of CET tearing, PRP injections may be a better option as the PRP group had no association between tearing and failure. The Worker’s Compensation cases’ association with failure was consistent with previous literature showing worse outcomes for upper extremity conditions in Worker’s Compensation claims.20, 21, 22 The number of Worker’s Compensation cases were lower for PRP, but we were establishing risk factors within groups instead of comparing risk between groups. So, differences between groups would not affect these results.
The risk factors for failure in the PRP group were LCL complex involvement and LCL complex injection. At this study’s institution, the LCL complex is typically only injected when there is LCL complex tearing or other overt LCL complex involvement. Only one patient received LCL complex injection without major LCL complex involvement. Based on this, it is likely that LCL complex involvement is the true risk factor for the failure of PRP, with LCL complex injection being a reflection of this variable. Deal et al showed that PRP injections are an effective treatment for partial medial ulnar collateral ligament tears.23 Although treating ligament-only injury with PRP injections may be effective, our findings show that when combined with CET tendinosis, ligament injury led to poor outcomes with PRP.
A factor that was not significantly associated with the failure of either procedure (P = .26 for USTD and P = .63 for PRP) was prior corticosteroid injections for the affected elbow. Recent studies have found corticosteroid injections to be a risk factor for worse clinical outcomes in patients with LET.22,24 However, our study did not find this same significant association with LET. Given the variability in outcomes with corticosteroid injections, further study to examine the outcomes and effects of these injections for LET would be beneficial.
Both procedures showed strong efficacy in treating LET. With 87.7% of USTD and 84.6% of PRP procedures not needing surgery, patients and physicians can have a better understanding of the likelihood of successful treatment. These are high rates of success, but they are lower than the reported rate of surgical avoidance of 98.4% for the two procedures combined in Boden et al.7 However, the follow-up period reported in the study by Boden et al7 is less strict than in the current study. There was also no description of the severity of LET treated in subjects such as LCL complex involvement or CET tearing, as reported in our study. With the success rates, least squares mean PIP of roughly 70% to 80% for both procedures, and significant differences (P < .01) in Ortho Pain 4 scores from baseline to a 12-week follow-up, we believe that PRP and USTD are effective treatments. Comparatively, studies on steroid injections for LET have shown variable results, with more recent studies showing roughly 25% to 30% improvement in visual analog scale pain scores.18 Physiotherapy or exercise programs at a 12-week follow-up have 50% to 60% improvement in pain on the visual analog scale.25,26 The wait-and-see approach showed roughly 40% to 50% improvement at 12 weeks.25 In regard to surgical avoidance, the historical rate of successful nonsurgical treatment has been approximately 90%.3,27 However, a more recent study showed only 67% of patients treated with physiotherapy and 75% of patients treated with splinting avoided surgery.22
Interestingly, the PIP PRO showed significantly more improvement (P = .006) with USTD than PRP at 2 weeks. It is possible that the USTD’s specificity to and removal of pathologic tissue may be responsible for the initial large improvement in pain, while PRP still requires the body’s natural mechanisms to heal and regenerate the tendon tissue.6,10 At 12 weeks, there was no significant difference (P = .32) in PIP between the two treatments.
Percentage Improvement in Pain was significantly different (P = .04 for PRP and P = .005 for USTD) between successful and failed outcomes at the 12-week follow-up for both PRP and USTD. The difference in PIP was statistically significant (P = .02) at the 6-week follow-up only for the USTD group. These measures could act as additional tools in assessing the likelihood or necessity of surgical intervention at an earlier follow-up than previously done. A 12-week follow-up would be earlier than the minimum time from the procedure to surgical indication for both groups and much earlier than the meantime for both groups.
There are some limitations in this study. First, the response rate for PROs, specifically Ortho Pain 4, was weak. However, the response rates for PIP were much better. Second, the lack of surgical indication is not a typical definition of procedural success, but we believe that much of the value with PRP and USTD is in avoiding the need for open debridement. Additionally, there was no control group for the two procedures. Because this was a retrospective review of patients treated, a control was not possible. Finally, all procedures were performed by the same two physicians. This makes it possible that physician skills could influence the results of the study.
Future directions for study regarding PRP and USTD could include expansion on the evaluation of other variables as risk factors for failure or poor outcomes. Additionally, predictive modeling for patient outcomes or risk stratification would be beneficial for both physicians and patients. A cost analysis for the two procedures would also be helpful to assist in patient and physician decision making.
Footnotes
Declaration of interests: No benefits in any form have been received or will be received related directly to this article.
References
- 1.Sanders T.L., Jr., Maradit Kremers H., Bryan A.J., Ransom J.E., Smith J., Morrey B.F. The epidemiology and health care burden of tennis elbow: a population-based study. Am J Sports Med. 2015;43(5):1066–1071. doi: 10.1177/0363546514568087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanders T.L., Maradit Kremers H., Bryan A.J., Ransom J.E., Morrey B.F. Health care utilization and direct medical costs of tennis elbow: a population-based study. Sports Health. 2016;8(4):355–358. doi: 10.1177/1941738116650389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sims S.E., Miller K., Elfar J.C., Hammert W.C. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (N Y) 2014;9(4):419–446. doi: 10.1007/s11552-014-9642-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma K.L., Wang H.Q. Management of lateral epicondylitis: a narrative literature review. Pain Res Manag. 2020;2020 doi: 10.1155/2020/6965381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lenoir H., Mares O., Carlier Y. Management of lateral epicondylitis. Orthop Traumatol Surg Res. 2019;105(8S):S241–S246. doi: 10.1016/j.otsr.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Mishra A., Woodall J., Jr., Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28(1):113–125. doi: 10.1016/j.csm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Boden A.L., Scott M.T., Dalwadi P.P., Mautner K., Mason R.A., Gottschalk M.B. Platelet-rich plasma versus Tenex in the treatment of medial and lateral epicondylitis. J Shoulder Elbow Surg. 2019;28(1):112–119. doi: 10.1016/j.jse.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 8.Thanasas C., Papadimitriou G., Charalambidis C., Paraskevopoulos I., Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39(10):2130–2134. doi: 10.1177/0363546511417113. [DOI] [PubMed] [Google Scholar]
- 9.Peerbooms J.C., Sluimer J., Bruijn D.J., Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255–262. doi: 10.1177/0363546509355445. [DOI] [PubMed] [Google Scholar]
- 10.Peck E., Jelsing E., Onishi K. Advanced ultrasound-guided interventions for tendinopathy. Phys Med Rehabil Clin N Am. 2016;27(3):733–748. doi: 10.1016/j.pmr.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Seng C., Mohan P.C., Koh S.B., et al. Ultrasonic percutaneous tenotomy for recalcitrant lateral elbow tendinopathy: sustainability and sonographic progression at 3 years. Am J Sports Med. 2016;44(2):504–510. doi: 10.1177/0363546515612758. [DOI] [PubMed] [Google Scholar]
- 12.Chalian M., Nacey N.C., Rawat U., et al. Ultrasound-guided percutaneous needle tenotomy using Tenex system for refractory lateral epicondylitis; short and long-term effectiveness and contributing factors. Skeletal Radiol. 2021;50(10):2049–2057. doi: 10.1007/s00256-021-03778-9. [DOI] [PubMed] [Google Scholar]
- 13.Barnes D.E., Beckley J.M., Smith J. Percutaneous ultrasonic tenotomy for chronic elbow tendinosis: a prospective study. J Shoulder Elbow Surg. 2015;24(1):67–73. doi: 10.1016/j.jse.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Alessio-Mazzola M., Lovisolo S., Sonzogni B., et al. Clinical outcome and risk factor predictive for failure of autologous PRP injections for low-to-moderate knee osteoarthritis. J Orthop Surg (Hong Kong) 2021;29(2) doi: 10.1177/23094990211021922. [DOI] [PubMed] [Google Scholar]
- 15.Shen Y.P., Li T.Y., Chou Y.C., Chen L.C., Wu Y.T. Outcome predictors of platelet-rich plasma injection for moderate carpal tunnel syndrome. Int J Clin Pract. 2021;75(10) doi: 10.1111/ijcp.14482. [DOI] [PubMed] [Google Scholar]
- 16.Degen R.M., Bernard J.A., Oliver K.S., Dines J.S. Commercial separation systems designed for preparation of platelet-rich plasma yield differences in cellular composition. HSS J. 2017;13(1):75–80. doi: 10.1007/s11420-016-9519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gridley L., van den Dolder P.A. The percentage improvement in pain scale as a measure of physiotherapy treatment effects. Aust J Physiother. 2001;47(2):133–138. doi: 10.1016/s0004-9514(14)60304-4. [DOI] [PubMed] [Google Scholar]
- 18.Krogh T.P., Fredberg U., Stengaard-Pedersen K., Christensen R., Jensen P., Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625–635. doi: 10.1177/0363546512472975. [DOI] [PubMed] [Google Scholar]
- 19.Mishra A.K., Skrepnik N.V., Edwards S.G., et al. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014;42(2):463–471. doi: 10.1177/0363546513494359. [DOI] [PubMed] [Google Scholar]
- 20.Higgs P.E., Edwards D., Martin D.S., Weeks P.M. Carpal tunnel surgery outcomes in workers: effect of workers’ compensation status. J Hand Surg Am. 1995;20(3):354–360. doi: 10.1016/S0363-5023(05)80086-3. [DOI] [PubMed] [Google Scholar]
- 21.Sotereanos D.G., Varitimidis S.E., Giannakopoulos P.N., Westkaemper J.G. Results of surgical treatment for radial tunnel syndrome. J Hand Surg Am. 1999;24(3):566–570. doi: 10.1053/jhsu.1999.0566. [DOI] [PubMed] [Google Scholar]
- 22.Knutsen E.J., Calfee R.P., Chen R.E., Goldfarb C.A., Park K.W., Osei D.A. Factors associated with failure of nonoperative treatment in lateral epicondylitis. Am J Sports Med. 2015;43(9):2133–2137. doi: 10.1177/0363546515590220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deal J.B., Smith E., Heard W., O’Brien M.J., Savoie F.H. Platelet-rich plasma for primary treatment of partial ulnar collateral ligament tears: MRI correlation with results. Orthop J Sports Med. 2017;5(11) doi: 10.1177/2325967117738238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coombes B.K., Bisset L., Brooks P., Khan A., Vicenzino B. Effect of corticosteroid injection, physiotherapy, or both on clinical outcomes in patients with unilateral lateral epicondylalgia: a randomized controlled trial. JAMA. 2013;309(5):461–469. doi: 10.1001/jama.2013.129. [DOI] [PubMed] [Google Scholar]
- 25.Bisset L., Beller E., Jull G., Brooks P., Darnell R., Vicenzino B. Mobilisation with movement and exercise, corticosteroid injection, or wait and see for tennis elbow: randomised trial. BMJ. 2006;333(7575):939. doi: 10.1136/bmj.38961.584653.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson M., Butler S., Eriksson M., Svärdsudd K. A randomized controlled trial of exercise versus wait-list in chronic tennis elbow (lateral epicondylosis) Ups J Med Sci. 2011;116(4):269–279. doi: 10.3109/03009734.2011.600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coonrad R.W., Hooper W.R. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg Am. 1973;55(6):1177–1182. [PubMed] [Google Scholar]