Abstract
Objectives
This umbrella review aimed to review the effects of resistance training on patients with end-stage renal disease and assess the methodological quality of the available literature.
Methods
An umbrella review and meta-meta-analysis was performed. A systematic search was conducted until May 2022. Article selection, quality assessment, and risk of bias assessment were performed by two independent reviewers. The meta-meta-analyses were performed with a random-effects model and the summary statistics were presented in the form of a forest plot with a weighted compilation of all standardized mean differences and corresponding 95% confidence interval. Twenty-four reviews were eventually included. The protocol was registered in the international registry PROSPERO (CRD42022321702).
Results
Resistance training showed positive effects on functional capacity (g = 0.614), aerobic capacity (g = 0.587), health-related quality of life (g = 0.429), and peak force (g = 0.621). Fifteen of the included studies (63%) presented low risk of bias, and the remaining studies (37%) showed unclear risk of bias.
Conclusion
Resistance training in patients undergoing hemodialysis is an intervention that shows positive results regarding physical and functional outcomes. The quality level of the literature is inconclusive, but the included studies present low risk of bias.
Graphical abstract
Keywords: Dialysis, Exercise, Meta-analysis, Resistance training
Introduction
End-Stage Renal Disease (ESRD) is an increasing worldwide concern that involves significant healthcare costs [1]. Global estimates reveal 8–16% prevalence rates that are progressively growing and will end up presenting a major challenge for healthcare systems [2].
Among the possible treatment options, most patients suffering from ESRD receive hemodialysis [3], a treatment that is often related to several impairments, such as decreased functional capacity, decreased levels of physical activity and increased mortality rates [4]. The most significant predictor of mortality rates in this population is skeletal muscle wasting [5, 6]. This is very common in patients with ESRD due to sedentary behavior [7], acidosis, comorbid illnesses, corticosteroid usage, aging, oxidative stress, insulin resistance, chronic inflammation and problems related to restrictions in dietary protein intake [8]. All these factors lead to loss of muscle strength and the development of functional impairments [7, 9].
To revert and prevent the development of skeletal muscle wasting, exercise has been extensively introduced as a complement to daily dialysis care routines. Exercise modalities include aerobic, resistance and combined programs, which are delivered during dialysis sessions or on non-dialysis days. Among them, resistance training has become a well-established, safe, and effective modality to overcome skeletal muscle wasting [10, 11], being the choice of preference of many authors when implementing exercise sessions during dialysis.
Several resistance training programs [10], such as the “Progressive Exercise for Anabolism in Kidney Disease (PEAK)” or clinical exercise guidelines such as the one developed by The Life Options Rehabilitation Advisory Council (LORAC), “Exercise. A guide for people on dialysis” have also been created to support these implementations [12]. To better understand the benefits and the effects of resistance training in patients with ESRD, several clinical trials, and further systematic reviews and meta-analyses, have been conducted. However, to the best of our knowledge, there is no review that involves the assessment of the overall effect and quality of these studies, which could help to establish guidelines and recommendations when implementing resistance training in these patients. Therefore, our research team conducted an umbrella review based on the evidence found involving resistance training in ESRD patients. This study aims to provide an overview of the benefits of resistance training in patients suffering from ESRD and an assessment of the quality of the published literature.
Methods
Design
A systematic review of reviews was conducted in accordance with the Preferred Reporting Items for Overviews of Systematic Reviews including harm checklist (PRIO-harms), which consists of 27 items and 56 sub-items, followed by a 5-stage process flow diagram [13]. The protocol was registered in the international registry PROSPERO (CRD42022321702).
Inclusion criteria
The criteria used in this search involved methodological and clinical outcome factors, including population, intervention, comparison, and study type (PICOS) criteria [14].
Participants (population) included in this search were adult (> 18 years old) patients that suffered from CKD who were undergoing maintenance hemodialysis. The trial (intervention) had to include either a resistance exercise training program or a combined exercise program that included resistance exercises. Comparator groups (control) received no-exercise, sham, or placebo exercise interventions. Assessments (outcomes) involved functional capacity assessed through the 6-Minute Walk Test (6MWT), aerobic capacity assessed through maximum oxygen intake (VO2max), peak force assessed through dynamometry, health-related quality of life (HRQOL) assessed through the Short-Form 36 (SF-36) questionnaire, depression symptoms assessed through the Beck Depression Inventory (BDI), and changes in systolic and diastolic blood pressures. Papers included systematic reviews with a meta-analysis of randomized controlled, randomized uncontrolled or controlled clinical trials.
Search strategy
A systematic search was carried out in the following scientific databases for related papers published by May 27, 2022: MEDLINE (Pubmed), The Cochrane Library (CENTRAL), EMBASE, CINAHL, PEDro and Google Scholar. The search strategy combined medical subject headings (MeSH) through Boolean operators “OR” and/or “AND”. The following headings were applied: “hemodialysis”, “renal dialysis”, “dialysis” “kidney failure, chronic”, “renal insufficiency, chronic”, “strength training” and “resistance training”.
Two independent researchers (BPD and LSM) carried out the search strategy following the same methods, and differences were resolved by consensus, mediated through a third researcher (JCG). Researchers manually searched journals that had published related papers as well as the reference lists of the included papers. Duplicates were hand-checked manually before being removed.
Selection criteria and data extraction
Two independent researchers conducted a data analysis to assess the relevance of the reviews according to the search question. Initially, this analysis was carried out by extracting information regarding the title, abstract and keywords of the systematic reviews. If there was no consensus between the researchers or if the title and abstract contained insufficient information, the full text was reviewed. After this first analysis, the researchers screened the reviews to assess whether they met the inclusion criteria. Differences between researchers were resolved through discussion and mediation by a third researcher.
The extraction protocol was based on the Cochrane Review Groups: (1) study characteristics, including author, title, and year of publication, (2) review information, including sample size, patients, and types of trials, (3) description of the intervention and control, including types of exercise, parameters of frequency, intensity and time, and study outcomes such as functional capacity, aerobic capacity, strength, health-related quality of life, depression symptoms, and blood markers.
Risk of bias analysis
Two independent researchers (BPD and LSM) assessed the quality of the included reviews by analyzing their risk of bias through the Risk of Bias in Systematic Reviews (ROBIS) tool [15]. This tool assesses quality across four domains: (1) study eligibility, (2) study identification and selection, (3) data collection and study appraisal and (4) synthesis of findings. ROBIS provides an overall assessment of high, low, or unclear risk of bias. Both researchers assessed the quality of the systematic reviews using the same method. Disagreements between the researchers were resolved by consensus mediated by a third researcher. Interrater reliability was determined using the kappa coefficient (κ), using κ > 0.7 to indicate a high level of agreement; κ = 0.5–0.7 to indicate a moderate level of agreement; and κ < 0.5 to indicate a low level of agreement [16].
Statistical analysis
Statistical analysis was performed using the “metaumbrella” app and its associated package in R, which is a tool that allows users to perform umbrella reviews with stratification of evidence [17]. Meta-analytical procedures were carried out following recommendations on how to conduct umbrella reviews [18]. We used the same inclusion criteria for the systematic review and meta-analysis but added two criteria: (1) The Results section contained detailed information on the comparative statistical data (mean, standard deviation, and/or 95% confidence interval [CI]) of the main variables, and (2) data for the analyzed variables were represented in at least three meta-analyses. Common effect sizes were estimated using standardized mean differences (SMDs), assessed through Hedge’s g to compare continuous variables, and their 95% confidence intervals using a random-effects model. The heterogeneity of each meta-analysis was estimated using the I-squared statistic (I2), establishing an I2 of 25% to represent a small degree of heterogeneity, 50% a moderate degree and 75% a large degree [19]. To obtain a pooled estimate of the effect in the meta-analysis of the heterogeneous studies, we performed a random-effects model, as described by DerSimonian and Laird [20]. The estimated SMDs were interpreted as described by Hopkins et al. an SMD of 4.0 was considered to represent an extremely large clinical effect; 2.0–4.0 a very large effect; 1.2–2.0 a large effect; 0.6–1.2 a moderate effect; 0.2–0.6 a small effect; and 0.0–0.2 a trivial effect [21].
Overall strength of the evidence
Strength of the evidence across the systematic reviews was assessed using the Physical Activity Guidelines Advisory Committee (PAGAC) [22]. For the PAGAC analysis, the findings were evaluated according to five criteria: (1) applicability of the study sample, exposures, and outcomes to the research question; (2) generalizability to the population of interest; (3) risk of bias or study limitations; (4) quantity and consistency of findings across studies; and (5) magnitude and precision of the effect. The strength of the evidence was classified according to the PAGAC as strong, moderate, limited or not assignable.
Overlapping analysis
To assess the overlap of primary studies among systematic reviews the GROOVE (Graphical Representation of Overlap for OVErviews) methodological approach was employed. This tool is intended to be used mainly by authors of overviews of systematic reviews. The tool was made in Microsoft Excel, which we think could boost its wide usage [23].
Results
Figure 1 presents the PRISMA flow diagram, showing the different stages of the review process, including reasons for exclusion. One hundred fifteen initial studies were identified through databases and nine additional studies were identified through other sources. After removing duplicates and eliminating studies that the authors were unable to retrieve, 88 studies were screened for eligibility. Studies were excluded if they were not related to a population of patients suffering from ESRD that underwent treatment with dialysis or if the intervention did not include a form of resistance training. After excluding all the papers that did not meet the criteria, 24 studies were finally included in the analysis.
Fig. 1.
PRISMA flow Diagram
Characteristics of the included studies
Table 1 reports the characteristics of the studies included in the umbrella analysis (n = 24). Studies were published between 2010 and 2021 and included a total of 13,415 participants. Participants included in the studies were adults (> 18 years) suffering from ESRD under maintenance dialysis, who received an intervention of either a resistance training program or a combined training program that included resistance exercises. Specific details of the included interventions are summarized in Table 2. Exercise interventions lasted from 4 weeks to 1 year, exercise was mostly performed 2–3 times per week and lasted from 10 min to 2 h. Exercise intensity was mainly assessed with Borg’s Rating of Perceived Exertion scale. Control or comparison groups were defined as patient’s receiving regular dialysis treatment, considered to be usual care, or placebo or sham exercise, that consisted in stretching exercises or passive range of movement exercises.
Table 1.
Description of meta-analysis studies included
| Study | Number of studies | Design of studies | Sample in RT interventions | CKD Type and patient characteristics | Intervention and control group | Outcomes | ||
|---|---|---|---|---|---|---|---|---|
| Number of RT studies included in meta-analysis | Scales of measurement | Results | ||||||
| Andrade et al. [24] | 15 | RCTs | n = 243 |
CKD patients who underwent intradialytic exercise protocols Mean age range: 42–68 years |
Intervention: RT + AE or RT + AE + Flexibility Control: RDT |
5 | Functional aerobic capacity (VO2max) | Significant improvements (MD = −1.64, CI 95% −3.21 to −0.07) |
| Bernier-Jean et al. [25] | 89 | RCTs and quasi-RCTs (allocation to treatment was obtained through alternation) | n = 1.245 |
Adults receiving maintenance dialysis treatment Mean age range: 30–72 years |
Intervention: RT, RT + AE or Pilates Control: No exercise or placebo exercise |
36 | Functional capacity (6MWT) |
Improved scores RT (MD: 44.7, CI 95% 27.0–62.4) Improved scores RT + AE (MD = 53.6, CI 95% 39.4–67.9) |
| HRQOL (SF-36) |
Improved scores RT (MD = 2.5, CI 95% 1.3–6.3) Improved scores RT + AE (MD = 4.4, CI 95% 1.9–6.8) |
|||||||
| HRQOL (SF-36) |
Improved scores RT (MD = 0.7, CI 95% −5.9 to 4.6) Improved scores RT + AE (MD = 2.6, CI 95% 1.7–6.9) |
|||||||
| HRQOL (SF-36) |
Improved scores RT (MD = 10.7, CI 95% 6.5–28.0) Improved scores RT + AE (MD = 4.0, CI 95% 2.5–10.5) |
|||||||
| Depression (BDI) |
Improved scores RT (SMD = 0.52, CI 95% 0.92 to 0.12) Improved scores RT + AE (SMD = -1.0, CI 95% -1.7 to -0.3) |
|||||||
| Bogataj et al. [26] | 104 | RCTs with a pre-post intervention lasting > 8 weeks | n = 909 |
Adult end-stage kidney disease HD patients Average age: 55.7 ± 9.6 years |
Intervention: RT or RT + AE Control: Usual care |
20 | Functional Capacity (6MWT) |
Moderate effects RT (ES = 0.10, CI 95% −0.19 to 0.39) Moderate effects RT + AE (ES = 0.71, CI 95% −0.06 to 1.48) |
| Functional aerobic capacity (VO2max) |
Moderate effects RT + AE (ES = 0.67, CI 95% 0.33–1.01) |
|||||||
| Cheema et al. [27] | 23 | RCTs | n = 271 |
Adults with CKD (Stage 1–5) receiving maintenance HD Mean age range: 43–69 years |
Intervention: RT Control: Usual care or stretching exercises |
7 | Peak force (dynamometry) |
Significant improvement RT (SMD = 1.15, CI 95% 0.80–1.49) |
| HRQOL (SF-36) |
Significant improvement RT (SMD = 0.83, CI 95% 0.51–1.16) |
|||||||
| Chung et al. [28] | 17 | RCTs | n = 284 |
Participants aged > 18 years with ESRD requiring HD > 3 months Mean age range: Not reported |
Intervention: RT, RT + AE Control: No exercise |
5 | Functional aerobic capacity (VO2max) |
Significant improvement RT + AE (SMD = 0.50, CI 95% 0.26–0.74) |
| Depression (BDI) |
Significant improvement RT + AE (SMD = −0.80, CI 95% −1.10 to −0.50) |
|||||||
| HRQOL (SF-36) |
Significant improvement RT + AE (SMD = 0.46, CI 95% 0.20–0.73) |
|||||||
| Clarkson et al. [29] | 27 | RCTs | n = 616 |
Patients aged years with stage 5 CKD undergoing HD Mean age range: Not reported |
Intervention: RT, RT + AE, EMS Control: Usual care, non-exercising or undergoing only range of motion passive exercises |
15 | Functional capacity (6MWT) |
Significant improvement RT (ES = 23.62, CI 95% 6.45–40.79) |
| Ferrari et al. [30] | 50 | RCTs | n = 782 |
Adults years with ESRD undergoing HD Mean age range: 20–73.9 years |
Intervention: RT, RT + AE Control: Usual care or sham exercises |
20 | Functional capacity (6MWT) |
Significant improvement RT (MD = 68.50, CI 95% 29.05–107.96) |
| Functional aerobic capacity (VO2max) |
Significant improvement RT + AE (MD = 5.41, CI 95% 4.03–6.79) |
|||||||
| Diastolic BP (mmHg) |
Significant reduction RT + AE (MD = −5.76, CI 95% −8.83 to −2.70) |
|||||||
| Ferreira et al. [31] | 8 | RCTs | n = 179 |
Adults > 18 years with CKD on maintenance hemodialysis Mean age range: 18–70 years |
Intervention: RT + AE Control: Routine care or education exercise |
4 | Depression (BDI) |
Significant improvement RT + AE (SMD = −0.66, CI 95% −1.00 to −0.33) |
| Gomes Neto et al. [32] | 56 | RCTs | n = 1.255 |
Maintenance HD patients Mean age range: 20–73.9 years |
Intervention: RT, RT + AE Control: No exercise |
27 | Functional aerobic capacity (VO2max) |
Significant improvement RT + AE (MD = 5.1, CI 95% 3.4–6.8) |
| Functional capacity (6MWT) |
Significant improvement RT (MD = 30.2, CI 95% 24.6–35.9) Significant improvement RT + AE (MD = 45.2, CI 95% 7.8–82.6) |
|||||||
| Peak force (dynamometry) |
Significant improvement RT (MD = 12.5, CI 95% 0.9–24.0) |
|||||||
| HRQOL (SF-36) |
Significant improvement RT Physical Component Score (MD = 9.53, CI 95% −3.09 to 22.15) Significant improvement RT + AE Physical Functioning (MD = 10.67, CI 95% 1.08–20.25) Significant improvement RT + AE Vitality (MD = 10.01, CI 95% 4.30–15.72) |
|||||||
| Depression (BDI) |
Significant reduction RT + AE (MD = −7.3, CI 95% −9.3 to −5.3) |
|||||||
| Heiwe et al. [33] | 41 | RCTs | n = 787 |
Adults with CKD (Stage 2–5) Mean age range: 36–71 years |
Intervention: RT, RT + AE Control: No exercise or sham exercise |
18 | Functional aerobic capacity (VO2max) |
Significant improvement RT + AE (SMD = −0.77, CI 95% −1.06 to −0.48) |
| Diastolic BP (mmHg) |
Significant improvement RT + AE (SMD = 0.41, CI 95% 0.02–0.80) |
|||||||
| Systolic BP (mmHg) |
Significant improvement RT + AE (SMD = 0.33, CI 95% 0.04–0.62) |
|||||||
| Peak force (dynamometry) |
Significant improvement RT + AE (SMD = −0.43, CI 95% −0.79 to −0.08) Significant improvement RT (SMD = −0.55, CI 95% −0.90 to −0.19) |
|||||||
| Hu et al. [34] | 33 | RCTs | n = 992 |
Adults 18 years diagnosed with ESRD under HD > 3 months Mean age range: Not reported |
Intervention: RT, RT + AE Control: Usual care, sham exercise, or no exercise |
21 |
HRQOL (SF-36) |
Significant improvement RT Physical Component Scale (SMD = 0.23, CI 95% 0.02–0.44) Significant improvement RT + AE Physical Component Scale (SMD = 0.50, CI 95% 0.22–0.77) Significant improvement RT Mental Component Scale (SMD = 0.28, CI 95% 0.03–0.54) Significant improvement RT Physical Functioning (SMD = 0.53, CI 95% 0.16–0.90) Significant improvement RT + AE Physical Functioning (SMD = 0.49, CI 95% 0.05–0.92) Significant improvement RT + AE Role Physical (SMD = 0.21, CI 95% −0.09–0.50) Significant improvement RT Vitality (SMD = 0.37, CI 95% 0.12–0.63) Significant improvement RT + AE Role Emotional (SMD = 0.34, CI 95% 0.04–0.64) Significant improvement RT Mental Health (SMD = 0.44, CI 95% 0.13–0.74) |
| Huang et al. [35] | 20 | RCTs | n = 435 |
Participants > 18 years diagnosed with ESRD under HD > 3 months Mean age range: 30–70 years old |
Intervention: RT, RT + AE Control: Usual care or sham exercise |
10 | Functional aerobic capacity (VO2max) |
Significant improvement RT + AE (SMD = 0.78, CI 95% 0.51–1.05) |
| Lu et al. [36] | 21 | RCTs | n = 539 |
Adult participants (age > 18 years old) with clinical diagnosis of kidney failure undergoing HD > 3 months Mean age range: Not reported |
Intervention: RT, RT + AE Control: Usual care, no exercise, sham exercise, attention care or placebo |
14 | Physical performance (6MWT) |
Significant improvement RT (MD = 41.92, CI 95% 8.06–75.75) |
| Peak force (dynamometry) |
Significant improvement RT (MD = 3.93, CI 95% 0.59–7.28) |
|||||||
| Matsuzawa et al. [37] | 39 | RCTs | n = 759 |
Participants at least 18 years of age undergoing hemodialysis Mean age range: Not reported |
Intervention: RT, RT + AE Control: Usual care or no exercise |
19 | Physical performance (6MWT) |
Significant improvement RT + AE (SMD = 0.58, CI 95% 0.24–0.93) |
| Functional aerobic capacity (VO2max) |
Significant improvement RT + AE (SMD = 0.62, CI 95% 0.38–0.87) |
|||||||
|
Peak force (dynamometry) |
Significant improvement RT + AE (SMD = 0.94, CI 95% 0.67–1.21) |
|||||||
| HRQOL (SF-36) |
Significant improvement RT + AE (SMD = 0.53, CI 95% 0.52–0.82) |
|||||||
| Molsted et al. [38] | 8 | RCTs | n = 290 |
Participants 18 years undergoing HD 1 month Mean age range: 40.2–71.1 years |
Intervention: RT Control: Usual care or stretching |
8 | HRQOL (SF-36) |
Significant improvement RT Physical Component Scale (MD = 10.05, CI 95% 2.95–17.14) Significant improvement RT Physical Function (MD = 9.38, CI 95% 0.79–17.97) |
| Pu et al. [39] | 27 | RCTs | n = 499 |
Adult patients on HD > 3 months Mean age: 53 years |
Intervention: RT, RT + AE Control: No exercise |
11 | Functional aerobic capacity (VO2max) |
Significant improvement RT + AE (MD = 4.11, CI 95% 2.94–5.27) |
| Depression (BDI) |
Significant reduction RT + AE (SMD = −1.16, CI 95% −1.86 to −0.45) |
|||||||
| HRQOL (SF-36) |
Significant improvement RT + AE (MD = 7.72, CI 95% 1.93–13.51) |
|||||||
| Diastolic BP (mmHg) |
Significant improvement RT + AE (MD = −4.11, CI 95% −6.50 to −1.72) |
|||||||
| Systolic BP (mmHg) |
Significant improvement RT + AE (MD = −4.87, CI 95% −9.20 to −0.55) |
|||||||
| Scapini et al. [40] | 33 | Controlled trials | n = 782 |
Adults requiring HD for ESRD Mean age range: Not reported |
Intervention: RT, RT + AE Control: No exercise or placebo |
15 | Functional aerobic capacity (VO2max) |
Significant improvement RT + AE (MD = 5.00, CI 95% 3.50–6.50) |
| Diastolic BP (mmHg) |
Significant reduction RT + AE (MD = −5, CI 95% −6 to −3) |
|||||||
| Systolic BP (mmHg) |
Significant reduction RT + AE (MD = −9, CI 95% −13 to −4) |
|||||||
| Schardong et al. [41] | 10 | RCTs and controlled clinical trials | n = 224 |
Patients with CKD undergoing HD > 3 months Mean age range: 46.4–68.6 years |
Intervention: RT Control: Control, placebo, or exercise with no EMS |
8 | Peak force (dynamometry) |
Significant improvement RT Lower limb (SMD = 1.46, CI 95% 0.86–2.07) Significant improvement RT Upper limb (MD = 10.02, CI 95% 0.78–19.27) |
| Functional capacity (6MWT) |
Significant improvement RT (MD = 30.11, CI 95% 15.57–44.65) |
|||||||
| Segura-Orti [42] | 14 | RCTs | n = 364 |
Adults (> 18 years) undergoing HD Mean age range: 32–65 years |
Intervention: RT, RT + AE Control: No exercise or placebo |
6 | HRQOL (SF-36) |
Significant improvement RT Physical Component Scale (SMD = 11.03, CI 95% 5.63–16.43) |
| Functional aerobic capacity (VO2max) |
Significant improvement RT + AE (SMD = 5.57, CI 95% 2.52–8.61) |
|||||||
| Sheng et al. [43] | 24 | RCTs | n = 451 |
HD patients Mean age range: 46.6–69 years |
Intervention: RT, RT + AE Control: No exercise |
11 | Functional aerobic capacity (VO2max) |
Significant improvement RT + AE (SMD = 0.92, CI 95% 0.53–1.31) |
| Wu et al. [44] | 12 | RCTs and quasi-randomized controlled trials | n = 745 |
Adults 18 years with CKD stages 1–5 Mean age range: 47.5–80.3 years |
Intervention: RT + AE Control: Usual care and no exercise |
12 | Diastolic BP (mmHg) |
Significant improvement RT + AE (MD = −3.63, CI 95% −5.35 to −1.91) |
| Systolic BP (mmHg) |
Significant improvement RT + AE (MD = −5.24, CI 95% −7.93 to −2.54) |
|||||||
| Zhang et al. [45] | 14 | RCTs | n = 594 |
Adults (> 18 years) undergoing HD > 3 months Mean age range: 28.5 to 81.4 years |
Intervention: RT Control: No exercise or stretching |
14 | Functional capacity (6MWT) |
Significant improvement RT (SMD = 0.52, CI 95% 0.28–0.75) |
ADLSS activity daily living summary score; AE aerobic exercise; BAI Beck Anxiety Index; BALP bone-specific alkaline phosphatase; BDI Beck depression inventory; BMD bone mineral density; BP blood pressure; CI confidence interval; CKD chronic kidney disease; CPET cardiopulmonary exercise test; CRP C-reactive protein; CSA cross sectional area; DASS Depression Anxiety Stress Scale; DBS diastolic blood pressure; EMS electrical myostimulation; ES effect size; GXT graded exercise test; ESRD end stage renal disease; HADS Hospital Anxiety Depression Scale; HD hemodialysis; HRQOL health-related quality of life; L left; LBM lean body mass; MD mean difference; MRI magnetic resonance imaging; OPG osteoprotegerin; PASE physical activity scale elderly; RCT randomized controlled trial; RDT regular dialysis treatment; R right; RT resistance training; SBP systolic blood pressure; SD standard deviation; SF-36 short-form 36 questionnaire; STS sit-to stand-to sit test; 6MWT 6 Minute Walking Test
Table 2.
Interventions included in each of the systematic reviews
| Study | Group | Exercise modality | Intervention characteristics | Intervention duration | Frequency | Session duration | ||
|---|---|---|---|---|---|---|---|---|
| RT Protocol (distribution and exercise type) | RT Work interval (duration and intensity) | RT Rest interval (duration and intensity) | ||||||
| Andrade et al. [24] | Exercise | 2 RT + AE, 3 RT + AE + Flexibility |
- Prior to dialysis or during dialysis - 2–10 min WU - From 2 sets × 8 repetitions to 3 sets × 15 repetitions - Exercises with thera-bands and weights - Isotonic and isometric exercises for abdomen and lower limbs |
- Not reported - Intensity Borg scale (RPE) 13 or 60–70% of the HRMAX |
Not reported | 3–12 months | Three times per week | 30–90 min or not reported |
| Control | RDP | |||||||
| Bernier-Jean et al. [25] | Exercise | 21 RT, 19 RT + AE |
-Prior to dialysis or during dialysis - 2–10 min WU - From 1 to 4 sets × 8 to 30 repetitions - Solely lower body exercises or both upper and lower limb exercises - Closed and open chain strengthening exercises - Weights, resistance bands, both, Response Seated Leg Curl Thigh Extension pulley or leg press machine |
- Concentric phase as fast as possible - Concentric phase 1.5 s, isometric phase 0.5 s and eccentric phase 3 s - 5 s isometric contraction - Intensity Borg scale (RPE), Omni scale, % of 1, 3 or 5RM, 60–70% of the HRMAX - Not reported |
- 1–2-min rest between sets - 1 min between sets, 3 min between exercises - 3-min rest between exercise periods - According to the necessity of the patient - Not reported |
8 weeks to 2 years | 2–5 times per week | 30–110 min or not reported |
| Control | No exercise or placebo exercise | |||||||
| Bogataj et al. [26] | Exercise | 9 RT, 12 RT + AE |
- Prior to dialysis or during dialysis - 2–10 min WU - From 2 to 3 sets × 8 to 10 repetitions - Free-weight dumbbells and weighted ankle cuffs - Equipment designed to fit the end of the dialysis chair - Response Seated Leg Curl Thigh Extension pulley - Calisthenics, steps and low weight exercises |
- 4:1 work/rest intervals or not reported - Intensity Borg scale (RPE), % of the 1-5RM, 50–60% of VO2max, 60–70% of HRmax or subjective assessment |
- 1–2 min between sets and exercise - Not reported |
8–40 weeks | 2–4 times per week | 13–90 min or not reported |
| Control | Usual care, stretching, walking program at home or ROM exercises | |||||||
| Cheema et al. [27] | Exercise | 7 RT |
- Prior to dialysis or during dialysis - From 2 to 3 sets × 8 to 15 repetitions - Both upper and lower limb exercises - Exercise with weights, elastic bands or sandbags - Exercise using weighted dumbbells, ankle cuffs and elastic tubing - Standard machine weights -Pneumatic resistance equipment - Exercises based con the DPGE |
- 1.5 s concentric phase, 0.5 s isometric and 3 s eccentric phase or not reported - Intensity 40–80% of 1-3RM or 15–17 Borg’s scale |
1–2 min between sets or not reported | From 8 to 24 weeks | From 2 to 3 times per week | 20–45 min or not reported |
| Control | Usual care or stretching exercises | |||||||
| Chung et al. [28] | Exercise | 4 RT, 1 RT + AE |
- During dialysis or immediately before - From 2 to 3 sets × 8 repetitions - Both upper and lower limbs - Exercise with ankle weights, Thera-band tubing or free-weight dumbbells - EMS |
- 20 s stimulation/ 20 s rest - Not reported - Intensity 60% of 3RM or 15–17 Borg’s scale |
- 1–2 min between sets and exercises - Not reported |
From 8 to 20 weeks | From 2 to 3 times per week | 10–60 min or not reported |
| Control | No exercise | |||||||
| Clarkson et al. [29] | Exercise | 9 RT, 3 RT + AE, 3 EMS |
- From 1 to 3 sets × 8 to 15 repetitions - Free-weight exercises with dumbbells for the upper body and ankle weights for the lower body - Sets of 10 to 12 exercises, combining upper body, lower body and core training |
- From 2 to 20 s EMS stimulation - Not reported - Intensity % of 1–3 RM or 9–17 Borg’s scale |
- 10–50 s rest between EMS stimulation - Not reported |
From 8 to 22 weeks | From 2 to 3 times per week | 15–60 min or not reported |
| Control | Usual care, non-exercising or undergoing only range of motion passive exercises | |||||||
| Ferrari et al. [30] | Exercise | 14 RT, 7 RT + AE |
- During dialysis or on non-dialysis days - 5-to-10-min WU - From 2 to 3 sets × 8 to 20 repetitions - Isometric and isotonic exercises for the abdomen and lower limbs - Participants asked to perform as many repetitions as possible - Exercises in both lower limbs and the non-dialyzed upper limb - Exercise with ankle weights, dumbbells and elastic bands - Leg press equipment |
- 3–5 s isometric contraction - Co-isometric contraction held for 10 s - 2 s concentric and 2 s eccentric for each repetition - Not reported - Intensity 40–80% of the 1-3RM, according to participant’s subjective tolerance or 15–17 Borg’s scale |
- 1-to-2-min rest between sets - 1 min rest between sets and 3-min rest between exercises - 1 min rest between sets and exercises - Rest according to the necessity of the participants - Not reported |
From 8 weeks to 1 year | From 2 to 3 times per week | From 10 min to 2 h |
| Control | Usual care or sham exercises | |||||||
| Ferreira et al. [31] | Exercise | 4 RT + AE |
- During dialysis or non-dialysis days - From 2 to 3 sets × 10 to 15 repetitions - 7 exercises involving lower and upper limbs - Dynamic closed and open-chained exercises - Exercise with Thera-band resistive bands and soft weights |
- Not reported - Intensity 50% of 1RM or 13–15 Borg’s scale |
- Not reported | From 4 weeks to 1 year or not reported | 3–4 times per week | From 15 to 90 min |
| Control | Control: Routine care or education exercise | |||||||
| Gomes Neto et al. [32] | Exercise | 16 RT, 10 RT + AE |
- Before, after or during dialysis - From 2 to 5-min WU - From 4 to 10 types of exercises - From 2 to 4 sets × 8 to 30 repetitions - Concentric and eccentric contractions - Isotonic and isometric exercises for lower limbs and abdomen - Exercises with free-weight dumbbells, thick colored elastic bands, ankle cuffs, sandbags and leg press equipment - Response Seated Leg Curl Thigh Extension pulley |
- 1.5 s concentric phase, 0.5 s isometric and 3 s eccentric phase - 5 s isometric contraction - Co-isometric contraction held for 10 s - Not reported - Intensity 60–80% of 1-3RM, 11–17 Borg’s scale, according to participant’s tolerance or not reported |
-1-to-2-min rest between sets and exercises - 3-min rest between exercises - 60 s rest between sets - Not reported |
From 8 weeks to 1 year | From 2 to 3 times per week | From 20 to 90 min or not reported |
| Control | No exercise | |||||||
| Heiwe et al. [33] | Exercise | 13 RT, 5 RT + AE |
- During dialysis or on non-dialysis days - 2–10 min WU - From 1 to 3 sets × 8 to 15 repetitions - Exercise with resistance training equipment - Only lower body exercises using ankle weights - Isometric and isotonic exercises for the lower body and abdomen - Response Seated Leg Curl Thigh Extension pulley - Pneumatic leg press machine |
- 1.5 s concentric phase, 0.5 s isometric and 3 s eccentric phase - 2 s concentric and 2 s eccentric - Not reported - Intensity 60–80% of 1-5RM, individual basis to 60–70% of HRMax, 40–60% of VO2PEAK or 12–17 Borg’s scale |
- 1-to-2-min rest between sets and exercises - 1 min between sets - Not reported |
From 12 weeks to 1 year | From 2 to 5 times per week | From 30 to 110 min or not reported |
| Control | No exercise or sham exercise | |||||||
| Hu et al. [34] | Exercise | 11 RT, 10 RT + AE |
- During dialysis - From 3 to 5-min WU - From 1 to 3 sets × 8 to 20 repetitions - As many repetitions as possible - Repetitions until momentary failure occurred - From 4 to 11 types of exercises using elastic bands and soft weights - 10 sets of 10 repetitions lower limb raising - Exercises in both lower limbs and the contralateral arteriovenous fistula upper limb |
- 1.5 s concentric phase, 0.5 s isometric and 3 s eccentric phase - Not reported - Intensity 60% of 1-3RM, 10–17 Borg’s scale or according to participant’s tolerance |
- 1–2-min rest between each set - 2-to-3-min rest between each exercise - Rest according to the necessity of the participants - Not reported |
From 8 weeks to 10 months | From 2 to 3 times per week | From 20 to 60 min or not reported |
| Control | Usual care, sham exercise or no exercise | |||||||
| Huang et al. [35] | Exercise | 4 RT, 7 RT + AE |
- Prior, after or during dialysis - 2-to-5-min WU - From 1 to 3 sets × 8 to 15 repetitions - 10 types of exercise with free-weight dumbbells, weighted ankle cuffs and elastic band tubing - Response Seated Leg Curl Thigh Extension pulley |
- Not reported - Intensity 50–60% of the 1-3RM, 12–17 Borg’s scale or according to participant’s tolerance |
- 60 s between sets - Not reported |
From 8 weeks to 1 year | From 2 to 3 times per week | From 10 to 90 min or not reported |
| Control | Usual care or sham exercise | |||||||
| Lu et al. [36] | Exercise | 10 RT, 4 RT + AE |
- During dialysis - 5-to-10-min WU - From 1 to 2 sets × 8 to 20 repetitions - From 10 to 11 types of exercise - Exercises with grip ring, free weight dumbbells, ankle cuffs or leg press equipment - Training with chair stand exercise - Response Seated Leg Curl Thigh Extension pulley |
- 10 s isometric contractions - 1.5 s concentric phase, 0.5 s isometric and 3 s eccentric phase - 5 to 10 s held isometric contractions - Standing up in 3 s and sitting down in 3 s - Intensity 50–80% of 1-5RM, 60% of individual’s maximal capacity, 12–17 Borg’s scale or not reported |
- 1 to 2 min between sets and exercises - 2-min rest between sets - According to the necessity of the participants - Not reported |
From 8 weeks to 5 months | From 2 to 3 times per week | From 15 to 60 min or not reported |
| Control | Usual care, no exercise, sham exercise, attention care or placebo | |||||||
| Matsuzawa et al. [37] | Exercise | 13 RT, 6 RT + AE |
- Prior, after or during dialysis - 5-min WU - From 1 to 3 sets × 10 to 15 repetitions - Exercises with a pneumatic leg press machine - Exercises for upper and lower body using elastic bands, sandbags and dumbbells - Response Seated Leg Curl Thigh Extension pulley |
- 1.5 s concentric phase, 0.5 s isometric and 3 s eccentric phase - Not reported - Intensity 50–80% of 1-5RM, 60% of individual’s maximal capacity, 12–17 Borg’s scale or not reported |
- 1 to 2 min between sets - 1 min between sets - Not reported |
From 8 weeks to 12 months | From 2 to 3 times per week or not reported | From 20 to 90 min or not reported |
| Control | Usual care or no exercise | |||||||
| Molsted et al. [38] | Exercise | 8 RT |
- Prior, after or during dialysis - 5-min WU - From 1 to 3 sets × 10 to 15 repetitions - Exercises with a pneumatic leg press machine - Exercises for upper and lower body using elastic bands, sandbags and dumbbells - Response Seated Leg Curl Thigh Extension pulley |
- 1.5 s concentric phase, 0.5 s isometric and 3 s eccentric phase - Not reported - Intensity 50–80% of 1-5RM or 15–17 Borg’s scale |
- 1–2 min between sets - 1 min between sets - 2-min rest period between sets - Not reported |
From 12 weeks to 6 months | 3 times per week or not reported | 30 min or not reported |
| Control | Usual care or stretching | |||||||
| Pu et al. [39] | Exercise | 4 RT, 7 RT + AE |
- Prior, after or during dialysis - 5-min WU - Isotonic and isometric exercises for the lower limb - Exercises for upper and lower body using elastic bands, sandbags and dumbbells |
- 1.5 s concentric phase, 0.5 s isometric and 3 s eccentric phase Intensity 50 to 80% of 1–5 RM or 15–17 Borg’s scale - Not reported |
- 1–2 min between sets - 1 min between sets |
From 8 weeks to 12 months | From 2 to 3 times per week or not reported | From 10 to 90 min or not reported |
| Control | No exercise | |||||||
| Scapini et al. [40] | Exercise | 5 RT, 10 RT + AE |
- Prior to, after, during dialysis or on non-dialysis days - 5-to-10-min WU - From 2 to 3 sets × 8 to 15 repetitions - Isotonic and isometric exercises for the lower limb and abdomen - 4 lower limb exercises with ankle cuffs and elastic bands -5 upper limb exercises using dumbbells and 5 lower limb exercises using ankle weights - Close and open-chained exercises - Apparatus for leg extension and flexion - Response Seated Leg Curl Thigh Extension pulley |
- Intensity 50–80% of the 1-5RM, 60–70% HRMax or 12–17 Borg’s scale |
- 1 min rest between sets - Not reported |
From 8 weeks to 1 year | From 2 to 4 times per week | From 20 to 90 min or not reported |
| Control | No exercise or placebo | |||||||
| Schardong et al. [41] | Exercise | 8 EMS |
- During dialysis - 5-min WU - Exercise with dual-channeled portable stimulators, a calibrated electrical stimulator or a neoprene garment |
- From 1.5 to 20 s stimulation - Intensity according to patient’s tolerance or maximum painless level of stimulation |
- From 0.75 to 20 s rest between stimulations - Rest time decreasing as the protocol advances (from 50 to 10 s) - Not reported |
From 8 to 20 weeks | From 2 to 3 times per week | From 20 to 60 min or not reported |
| Control | Control, placebo or exercise with no EMS | |||||||
| Segura-Orti [42] | Exercise | 2 RT, 4 RT + AE |
- Before, after, during dialysis or on non-dialysis days - 2-to-10-min WU - From 1 to 3 series × 8 to 10 repetitions - 10 exercises for lower and upper body - 14 types of low resistance - Response Seated Leg Curl Thigh Extension pulley |
- Not reported - Intensity 50–60% of the 3-5RM or Borg’s scale 15–17 |
- Not reported | From 12 to 24 weeks | From 2 to 4 times per week | From 30 to 90 min or not reported |
| Control | No exercise or placebo | |||||||
| Sheng et al. [43] | Exercise | 7 RT, 4 RT + AE |
- Prior, after or during dialysis - From 2 to 5-min WU - From 2 to 3 series × 8 to 15 repetitions - Lower body exercises using ankle weights - Upper and lower body exercises using elastic bands and sandbags - 10 types of exercises - isotonic quadriceps and hamstring exercises - Isotonic and isometric exercises for the lower limbs and abdomen - Response Seated Leg Curl Thigh Extension pulley |
- 1.5 s concentric phase, 0.5 s isometric and 3 s eccentric phase - Intensity 40–60% of the 1-5RM, 65–85% of VO2max or 11–17 Borg’s scale |
- 60 s rest between sets - 1 to 2 min between sets - Not reported |
From 8 weeks to 10 months | From 2 to 3 times per week | From 10 to 60 min or not reported |
| Control | No exercise | |||||||
| Wu et al. [44] | Exercise | 12 RT + AE |
- During dialysis or on non-dialysis days - 10-min WU - From 1 to 3 set × 8 to 30 repetitions - Exercises for a variety of upper and lower body muscle groups - Exercises with a handgrip strengthening device and mid-level load exercises - Exercises in fixed resistance machines |
- Not reported - Intensity 70–80% of the 1RM, 12–14 Borg’s scale, 50–80% of heart rate reserve or not reported |
- Not reported | From 12 weeks 1 year |
- From 2 to 3 times per week - 6 times per month |
From 60 to 120 min or not reported |
| Control | Usual care and no exercise | |||||||
| Zhang et al. [45] | Exercise | 14 RT |
- Prior to or during dialysis - 5-to-10-min WU - From 3 to 4 sets × 8–30 repetitions - Leg press exercises - 6 upper body and 6 lower body exercises with elastic bands and sandbags - 11 different types of exercises |
- 1.5 s concentric phase, 0.5 s isometric and 3 s eccentric phase - Not reported - Intensity 70–80% of the 1-5RM, 12–17 Borg’s scale |
-2-min rest period between sets - 1-to-2-min rest between sets - 1 min rest between sets - 3-min rest between exercises - Rest according to the necessity of the patient - Not reported |
From 12 to 24 weeks | - From 2 to 3 times per week | From 30 to 50 min or not reported |
| Control | No exercise or stretching | |||||||
AE aerobic exercise; DPGE dialysis patient’s guide exercise; EMS electrical myostimulation; HRMAX maximum heart rate; MIP maximum inspiratory pressure; RDT regular dialysis treatment; RM repetition maximum; ROM range of motion; RPE rate perceived exertion; RT resistance training; WU warm-up
Methodological quality analysis
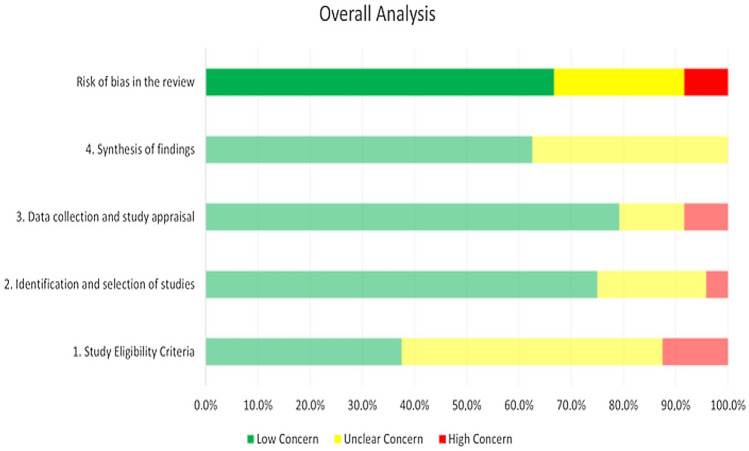
To assess the level of risk of bias, two independent reviewers used the ROBIS tool, and discrepancies were resolved by discussion and consensus. Figure 2 shows a summary plot graph summarizing the results obtained for each of the included reviews. Overall results showed that most of the studies had low risk of bias (67.5%). The risk of bias in the remaining studies was unclear (25%) or high (7.5%). The level of agreement between the reviewers in the assessment was high (κ = 0.820).
Fig. 2.
Summary plot graph of the ROBIS Scale
Main findings
Functional capacity
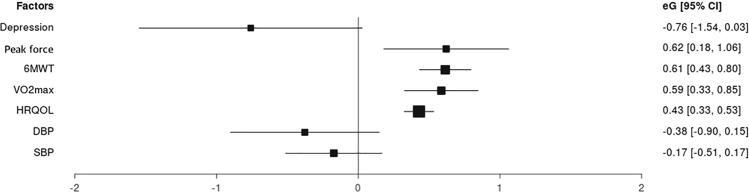
Nine studies (38%) [25, 26, 29, 30, 32, 36, 37, 41, 45] including 2290 participants reported effects of resistance training on functional capacity. Functional capacity was assessed in every study through the 6MWT. Overall effect size of resistance training with respect to functional capacity showed statistically significant differences with a moderate clinical effect (SMD = 0.614, CI 95% 0.433–0.795), and heterogeneity among the studies was high (I2 = 74.43%) (Fig. 3). The certainty of evidence was moderate, showing that resistance training increases functional capacity in patients with ESRD (Table 3).
Fig. 3.
Forest plot
Table 3.
Summary of findings and quality of evidence according to Physical Activity Guidelines Advisory Committee Grading Criteria (PAGAC)
| Systematic review research questions | Applicability | Generalizability | Risk of bias or study limitations | Quantity and consistency | Magnitude and precision of effect | Effect [95% CI] | Evidence |
|---|---|---|---|---|---|---|---|
| Depression | Strong | Strong | Limited | Limited | Strong | −0.76 [−1.54, 0.03] | Moderate |
| Peak force | Strong | Strong | Limited | Limited | Strong | 0.62 [0.18, 1.06] | Moderate |
| 6MWT | Strong | Strong | Limited | Limited | Strong | 0.61 [0.43, 0.80] | Moderate |
| VO2 max | Strong | Strong | Limited | Limited | Strong | 0.59 [0.33, 0.85] | Moderate |
| HRQOL | Strong | Strong | Limited | Limited | Strong | 0.43 [0.33, 0.53] | Moderate |
| DBP | Strong | Strong | Limited | Limited | Strong | −0.38 [−0.90, 0.15] | Moderate |
| SBP | Strong | Strong | Limited | Limited | Strong | −0.17 [−0.51, 0.17] | Moderate |
95% CI: 95% Confidence interval; HRQOL: Health Related Quality of Life; 6MWT: 6 Minute Walking Test; SBP: Systolic Blood Pressure; DBP: Diastolic blood pressure; VO2 max: Maximal oxygen uptake
Aerobic capacity
Twelve studies (50%) [24, 26, 28, 30, 32, 33, 35, 37, 39, 40, 42, 43] including 4037 participants reported the effects of resistance training on aerobic capacity. Every included study assessed aerobic capacity through the VO2max. Overall effect size of resistance training with respect to aerobic capacity showed statistically significant results with small clinical effects (SMD = 0.587, CI 95% 0.328–0.845) and high heterogeneity among the studies (I2 = 89.82%) (Fig. 3). The certainty of evidence was moderate, showing that resistance training increases aerobic capacity in patients with ESRD (Table 3).
Peak force
Six studies (25%) [27, 32, 33, 36, 37, 41] including 2160 participants reported the effects of resistance training on peak force. Every included study assessed peak force through dynamometry. Overall effect size of resistance training with respect to muscle strength showed statistically significant differences with moderate clinical effects (SMD = 0.621, CI 95% 0.181–1.061) and heterogeneity among the studies was very high (I2 = 90.752%) (Fig. 3). The certainty of evidence was moderate, showing that resistance training increases muscle strength in patients with ESRD (Table 3).
Depression
Five studies (21%) [25, 28, 31, 32, 39] including 960 participants reported the effects of resistance training on depression symptoms. Depression was assessed through the Beck Depression Inventory. Overall effect size of resistance training with respect to depression did not show statistically significant differences (SMD = −0.759, CI 95% −1.545–0.027) and heterogeneity among the studies was high (I2 = 93.75%) (Fig. 3). The certainty of evidence was moderate, showing that resistance training does not decrease depression in patients with ESRD (Table 3).
Health-related quality of life
Ten studies (42%) [25, 27, 28, 32, 34, 37–39, 42, 43] including 2481 participants assessed the effects of resistance training on HRQOL, which was assessed using the Short-Form 36 questionnaire (SF-36), specifically with its Physical Component Scale. Overall effect size of resistance training with respect to HRQOL showed statistically significant differences with small clinical effects (SMD = 0.429, CI 95% 0.326–0.532) and small levels of heterogeneity among the studies (I2 = 36.09%) (Fig. 3). The certainty of evidence was moderate, showing that resistance training increases HRQOL in patients with ESRD (Table 3).
Systolic and diastolic blood pressures
Four studies (17%) [30, 33, 39, 40] including 1067 participants reported effects of resistance training on systolic blood pressure and diastolic blood pressure. Changes were assessed by analyzing the change in values in mmHg for both systolic and diastolic blood pressures. Overall effect size of resistance training with respect to systolic blood pressure did not show statistically significant differences (SMD = −0.173, CI 95% −0.512–0.167) and heterogeneity among the studies was high (I2 = 82.61%). Overall effect size of resistance training with respect to diastolic blood pressure did not show statistically significant differences (SMD = −0.377, CI 95% −0.902–0.147) and heterogeneity among the studies was high (I2 = 88.17%) (Fig. 3). The certainty of evidence was moderate, showing that resistance training does not influence systolic and diastolic blood pressures in patients with ESRD (Table 3).
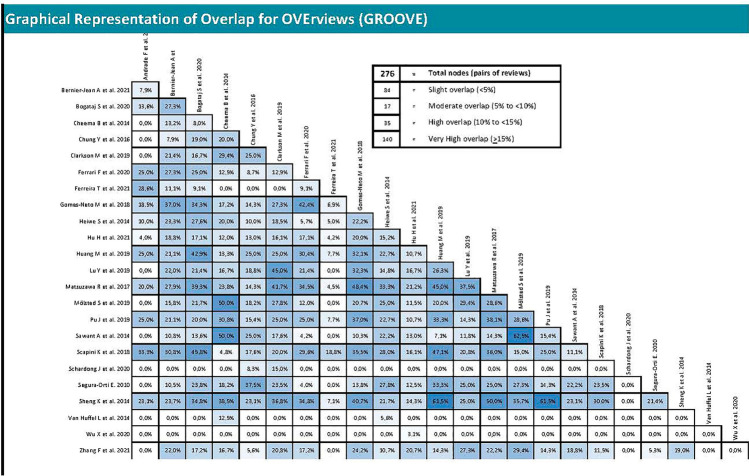
Overlapping analysis
The overlap analysis performed using the GROOVE tool revealed a total corrected overlap area of 9.45%, showing a moderate level of overlap. The detail of the overlap is shown in Fig. 4.
Fig. 4.
Graphical representation of overlap for Overviews
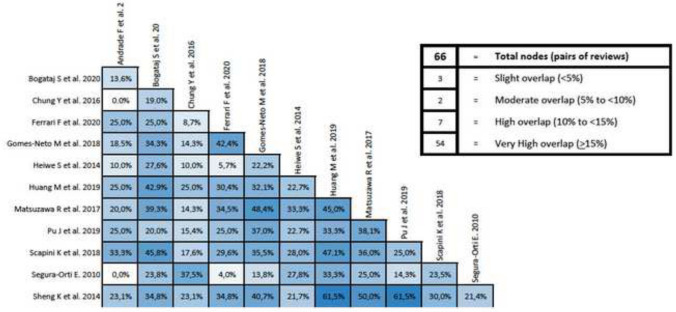
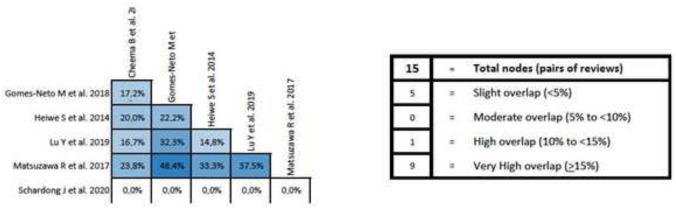
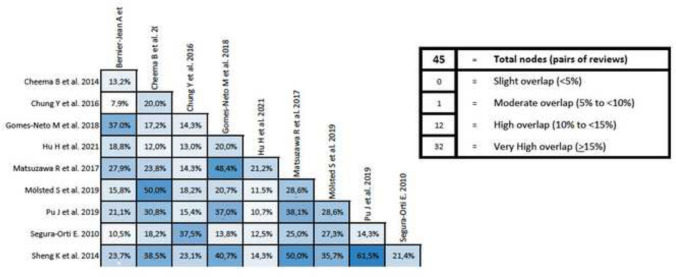
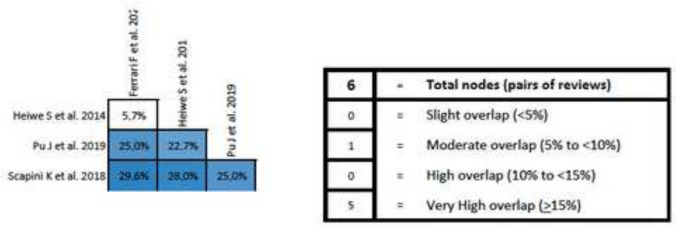
In addition, an overlap analysis was performed for each variable. In the 6MWT, a total of nine reviews including 70 different primary studies were included, which represented a corrected covered area of 18.39% (very high overlap) (Fig. 5). Regarding VO2max, 12 reviews were included with 165 different primary studies, representing a corrected covered area of 19.21% (very high overlap) (Fig. 6). Similar results were found in peak force outcome, including a total of six reviews, 51 primary studies and a corrected covered area of 16.08% (very high overlap) (Fig. 7). In relation to HRQOL, a total of 10 reviews and 63 different primary studies were included, representing a corrected covered area of 15.34% (very high overlap) (Fig. 8). Regarding the depression variable, five reviews and 47 primary studies were included, representing a corrected covered area of 18.62% (very high overlap) (Fig. 9). Finally, in SBP and DBP, four reviews and 39 primary studies were included, representing a corrected covered area of 19.66% (very high overlap) (Fig. 10).
Fig. 5.
Overlap analysis for the 6MWT
Fig. 6.
Overlap analysis for the VO2max
Fig. 7.
Overlap analysis for peak force
Fig. 8.
Overlap analysis for the HRQOL
Fig. 9.
Overlap analysis for depression
Fig. 10.
Overlap analysis for the SBP and DBP
Discussion
This umbrella review analyzed the benefits and the effects of resistance training in patients with ESRD undergoing dialysis and assessed the quality of the published literature. Multiple outcomes that relate to patient’s overall well-being were analyzed, and positive effects were found in functional capacity, aerobic capacity, peak force and HRQOL. Twenty-four studies were eventually included in the review, presenting a substantial quantity of literature evidence that outlines the benefits of resistance training in patients undergoing dialysis.
Physical factors, such as functional capacity, aerobic capacity, and peak force, as well as psychosocial factors, such as HRQOL, showed positive results, reinforcing the idea that in this usually sedentary population, exercise interventions have an effect on several dimensions that intervene in the biopsychosocial well-being of these patients. Nonetheless, some of them showed small clinical effects, but still had a considerable margin of improvement, so developing adapted exercise interventions that are controlled and that follow a specific protocol for patients on dialysis could potentially result in even greater improvements.
Both functional and aerobic capacities, assessed through the 6MWT and VO2max, respectively, showed positive results. These assessments are widely used in the literature and are closely correlated [36], therefore it is logical to assume that an improvement in one will potentially generate improvement in the other. Because functional capacity showed bigger differences than aerobic capacity, the inclusion of aerobic exercise interventions would be relevant to more specifically improve this outcome [46].
HRQOL also showed positive effects, albeit with a small clinical effect. Functional impairment also contributes to impaired HRQOL [27], therefore, an improvement in functional outcomes could also imply an improvement in HRQOL, as shown in this review. Even though there is no standard definition of HRQOL, it is widely accepted that it includes physical, psychological and social domains of life [34], and therefore an improvement in this outcome could be more limited solely by performing exercise.
Significant differences were found regarding muscle peak force, with a moderate effect. The moderate effect might be due to insufficient stimulus in the training programs, that is unable to produce enough improvement. Results presenting smaller effects could require more complete programs that include sufficient exercise intensity in the interventions, together with adequate exercise volume (quantity of exercise measured in repetitions and sets), because of the overcautious approach when applying exercise in a frail population.
Additionally, these results might be influenced by other factors involved in muscle performance, such as dietary restrictions and metabolic inflammation. Even though resistance training is considered a potent insulin-like growth factor inducer [36], restrictions in protein intake, along with enhanced proteinuria, limit the synthesis of proteins in patients with ESRD, especially in those with associated prevalent metabolic comorbidities, such as diabetes mellitus [47]. Also, the associated inflammatory environment could create abnormalities in muscle fibers due to cellular adaptations, including enzymatic and contractile protein changes, also limiting protein synthesis [41] and impairing muscle performance.
No statistically significant effects were found in depression outcomes. This might be due to the biopsychosocial nature of depression, and the necessity to deal with this problem from a more multidisciplinary approach, including psychotherapy, for instance. Also, results in this analysis show high variability, meaning that, even though depression has high prevalence rates in this population [31, 32], patients on dialysis might not suffer from it, hence not perceiving the benefits of resistance training in this outcome.
No statistically significant changes were found in either systolic or diastolic blood pressure levels. Our results are consistent with those found by Heiwe et al. [33]. Even though intradialytic exercise interventions have been proposed to improve blood pressure levels [30, 39], these results should be cautiously interpreted because these reviews do not isolate different types of exercise interventions, so their conclusions are not strictly based on resistance training interventions alone. Also, resistance training protocols that elicit the most favorable blood pressure level benefits remain elusive, and populations taking antihypertensive medications, such as patients with ESRD, show smaller reductions in blood pressure levels [48]. In patients on hemodialysis, hypertension is closely related to overload, caused by retention due both to the dialytic treatment and the disease’s pathophysiology (uremic state, anemia, electrolyte imbalance) [24]. The authors of some studies even suggest that fluid overload (> 5% above dialysis body weight) must be considered an exclusion criterion, as they deem it makes patients unable to exercise during dialysis sessions [25].
Risk of bias was assessed with the ROBIS tool, a domain-based resource supported by signaling questions. Results from this analysis showed that most studies (63%) included had low risk of bias levels. However, it is important to outline that the ROBIS tool is not a fully objective assessment resource, and some of the items that the tool includes depend on the examiner’s interpretation, so careful conclusions must be extracted from this analysis.
The overlap analysis performed using the GROOVE tool showed a total moderate level of overlap. However, when analyzing the overlap for each individual outcome, high overlapping levels were observed in every outcome (6MWT (18.39% of overlap), VO2max (19.21% of overlap), peak force (16.08% of overlap), HRQOL (15.34% of overlap), Depression (18.62% of overlap), and SBP and DBP (19.66% of overlap)). When conducting clinical trials implementing exercise interventions in participants with ESRD, throughout the literature it is common to assess the same outcomes through the same assessment methods, and this increases the chances that the same clinical trial will be included in several reviews. This could potentially create an overpowering of the results in a specific outcome, therefore creating a bias that might lead to false assumptions about the results. However, overlap can also be considered an advantage as it simplifies the meta-reviewer’s work and reinforces the conclusions that can be extracted [49]. The assessment of study overlapping is important, as systematic reviews must have well-defined search strategies and should be conducted only when necessary, avoiding repetitive reviews that might lead to unreliable and overpowered clinical results.
Our study shows several strengths. There is a considerable number of included studies, and these show appropriate methodological quality levels. Studies included in the review were consistent with the assessment method choice when analyzing every outcome of this review (functional capacity was assessed with the 6MWT, aerobic capacity with the VO2max and so on), facilitating further analysis. Finally, even though resistance training programs were inconsistent due to the lack of following a standardized developed protocol, the included exercise programs were able to show positive results, implying that even more positive results can be potentially achieved if proper strength training protocols are followed.
Finally, this review also presents limitations. Even though the research was conducted in several scientific databases and other resources were also consulted, not every existing scientific database was accessed, meaning that not every study assessing resistance training in patients undergoing dialysis might have been included. Additionally, clinical characteristics of the participants enrolled in the exercise programs, detailed descriptions of the exercise programs used, and adherence rates were not thoroughly reported or should be carefully interpreted, and this may have influenced the interpretation of the results. For instance, even though we believe that this does not have a significant effect on the results, one of the studies included in this review [27] considered a study in pre-dialysis patients.
We believe that the meta-analytic results in this study reinforce the idea of recommending resistance training exercise interventions as part of the daily management of patients undergoing hemodialysis, due to the effectiveness in improving biopsychosocial dimensions of this disease. Future studies should focus on thoroughly reporting exercise parameters to determine the optimal loading.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. No funding was received to assist with the preparation of this manuscript.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Ethical approval was not required as this umbrella review is a synthesis and analysis of existing studies.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):73–90. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 2.Kramer A, Pippias M, Noordzij M, et al. The European renal association—European dialysis and transplant association (ERA-EDTA) registry annual report 2015: a summary. Clin Kidney J. 2018;11(1):108–122. doi: 10.1093/ckj/sfx149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 4.Saran R, Robinson B, Abbott KC, et al. US renal data system 2017 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3):A7. doi: 10.1053/j.ajkd.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bündchen DC, Sousa H, Afreixo V, Frontini R, Ribeiro O, Figueiredo D, Costa E. Intradialytic exercise in end-stage renal disease: an umbrella review of systematic reviews and/or meta-analytical studies. Clin Rehabil. 2021;35(6):812–828. doi: 10.1177/0269215520986784. [DOI] [PubMed] [Google Scholar]
- 6.Castaneda C. Muscle wasting and protein metabolism. J Anim Sci. 2002;80(Suppl. 2):E98–E105. doi: 10.2527/animalsci2002.80E-Suppl_2E98x. [DOI] [Google Scholar]
- 7.Desmeules S, Levesque R, Jaussent I, Leray-Moragues H, Chalabi L, Canaud B. Creatine index and lean body are excellent predictors of long-term survival in haemodiafiltration patients. Nephrol Dial Transplant. 2004;19:1182–1189. doi: 10.1093/ndt/gfh016. [DOI] [PubMed] [Google Scholar]
- 8.Johansen KL, Chertow GM, Ng AV, et al. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int. 2000;57:2564–2570. doi: 10.1046/j.1523-1755.2000.00116.x. [DOI] [PubMed] [Google Scholar]
- 9.Caso G, Garlick P. Control of muscle protein kinetics by acidbase balance. Curr Opin Clin Nutr Metab Care. 2005;8(1):73–76. doi: 10.1097/00075197-200501000-00011. [DOI] [PubMed] [Google Scholar]
- 10.John SG, Sigrist MK, Taal MW, et al. Natural history of skeletal muscle mass changes in chronic kidney disease stage 4 and 5 patients: an observational study. PLoS ONE. 2013;8(5):e65372. doi: 10.1371/journal.pone.0065372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansen KL, Shubert T, Doyle J, et al. Muscular atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int. 2003;63:291–297. doi: 10.1046/j.1523-1755.2003.00704.x. [DOI] [PubMed] [Google Scholar]
- 12.Cheema BS, O'Sullivan AJ, Chan M, Patwardhan A, Kelly J, Gillin A, Fiatarone Singh MA. Progressive resistance training during hemodialysis: rationale and method of a randomized-controlled trial. Hemodial Int. 2006;10(3):303–310. doi: 10.1111/j.1542-4758.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- 13.Chan M, Cheema B, Fiatarone Singh M. Progressive resistance training and nutrition in renal failure. J Ren Nutr. 2007;17:84–87. doi: 10.1053/j.jrn.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Cheema B, Abas H, Smith B, O'Sullivan A, Chan M, Patwardhan A, Kelly J, Gillin A, Pang G, Lloyd B, Singh MF. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007;18(5):1594–1601. doi: 10.1681/ASN.2006121329. [DOI] [PubMed] [Google Scholar]
- 15.Painter PL. Exercise for dialysis patients. In: Hörl WH, Koch KM, Lindsay RM, Ronco C, Winchester JF, editors. Replacement of renal function by dialysis. Dordrecht: Springer; 2004. [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and metanalyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;6(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Stone PW. Popping the (PICO) question in research and evidence-based practice. Appl Nurs Res. 2002;15(3):197–198. doi: 10.1053/apnr.2002.34181. [DOI] [PubMed] [Google Scholar]
- 18.Whiting P, Savović J, Higgins JPT, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–234. doi: 10.1016/j.jclinepi.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McHugh ML. Interrater reliability: the kappa statistic. Biochem Medica. 2012;22(3):276–282. doi: 10.11613/bm.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fusar-Poli P, Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health. 2018;21(3):95–100. doi: 10.1136/ebmental-2018-300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen J. Statistical power analysis for the behavioral sciences. 2. Milton Park: Routledge; 1988. [Google Scholar]
- 22.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. London: The Cochrane Collaboration; 2011. [Google Scholar]
- 23.Pérez-Bracchiglione J, Meza N, Bangdiwala SI, et al. Graphical Representation of overlap for overviews: GROOVE tool. Res Syn Methods. 2022;13:381–388. doi: 10.1002/jrsm.1557. [DOI] [PubMed] [Google Scholar]
- 24.Andrade FP, de Rezende P, de Ferreira T, Borba GC, Müller AM, Rovedder PM. Effects of intradialytic exercise on cardiopulmonary capacity in chronic kidney disease: systematic review and meta-analysis of randomized clinical trials. Sci Rep. 2019 doi: 10.1038/s41598-019-54953-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernier-Jean A, Beruni NA, Bondonno NP, Williams G, Teixeira-Pinto A, Craig JC, Wong G. Exercise training for adults undergoing maintenance dialysis. Cochrane Database Syst Rev. 2022 doi: 10.1002/14651858.cd014653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogataj P, Pajek M, Pajek J, ButurovićPonikvar J, Paravlic AH. Exercise-based interventions in hemodialysis patients: a systematic review with a meta-analysis of randomized controlled trials. J Clin Med. 2019;9(1):43. doi: 10.3390/jcm9010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheema BS, Chan D, Fahey P, Atlantis E. Effect of progressive resistance training on measures of skeletal muscle hypertrophy, muscular strength, and health-related quality of life in patients with chronic kidney disease: a systematic review and meta-analysis. Sports Med. 2014;44(8):1125–1138. doi: 10.1007/s40279-014-0176-8. [DOI] [PubMed] [Google Scholar]
- 28.Chung YC, Yeh ML, Liu YM. Effects of intradialytic exercise on the physical function, depression, and quality of life for haemodialysis patients: a systematic review and meta-analysis of randomised controlled trials. J Clin Nurs. 2017;26(13–14):1801–1813. doi: 10.1111/jocn.13514. [DOI] [PubMed] [Google Scholar]
- 29.Clarkson MJ, Bennett PN, Fraser SF, Warmington SA. Exercise interventions for improving objective physical function in patients with end-stage kidney disease on dialysis: a systematic review and meta-analysis. Am J Physiol Renal Physiol. 2019;316(5):F856–F872. doi: 10.1152/ajprenal.00317.2018. [DOI] [PubMed] [Google Scholar]
- 30.Ferrari F, Helal L, Dipp T, Soares D, Soldatelli N, Mills AL, Paz C, Tenório MCC, Motta MT, Barcellos FC, Stein R. Intradialytic training in patients with end-stage renal disease: a systematic review and meta-analysis of randomized clinical trials assessing the effects of five different training interventions. J Nephrol. 2019;33(2):251–266. doi: 10.1007/s40620-019-00687-y. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira TL, Ribeiro HS, Ribeiro ALA, Bonini-Rocha AC, Lucena JMS, de Oliveira PA, Amorim FRS, Ferreira AP, Magno LAV, Martins WR. Exercise interventions improve depression and anxiety in chronic kidney disease patients: a systematic review and meta-analysis. Int Urol Nephrol. 2020;53(5):925–933. doi: 10.1007/s11255-020-02612-w. [DOI] [PubMed] [Google Scholar]
- 32.Gomes Neto M, de Lacerda FFR, Lopes AA, Martinez BP, Saquetto MB. Intradialytic exercise training modalities on physical functioning and health-related quality of life in patients undergoing maintenance hemodialysis: systematic review and meta-analysis. Clin Rehabil. 2018;32(9):1189–1202. doi: 10.1177/0269215518760380. [DOI] [PubMed] [Google Scholar]
- 33.Heiwe S, Jacobson SH. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;64(3):383–393. doi: 10.1053/j.ajkd.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Hu H, Liu X, Chau PH, Choi EPH. Effects of intradialytic exercise on health-related quality of life in patients undergoing maintenance haemodialysis: a systematic review and meta-analysis. Qual Life Res. 2021;31(7):1915–1932. doi: 10.1007/s11136-021-03025-7. [DOI] [PubMed] [Google Scholar]
- 35.Huang M, Lv A, Wang J, Xu N, Ma G, Zhai Z, Zhang B, Gao J, Ni C. Exercise training and outcomes in hemodialysis patients: systematic review and meta-analysis. Am J Nephrol. 2019;50(4):240–254. doi: 10.1159/000502447. [DOI] [PubMed] [Google Scholar]
- 36.Lu Y, Wang Y, Lu Q. Effects of exercise on muscle fitness in dialysis patients: a systematic review and meta-analysis. Am J Nephrol. 2019;50(4):291–302. doi: 10.1159/000502635. [DOI] [PubMed] [Google Scholar]
- 37.Matsuzawa R, Hoshi K, Yoneki K, Harada M, Watanabe T, Shimoda T, Yamamoto S, Matsunaga A. Exercise training in elderly people undergoing hemodialysis: a systematic review and meta-analysis. Kidney Int Rep. 2017;2(6):1096–1110. doi: 10.1016/j.ekir.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molsted S, Bjørkman ASD, Lundstrøm LH. Effects of strength training to patients undergoing dialysis: a systematic review. Dan Med J. 2019;66(1):A5526. [PubMed] [Google Scholar]
- 39.Pu J, Jiang Z, Wu W, Li L, Zhang L, Li Y, Liu Q, Ou S. Efficacy and safety of intradialytic exercise in haemodialysis patients: a systematic review and meta-analysis. BMJ Open. 2019;9(1):e020633. doi: 10.1136/bmjopen-2017-020633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scapini KB, Bohlke M, Moraes OA, Rodrigues CG, Inácio JF, Sbruzzi G, Leguisamo CP, Sanches IC, Tourinho Filho H, Irigoyen MC. Combined training is the most effective training modality to improve aerobic capacity and blood pressure control in people requiring haemodialysis for end-stage renal disease: systematic review and network meta-analysis. J Physiother. 2019;65(1):4–15. doi: 10.1016/j.jphys.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Schardong J, Stein C, dellaMéaPlentz R. Neuromuscular electrical stimulation in chronic kidney failure: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2020;101(4):700–711. doi: 10.1016/j.apmr.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Segura-Ortí E. Ejercicio en pacientes en hemodiálisis: revisión sistemática de la literatura. Nefrología (Madrid) 2010;30(2):236–246. doi: 10.3265/Nefrologia.pre2010.Jan.10229. [DOI] [PubMed] [Google Scholar]
- 43.Sheng K, Zhang P, Chen L, Cheng J, Wu C, Chen J. Intradialytic exercise in hemodialysis patients: a systematic review and meta-analysis. Am J Nephrol. 2014;40(5):478–490. doi: 10.1159/000368722. [DOI] [PubMed] [Google Scholar]
- 44.Wu X, Yang L, Wang Y, Wang C, Hu R, Wu Y. Effects of combined aerobic and resistance exercise on renal function in adult patients with chronic kidney disease: a systematic review and meta-analysis. Clin Rehabil. 2020;34(7):851–865. doi: 10.1177/0269215520924459. [DOI] [PubMed] [Google Scholar]
- 45.Zhang F, Zhou W, Sun Q, Zhai Y, Zhang Y, Su H, Wang Z. Effects of intradialytic resistance exercises on physical performance, nutrient intake, and quality of life among haemodialysis people: a systematic review and meta-analysis. Nurs Open. 2019;8(2):529–538. doi: 10.1002/nop2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawant A, House AA, Overend TJ. Anabolic effect of exercise training in people with end-stage renal disease on hemodialysis: a systematic review with meta-analysis. Physiother Can. 2014;66(1):44–53. doi: 10.3138/ptc.2012-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Huffel L, Tomson CRV, Ruige J, Nistor I, van Biesen W, Bolignano D. Dietary restriction and exercise for diabetic patients with chronic kidney disease: a systematic review. PLoS ONE. 2014;9(11):e113667. doi: 10.1371/journal.pone.0113667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Macdonald HV, Johnson BT, Huedo-Medina TB, et al. Dynamic resistance training as stand-alone antihypertensive lifestyle therapy: a meta-analysis. JAHA. 2016 doi: 10.1161/jaha.116.003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hennessy EA, Johnson BT. Examining overlap of included studies in meta-reviews: guidance for using the corrected covered area index. Res Synth Methods. 2019;11(1):134–145. doi: 10.1002/jrsm.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.