Abstract
Purpose
Compare outcomes of acute versus delayed total elbow arthroplasty (TEA) following distal humerus fractures (DHF).
Methods
This retrospective study included 39 patients who underwent primary TEA with semiconstrained implants for DHF, either within 4 weeks of their injury or after failing initial open reduction and internal fixation (ORIF) or nonsurgical management, between June 1, 2003 and February 1, 2018 with minimum 1-year follow-up. Our outcome measures included QuickDASH (Disabilities of the Arm, Shoulder, and Hand) score, complications, reoperations, and range of motion (ROM). Demographics, clinical variables, and outcomes were compared using the Student’s t-test, Mann-Whitney U test, and Fisher’s exact test as appropriate. Kaplan-Meier curves for mortality, implant survivorship, and reoperation were created.
Results
Our patients were categorized into acute TEA (n = 22), ORIF to TEA (n = 10), and nonsurgical to TEA (n = 7) treatment groups. Additional analysis was performed comparing acute to delayed TEA, which combined data from failed ORIF and nonsurgical cohorts. The median follow-up, average age, and median Charlson comorbility index were similar between groups. The most common fracture pattern was AO13C. At median follow-up of 5.8 years, QuickDASH differed between cohorts: mean of 31 (SD 19) in acute TEA and 52 (SD 27) in delayed TEA, which further subdivided to 44.2 (SD 25) in failed ORIF and 76 (SD 23) in failed nonsurgical management. Poorer QuickDASH scores at final follow-up were associated with delayed TEA, initial nonsurgical management, and depression. Surgical complications were associated with delayed TEA. Higher Charlson comorbidity index was associated with death. No variables were associated significantly with ROM, revision, or reoperation.
Conclusion
Comminuted DHFs are difficult to treat in the elderly with high rates of complication and poor function after surgery. Our study suggests TEA performed acutely result in satisfactory outcomes and should be a consideration for patients at high risk of failing ORIF or nonsurgical management.
Type of Study/Level of Evidence
Therapeutic, III.
Key words: Distal humerus fracture, Elbow fracture, Malunion, Posttraumatic arthritis, Total elbow arthroplasty
Distal humerus fractures (DHF) have a bimodal age distribution, peaking in young male patients after high energy trauma and in older female patients after low energy falls.1 The estimated incidence of DHF in all adults is 6 per 100,000 people per year,1 but increases to 54 in 100,000 in patients over the age of 80,2 the majority of whom are female.3
Treatment of DHF depends on a multitude of factors, including age, functional status, and fracture pattern. Treatment options include nonsurgical management,4 open reduction and internal fixation (ORIF),5 and total elbow arthroplasty (TEA).3,6, 7, 8 It has been suggested that elderly patients with poor bone quality, severe comminution involving the articular surface or metaphysis, and disruption of the soft tissue often have high rates of failure after ORIF and may be managed better with primary TEA in the acute setting.3
Long-term follow-up after TEA estimates implant survival at 81% to 88% at 10 years.10, 11, 12, 9 Revision following trauma-related TEA is estimated to be as high as 4 times that of TEA for rheumatoid arthritis (RA).13 Many patients who undergo TEA for fractures are elderly and frail, and average patient survival is approximately 7.5 years after injury.6
The primary objective of our study was to compare patient-reported outcome measures (PROMs), range of motion (ROM), complication rates, and reoperation rates following semiconstrained TEA for DHF treated with acute TEA (≤28 days following injury), delayed TEA after failed initial nonsurgical treatment (>28 days following injury), or delayed TEA after failed initial ORIF. Our null hypothesis was that outcomes are no different among treatment groups. The secondary objective of our study was to identify any patient-, injury-, or surgery-related factors associated independently with PROMs, ROM, complication rates, and reoperation rates following TEA for DHF.
Methods
Study design
A retrospective study was conducted of all patients with DHF who were treated with TEA at 2 academic tertiary referral centers between June 1, 2003 and February 1, 2018. Approval was obtained from the institutional review board (Protocol 2010P002462). No funding was obtained for this study.
Patient selection
Cases were identified retrospectively by querying the hospital database using the Current Procedural Terminology code 24363 for total elbow (distal humeral and proximal ulnar) prosthetic arthroplasty. Inclusion criteria were TEA performed for DHF at 2 tertiary care referral centers in the same metropolitan region from 2003 to 2018. Exclusion criteria were incomplete medical records (n = 18), surgeries performed at outside institutions (n = 3), and TEA performed for other indications, such as RA or revision TEA (n = 174, Fig. 1). Patients who had baseline RA were included if the primary reason for presentation and treatment was because of traumatic injury resulting in a DHF. One patient sustained simultaneous bilateral fractures: only the first elbow treated with TEA was included to allow for the assumption of independence of observations. Thirty-nine patients were included in the study. Acute TEA (n = 22) was defined as primary TEA performed within 4 weeks of injury. The ORIF cohort (n = 10) was defined as conversion to delayed TEA after failed initial ORIF, while the nonsurgical cohort (n = 7) was defined as TEA after failed initial nonsurgical management of at least 4 weeks. Patients in the nonsurgical cohort all had fractures that had not demonstrated any healing at the time when TEA was performed. Some were treated with delayed TEA before the 9-month post-injury time period when the delayed unions could be classified officially as nonunion, based on Food and Drug Administration criteria,14 The degree of symptomology patients experienced directly from the elbow delayed union was unclear based on clinical documentations from the time of surgery. Additional analysis was performed combining data from the ORIF and nonsurgical cohorts into an aggregated delayed TEA data set.
Figure 1.

Flowchart for patient selection. Inclusion criteria were: primary TEA performed for distal humerus fracture, TEA performed at 2 tertiary care referral centers in the same metropolitan region, and TEA performed between 2003 and 2018. Exclusion criteria were incomplete medical records (n = 18), surgeries performed at outside institutions (n = 3), and TEA performed for other indications, such as RA or revision TEA (n = 174). A total of 39 patients met the above criteria and were included in this study.
Surgical approach
All TEA procedures were performed by hand and upper-extremity surgeons (n = 8), all of whom routinely perform TEA at 2 academic tertiary referral centers in a single health care network using 1 of 3 semiconstrained TEA systems: Discovery Total Elbow (DJO, LLC), Zimmer Coonrad-Morrey Total Elbow (Zimmer Biomet), or Tornier Latitude EV Total Elbow (Wright). No TEA was performed in an emergent or urgent setting by an on-call orthopedist. Surgical approach varied by surgeon preference, history of prior surgical treatments, and need for concurrent ORIF of additional elbow fractures. The ORIF of any additional fractures and ulnar neurolysis and/or transposition were performed at the discretion of the treating surgeon. Humeral and ulnar components were cemented with the limb exsanguinated under tourniquet. After surgery, patients were mobilized under the supervision of a therapist. Postoperative rehabilitation protocol and weightbearing restrictions were nonstandardized.
Variables
Baseline characteristics for patients (age, sex, and race) were recorded. Patient medical and social histories, including Charlson Comorbility index (CCI),15 depression, RA, employment status, worker’s compensation status, and injury-related explanatory variables, such as presence of ipsilateral upper-extremity fracture, open fracture status, and nerve injury were abstracted from the electronic medical record (EMR). Only variables and comorbidities present before the date of injury were included. Depression, like all other comorbidities, was collected from chart review and not specifically evaluated by the hand surgeon. Fracture classification was determined by the first author (CL) using initial injury radiographs. Six patients did not have viewable radiographs as they predated our EMR and were classified based on final radiologist reports of the injury films and cross-referenced with the orthopedic attending documentation, which either described the injury in detail or classified the fracture. Surgical variables, such as initial treatment (nonsurgical, ORIF, acute and delayed TEA), TEA operative techniques, and implants used were at the discretion of the treating surgeon and collected from the EMR.
Patient mortality data were obtained from our EMR as well as affiliated institutions for whom we have medical record access and the Social Security Index Master Death File, yielding complete mortality data. Final clinical follow-up was defined as the last clinic follow-up (n = 28) or virtual interview by video (n = 11) at the time of the study. All patients presumed to be living at the time of the study were contacted for virtual interview. Only 11 were able to be reached (n = 8 acute TEA, n = 2 ORIF, n = 1 nonsurgical). All patients with final virtual follow-up had all outcome variables collected (QuickDASH scores, complications, reoperations, and ROM). E-images were captured of each patient’s operative elbow and ROM was measured using the technique described by Meislin et al.16 Angle feature from Adobe Photoshop (Adobe) was used to measure elbow ROM angles on digital photos. For the 28 with no virtual follow-up, 17 were deceased at the time of the study and the remaining 11 were presumed to be living but unreachable. Final radiographic follow-up was defined as last elbow x-rays available in the EMR and did not necessarily coincide with the final clinical follow-up in cases where virtual interview was performed.
Response variables of this study were QuickDASH scores, complications, reoperations, revision surgeries, and objective ROM measurements, which were obtained from the EMR through clinic documentation at the last visit and confirmed on virtual video interview for the 11 patients we were able to reach at the time of the study. QuickDASH scores were obtained as part of routine clinical care for some of the patients and, thus, available for data collection. Fourteen patients lacked data points for QuickDASH scores. Two patients did not have complete elbow and forearm ROM documented in EMR and were obtained through e-image.16 x-rays from the last clinic visit or before revision surgery were used to determine implant loosening, heterotopic ossification, implant position, fracture, and other complications. Two patients did not have x-rays from their final follow-up. Revision TEA was defined as exchange or explant of the humeral and/or ulnar arthroplasty component; reoperation was defined as any surgery performed on the elbow after the index TEA procedure including implant revision. For all patients who underwent reoperation or revision, the type of secondary procedure(s) performed and the indication(s) were recorded.
Statistical methods
Bivariate analyses were used to identify explanatory variables associated with the chosen outcome measures. Student’s t-test was used for comparison of parametric variables, Mann-Whitney U test was used for nonparametric variables, and Fisher’s exact test was used for categorical variables. Continuous variables were compared across variables with multiple levels with 1-way analysis of variance or Kruskal–Wallis tests based on normality distributions. Survivorship analyses based on time-to-event were visualized with Kaplan-Meier curves. The standard significance criterion of P<.05 was used. A standard power criterion of (1-β) = 0.80 were used for all statistical tests. A convenience sample was used.
Results
Cohort demographics
Our study cohort comprised 39 patients treated with TEA following DHF: 22 were treated acutely with TEA within 4 weeks of injury, while 17 were treated with delayed TEA after failed ORIF (n = 10) or nonsurgical management (n = 7). All patients who failed nonsurgical management had delayed unions, while indications for conversion to TEA after ORIF included posttraumatic osteoarthritis (n = 3), hardware cutout (n = 1), malunion (n = 4), and nonunion (n = 2).
Patient demographic information, time to TEA, and follow-up time are presented in Table 1. Average age, White race, RA, and median CCI did not differ between cohorts. Average age of all cohorts was >65 years. Most patients identified as White in all cohorts. A third of patients in the acute TEA cohort had RA compared to only 10% of patients in the ORIF cohort. Median CCI of all patients was between 1 and 1.5 in all cohorts. All patients in the nonop cohort were female compared to only 60% in the ORIF cohort. There was a 5 times higher rate of depression in the nonsurgical cohort compared to acute TEA (13.6% acute vs 40.0% ORIF vs 71.0% nonsurgical). The acute TEA cohort had the lowest employment rate, while the ORIF cohort had the highest (9.0% acute vs 60.0% ORIF vs 14.0% nonsurgical). Female patients and patients with depression had the highest rate of initial nonsurgical treatment. Employed patients had the highest rate of initial ORIF. Clinical and radiographic follow-up were measured from time of TEA.
Table 1.
Patient Demographics and Patient-Specific Explanatory Variables
| All patients (n = 39) | Acute TEA (n = 22) | Failed ORIF (n = 10) | Failed Nonop (n = 7) | P value | |
|---|---|---|---|---|---|
| Age (y), mean (range) | 69.9 (48.9–88.1) | 71.6 (48.9–88.1) | 66.3 (53.0–84.1) | 69.6 (60.1–85.3) | .4 |
| Female, n (%) | 33 (84.6%) | 20 (91.0%) | 6 (60.0%) | 7 (100%) | .05 |
| White, n (%) | 37 (94.9%) | 20 (91.0%) | 10 (100%) | 7 (100%) | .9 |
| Employed, n (%) | 9 (23.1%) | 2 (9.0%) | 6 (60.0%) | 1 (14.0%) | .009 |
| CCI, median (IQR) | 1.0 (3.0) | 1.5 (3.0) | 1.0 (3.0) | 1.0 (4.0) | .9 |
| Depression, n (%) | 12 (30.8%) | 3 (13.6%) | 4 (40.0%) | 5 (71.0%) | .01 |
| RA, n (%) | 10 (25.6%) | 8 (36.4%) | 1 (10.0%) | 1 (14.0%) | .3 |
| Time to TEA (d), median (IQR) | 17 (130) | 9 (11) | 322 (316) | 195 (213) | <.001 |
| Clinical follow-up (y), median (IQR) | 5.8 (8.5) | 4.7 (8.9) | 8.0 (6.8) | 6.7 (7.5) | 1.0 |
| Radiographic follow-up (y), median (IQR) | 3.1 (4.0) | 3.1 (2.8) | 4.2 (1.2) | 0.8 (1.0) | .5 |
IQR, interquartile ratio.
Table 2 includes injury-related variables. Injuries tended to involve the dominant hand in all cohorts. One patient had an open fracture and 1patient had ulnar neuropraxia at the time of injury; both were in the ORIF cohort. Associated proximal forearm fractures were rare in the acute and nonsurgical cohorts but were present in half of patients undergoing ORIF. The majority of the fractures in all groups were AO13C.
Table 2.
Injury-related explanatory variables
| All patients (n = 39) | Acute TEA (n = 22) | Failed ORIF (n = 10) | Failed Nonop (n = 7) | P value | |
|---|---|---|---|---|---|
| AO Classification 13C, n (%) | 30 (76.9%) | 17 (77.3%) | 9 (90.0%) | 4 (57.1%) | .3 |
| AO Classification 13B, n (%) | 6 (15.4%) | 4 (18.2%) | 1 (10.0%) | 1 (14.3%) | .9 |
| AO Classification 13A, n (%) | 3 (7.7%) | 1 (4.5%) | 0 | 2 (28.6%) | .1 |
| Associated proximal forearm fracture, n (%) | 9 (23.1%) | 3 (13.6%) | 5 (50.0%) | 1 (14.3%) | .1 |
| Dominant hand injury, n (%) | 24 (63.2%) | 14 (63.6%) | 6 (60.0%) | 4 (57.0%) | .9 |
| Associated ulnar nerve injuries, n (%) | 1 (2.6%) | 0 | 1 (10.0%) | 0 | .4 |
| Open fractures, n (%) | 1 (2.6%) | 0 | 1 (10.0%) | 0 | .4 |
Table 3 describes surgery-related variables, none of which was associated with any outcome measures. Discovery total elbows were used in most cases. Olecranon ORIF was performed in 3 patients undergoing acute TEA and humerus ORIF was performed in 1 patient undergoing conversion TEA in the ORIF cohort.
Table 3.
Surgery-Related Explanatory Variables
| Implant type, n (%) | All patients (n = 39) | Acute TEA (n = 22) | Failed ORIF (n = 10) | Failed Nonop (n = 7) | P Value |
|---|---|---|---|---|---|
| Discovery total elbow | 35 (9.7%) | 19 (86.3%) | 10 (100%) | 6 (86.0%) | .6 |
| Conrad-Morrey total elbow | 3 (7.7%) | 2 (9.1%) | 0 | 1 (14.0%) | .7 |
| Tornier latitude total elbow | 1 (2.6%) | 1 (4.5%) | 0 | 0 | .9 |
| Olecranon ORIF, n (%) | 3 (7.7%) | 3 (13.6%) | 0 | 0 | .6 |
| Humerus ORIF, n (%) | 1 (2.6%) | 0 | 1 (10.0%) | 0 | .4 |
Outcomes after TEA
Average time to follow-up since TEA was 5.98 years (range, 0.38–15.42 years). The average QuickDASH score of the cohort at final follow-up was 41 (SD 25; range, 6.8–100, Table 4, Table 5, Table 6). Patients in the acute cohort had the shortest length of follow-up and patients in the ORIF cohort had the longest follow-up (4.7 years vs 8.0 years vs 6.7 years). QuickDASH scores were significantly better in the acute cohort and worst in the nonsurgical cohort (31 vs 44 vs 76). Moreover, worse QuickDASH scores were associated with depression and delayed TEA.
Table 4.
Patient Functional Outcomes Following Total Elbow Arthroplasty for Fracture Care
| No. patients | Acute TEA | Delayed TEA | P value | Failed ORIF | Failed Nonop | P value | |
|---|---|---|---|---|---|---|---|
| All patients | |||||||
| ROM, mean (SD) | |||||||
| Flexion∗ | 125.3 (16.9) | 129.4 (11.5) | 120.0 (21.4) | .1 | 123.6 (20.6) | 116.4 (23.2) | .5 |
| Extension∗ | 17.6 (25.2) | 16.9 (19.6) | 18.6 (31.8) | .9 | 8.6 (10.3) | 28.6 (43.0) | .4 |
| Pronation† | 78.4 (22.0) | 77.1 (20.5) | 80 (24.5) | .7 | 84.3 (11.3) | 75.7 (33.6) | .9 |
| Supination† | 76.5 (25.3) | 77.4 (22.6) | 75.4 (29.1) | .8 | 83.6 (11.1) | 67.1 (39.5) | .5 |
| QuickDASH, mean (SD)‡ | 41.3 (25.1) | 31.3 (18.8) | 52.1 (27.3) | .04 | 44.2 (24.8) | 75.7 (22.9) | .01 |
| Total No. pts. | 39 | 22 | 17 | 10 | 7 |
Missing 7 data points for flexion, extension.
Missing 8 data points for supination, pronation (1 data point described as “excellent” in clinic documentation, but no quantitative measurement provided).
Missing 14 data points for QuickDASH.
Table 5.
Patient functional outcomes following TEA for fracture care for patients over 60-years-old
| ROM, mean (SD)∗ | |||||||
| Flexion | 124.5 (17.3) | 128.7 (11.7) | 120.0 (21.4) | .2 | 123.6 (20.6) | 116.4 (23.2) | .3 |
| Extension | 16.9 (26.2) | 15.3 (20.7) | 18.6 (31.8) | .7 | 8.6 (10.3) | 28.6 (43.0) | .4 |
| Pronation | 29.8 (21.9) | 79.6 (19.9) | 80.0 (24.5) | 1.0 | 84.3 (11.3) | 75.7 (33.6) | .8 |
| Supination | 77.1 (24.9) | 78.9 (19.9) | 75.4 (29.1) | .7 | 83.6 (11.1) | 67.1 (39.5) | .5 |
| QuickDASH, mean (SD)† | 91.2 (24.2) | 30.2 (15.1) | 49.1 (29.1) | .008 | 37.7 (24.2) | 75.7 (22.9) | .002 |
| Total No. pts. >60 years old | 34 | 19 | 15 | 8 | 7 |
Missing 6 data points for ROM.
Missing 9 data points for QuickDASH.
Table 6.
Patient functional outcomes following TEA for fracture care for patients without RA
| ROM, mean (SD)∗ | |||||||
| Flexion | 134.5 (6.5) | 118.8 (22.9) | .2 | 122.5 (22.3) | 115.0 (25.1) | .4 | |
| Extension | 9.9 (12.6) | 21.7 (33.5) | .6 | 10.0 (10.5) | 33.3 (45.0) | .8 | |
| Pronation | 82.3 (15.4) | 78.3 (26.2) | .8 | 83.3 (12.1) | 73.3 (36.1) | 1.0 | |
| Supination | 85.5 (12.1) | 72.9 (30.9) | .2 | 82.5 (11.7) | 63.3 (41.8) | .5 | |
| QuickDASH, mean (SD)† | 21.5 (9.8) | 51.4 (28.5) | .002 | 42.3 (25.8) | 75.7 (22.9) | .004 | |
| Total Non-RA pts. | 29 | 14 | 9 | 6 |
Missing 6 data points for ROM.
Missing 13 data points for QuickDASH.
Forty-two percent of patients had a surgical complication and 36% underwent reoperation (Table 7). The most common complications were aseptic loosening as seen on x-ray (n = 7) and infection (n = 4). Three of the 4 cases of infection were in patients in the acute TEA cohort with RA. Six of the patients with loosening (n = 7) on x-rays were asymptomatic. Complication, revision, and reoperation rates were not statistically different between cohorts. However, when we consolidated all delayed TEA patients into 1 group (failed ORIF + failed nonop), we found more than double the rate of complications in the delayed TEA group compared to acute TEA cohort, most of which were due to aseptic loosening. There were 6 cases of aseptic loosening in the consolidated delayed TEA group compared to only 1 in the acute TEA cohort.
Table 7.
Mortality and Complication Rates Following TEA for Fracture Care in All Patients
| All patients (n = 39) | Acute TEA (n = 22) | Delayed TEA (n = 17) | P value | Failed ORIF (n = 10) | Failed Nonop (n = 7) | P value | |
|---|---|---|---|---|---|---|---|
| Death, n (%) | 17 (43.6%) | 11 (50.0%) | 6 (35.3%) | .7 | 2 (20.0%) | 4 (57.1%) | .4 |
| Complication, n (%) | 16 (42.1%) | 6 (22.3%) | 10 (58.8%) | <.05 | 6 (60.0%) | 4 (57.1%)∗ | .09 |
| Hardware irritation | 1 | 1 | 0 | 0 | 0 | ||
| Wound dehiscence† | 2 | 2 | 0 | 0 | 0 | ||
| Periprosthetic joint infection† | 2 | 1 | 1 | 0 | 1 | ||
| Stiffness | 2 | 1 | 1 | 1 | 0 | ||
| Aseptic loosening | 7 | 1 | 6 | 4 | 2 | ||
| Periprosthetic fracture | 1 | 0 | 1 | 1 | 0 | ||
| Ulnar nerve injury | 2 | 0 | 2 | 0 | 2 | ||
| Reoperation rate, n (%)‡ | 14 (35.9%) | 6 (27.0%) | 8 (47.1%) | .3 | 6 (60.0%) | 2 (28.6%) | .2 |
| Hardware irritation (removal of hardware) | 1§ | ||||||
| Fracture (ORIF) | 1 | 1 | |||||
| Heterotopic ossification (excision) | 1 | ||||||
| Wound dehiscence (irrigation and debridement) | 2 | ||||||
| Contracture (contracture release) | 1 | 1 | |||||
| Revision rate, n (%)¶ | 8 (23.1%) | 2 (9.1%) | 6 (35.3%) | .06 | 4 (40.0%) | 2 (28.6%) | .09 |
| Implant loosening | 1 | 5# | 4# | 1 | |||
| Periprosthetic joint infection (antibiotic spacer) | 1 | 1 | 1 |
One patient had both ulnar nerve injury and implant loosening.
3 of the 4 cases of infections occurred in patients with RA.
Includes revision cases.
This patient was under the age of 60 and had RA.
Revision defined as explant or exchange of TEA components.
All 4 cases of implant loosening in the ORIF to TEA cohort occurred in patients undergoing single stage conversion, ie removal of hardware was performed at the time of TEA. Two of the 4 cases were in patients under the age of 60. One of these patients had RA.
Postoperative ROM in flexion, extension, supination, and pronation were collected for the cohort (Table 4, Table 5, Table 6). Range of motion did not differ among treatment groups and was not associated with any explanatory variable in our bivariate analysis. In general, average flexion was 125° and average extension was −17° for all patients (lacking the terminal 17° of extension). This was similar between all 3 cohorts. Subgroup analyses of elderly patients aged ≥60 years older (Table 5) and patients without RA were performed and demonstrated similar findings (Table 6). Neither demonstrated any association between ROM and timing of TEA. Average ROM calculations did change dramatically when accounting for age or RA.
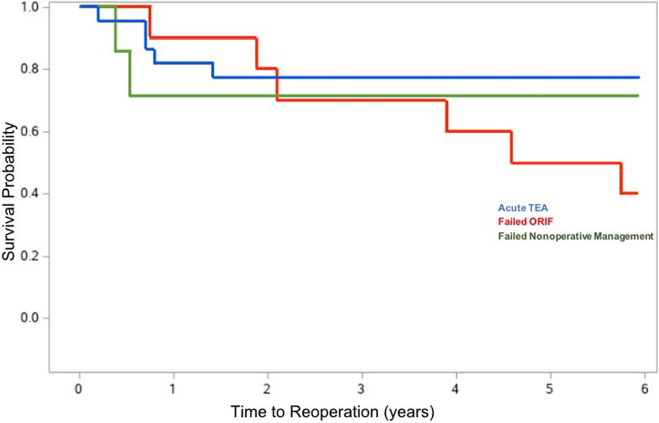
Seventeen of 39 patients were deceased at the time when study data were acquired; 11 in acute TEA, 2 in ORIF, and 4 in the nonsurgical group (Table 7; Figure 2, Figure 3, Figure 4 depict the Kaplan-Meier curves for mortality, reoperation, and revision).
Figure 2.
Kaplan-Meier curve for mortality following TEA for fracture. Mortality rate for the entire cohort was 43% at time of follow-up. Broken down by treatment groups, there was a 50% mortality rate in acute TEA, 20% in ORIF to TEA cohort, and 57.1% in nonsurgical to TEA cohort.
Figure 3.
Kaplan-Meier curve for reoperation following TEA for fracture. Overall reoperation rate was 35.9% for the entire cohort. Broken down by treatment groups, there was a 27% reoperation rate among patients in the acute cohort, 60% in the ORIF to TEA cohort, and 28.6% in the nonsurgical to TEA group.
Figure 4.
Kaplan-Meier curve for revision following TEA for fracture. Overall revision rate was 23.1% for the entire cohort. Broken down by treatment groups, there was a 9.1% revision rate in the acute TEA cohort, 40% in the ORIF to TEA cohort, and 28.6% in the nonsurgical to TEA group.
Discussion
In the last 2 decades, use of TEA in the treatment of DHF has increased in the acute and salvage settings.17 Recent studies have demonstrated similar outcomes after ORIF versus primary TEA for the acute treatment of DHF in the geriatric population.3,6,7,18, 19, 20, 21 In patients with low demand, RA, osteoporosis, and complex intraarticular fractures, acute TEA may result in superior outcomes compared to ORIF.18 Higher Mayo elbow performance score (MEPS) have been found in patients with underlying RA and DHF treated with acute TEA.22 McKee et al3 showed that acute TEA results in significantly better DASH scores at 6 months and better MEPS at 2 years compared to ORIF. Furthermore, 25% of DHF are not amenable to ORIF,3,18 while another 14% underwent conversion after ORIF to TEA.6 In general, there appears to be an approximately 10% revision rate after acute TEA, though this has varied from 4%–18%.23, 24, 25
It has been challenging to compare outcomes after acute and delayed TEA for DHF due to the low numbers seen in the clinical setting. Published data have varied with some showing better outcomes in the acute TEA group,26 while others showed no difference.24 In general, survivorship data for TEA following DHF has been limited by high mortality rates. Dehghan et al6 found that 60% of patients in their randomized controlled trial were deceased at 10 year follow-up and all had retained their initial implants.
Prasad et al24 followed 32 patients for 18 months and found no difference in outcomes. The data suggested that delayed TEA may have a higher rate of revision: acute TEA implant survivorship 93% at 88 months compared to 76% in the delayed TEA cohort. However, this was a small sample size and the difference was not statistically significant.24 Ellwein et al26 also published results from a small retrospective study of 23 patients (9 acute and 14 delayed TEA), showing significantly longer surgical time in the delayed cohort. Because of a small sample size, they, too, were not able to achieve statistical significance but the data suggested that delayed TEA may result in more pain and decreased function. Despite a larger sample size, Logli et al27 was not able to find a statistical difference in complication rates between acute and delayed TEA, though their data showed higher rates of loosening and infection in the salvage cohort.
While our sample size also was small, we did find a statistical difference in QuickDASH between patients undergoing acute versus delayed TEA. This difference was present in all 3 of our subgroup analysis: splitting the delayed TEA cohort into failed ORIF and nonsurgical, including only patients >60 years old, and excluding patients with RA. However, poorer QuickDASH scores also were associated with depression, which was higher in the delayed TEA cohort. This supports prior evidence of the association of worse PROMs with depression and poor coping28 as demonstrated by Cheng et al29 and Ring et al.30 In our study, depression was included if the diagnosis was documented in the chart before TEA. We were unable to determine if depression was present before DHF as not all patients were evaluated in our hospital system at the time of injury. Some patients had received their initial fracture care at outside hospitals and only presented to us for their TEA consultation.
In addition to a difference in QuickDASH scores, we also saw a statistical difference in complication rates with 58.8% of patients in the delayed TEA cohort having at least 1 complication compared to 22.3% in the acute TEA cohort. These rates are similar to those published in the literature.24,26,27 The high rate of infection (2 wound dehiscence, 2 prosthetic joint infection) in our study was likely because of patients with RA being on disease-modifying anti-rheumatic drugs, which was present in both cases of prosthetic joint infection and 1 of the cases of wound infection.
Reoperation and revision rates were not statistically different between cohorts, which may be because of the limited sample size. While the rate of revision in the acute TEA cohort is similar to that published in literature,23,24,31, 32, 33 the revision rate seen in the delayed cohort is higher than that noted by Prasad et also.24 We saw a high rate of aseptic loosening in the ORIF to TEA cohort (n = 4). All 4 patients had a single stage conversion with removal of hardware at the time of TEA. One patient was <60 years old. One patient had RA. The third patient had a nonunion following ORIF. It is possible that younger patients with higher functional demands and patients with poor bone stock are at higher risk of aseptic loosening after delayed TEA. Causes of loosening cited in the literature include particle wear resulting in osteolysis and postoperative weightbearing activity,34,35 which we were unable to study retrospectively.
Our study has several limitations including its retrospective design, unmatched cohorts, limited sample size, and involvement of many surgeons and implant designs. We were unable to reach 11 of the patients presumed to be living at the time of this study. We were able to use clinical documentation at the last clinic follow-up to fill in ROM and QuickDASH, which allowed us still to include these patients for follow-up. It is difficult to determine how these patients may have altered our outcomes data, though it is possible that if they were unsatisfied with their care they may have sought care elsewhere and, thus, our complication rate is underestimated. Furthermore, it is possible we may have reached statistical significance in some of our data points, such as complication, reoperation, and revision rates, if we were able to interview all patients virtually. Second, depression was higher in the delayed TEA cohort and may be a confounder in our QuickDASH analysis. Preoperative baseline ROM and QuickDASH scores were not available for comparison. Rehabilitation protocols were not recorded and likely varied. Additionally, we defined acute TEA as patients operated on within 28 days from injury. While similar definitions have been reported in the literature, this cutoff for acute TEA versus nonsurgical treatment is somewhat arbitrary.24,26 However, there was a minimum of 78 days between initial nonsurgical management and delayed TEA, and a minimum of 65 days between ORIF and delayed TEA, which helps to clearly distinguish the acute and delayed TEA groups. Lastly, the initial decision to pursue acute TEA versus ORIF versus nonsurgical management may have been influenced by the individual attending directing each patient’s care.
Our findings add to the current literature on the choice between delayed and acute TEA for DHF. Our findings suggest that delayed TEA may result in lower functional outcomes and higher complication rates compared to acute TEA for the treatment of DHF. Furthermore, patients with preoperative depression are at risk for worse QuickDASH scores. Future studies may focus on whether there is any combined interaction effect with pain interference or pain catastrophizing and whether this association is modifiable with cognitive behavioural therapy or other treatments.
Footnotes
Declaration of interests: No benefits in any form have been received or will be received related directly to this article.
References
- 1.Robinson C.M., Hill R.M.F., Jacobs N., Dall G., Court-Brown C.M. Adult distal humeral metaphyseal fractures: Epidemiology and results of treatment. J Orthop Trauma. 2003;17(1):38–47. doi: 10.1097/00005131-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Sheps David, Kemp Kyle, Hildebrand K. Population-based incidence of distal humeral fractures among adults in a Canadian urban center. Curr Orthop Pract. 2011;22(5):437–442. [Google Scholar]
- 3.McKee M.D., Veillette C.J.H., Hall J.A., et al. A multicenter, prospective, randomized, controlled trial of open reduction-internal fixation versus total elbow arthroplasty for displaced intra-articular distal humeral fractures in elderly patients. J Shoulder Elb Surg. 2009;18(1):3–12. doi: 10.1016/j.jse.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Brown R., Morgan R. Intercondylar T-Shaped fractures of the humerus. JBJS. 1971;53B(3):425–428. [PubMed] [Google Scholar]
- 5.Lauder A., Richard M.J. Management of distal humerus fractures. Eur J Orthop Surg Traumatol. 2020;30(5):745–762. doi: 10.1007/s00590-020-02626-1. [DOI] [PubMed] [Google Scholar]
- 6.Dehghan N., Furey M., Schemitsch L., et al. Long-term outcomes of total elbow arthroplasty for distal humeral fracture: results from a prior randomized clinical trial. J Shoulder Elb Surg. 2019;28(11):2198–2204. doi: 10.1016/j.jse.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Githens M., Yao J., Sox A.H.S., Bishop J. Open reduction and internal fixation versus total elbow arthroplasty for the treatment of geriatric distal humerus fractures: a systematic review and meta-analysis. J Orthop Trauma. 2014;28(8):481–488. doi: 10.1097/BOT.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 8.Obert L., Ferrier M., Jacquot A., et al. Distal humerus fractures in patients over 65: Complications. Orthop Traumatol Surg Res. 2013;99(8):909–913. doi: 10.1016/j.otsr.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Krukhaug Y., Hallan G., Dybvik E., Lie S.A., Furnes O.N. A survivorship study of 838 total elbow replacements: a report from the Norwegian Arthroplasty Register 1994-2016. J Shoulder Elb Surg. 2018;27(2):260–269. doi: 10.1016/j.jse.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Toulemonde J., Ancelin D., Azoulay V., Bonnevialle N., Rongières M., Mansat P. Complications and revisions after semi-constrained total elbow arthroplasty: a mono-centre analysis of one hundred cases. Int Orthop. 2016;40(1):73–80. doi: 10.1007/s00264-015-3008-z. [DOI] [PubMed] [Google Scholar]
- 11.Plaschke H.C., Thillemann T.M., Brorson S., Olsen B.S. Implant survival after total elbow arthroplasty: A retrospective study of 324 procedures performed from 1980 to 2008. J Shoulder Elb Surg. 2014;23(6):829–836. doi: 10.1016/j.jse.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Zhang D., Chen N. Total Elbow Arthroplasty. J Hand Surg Am. 2019;44(6):487–495. doi: 10.1016/j.jhsa.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Perretta D., van Leeuwen W.F., Dyer G., Ring D., Chen N. Risk factors for reoperation after total elbow arthroplasty. J Shoulder Elb Surg. 2017;26(5):824–829. doi: 10.1016/j.jse.2016.12.064. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham B.P., Brazina S., Morshed S., Miclau T. Fracture healing: a review of clinical, imaging and laboratory diagnostic options. Injury. 2017;48:S69–S75. doi: 10.1016/j.injury.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies : development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Meislin M.A., Wagner E.R., Shin A.Y. A comparison of elbow range of motion measurements: smartphone-based digital photography versus goniometric measurements. J Hand Surg Am. 2016;41(4):510–515.e1. doi: 10.1016/j.jhsa.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Klug A., Gramlich Y., Buckup J., Schweigkofler U., Hoffmann R., Schmidt-Horlohé K. Trends in total elbow arthroplasty: a nationwide analysis in Germany from 2005 to 2014. Int Orthop. 2018;42(4):883–889. doi: 10.1007/s00264-018-3818-x. [DOI] [PubMed] [Google Scholar]
- 18.Frankle M.A., Herscovici D., Dipasquale T.G., Vasey M.B., Sanders R.W. Frankle - ORIF versus TEA. 2003;17(7):1–8. doi: 10.1097/00005131-200308000-00001. papers2://publication/uuid/DF61E21B-D582-4376-B9E7-1E1BAC2E1DE1 [DOI] [PubMed] [Google Scholar]
- 19.Gambirasio R., Riand N., Stern R., Hoffmeyer P. Total elbow replacement for complex fractures of the distal humerus. J Bone Jt Surg Ser B. 2001;83(7):974–978. doi: 10.1302/0301-620x.83b7.11867. [DOI] [PubMed] [Google Scholar]
- 20.Kalogrianitis S., Sinopidis C., El Meligy M., Rawal A., Frostick S.P. Unlinked elbow arthroplasty as primary treatment for fractures of the distal humerus. J Shoulder Elb Surg. 2008;17(2):287–292. doi: 10.1016/j.jse.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Garcia J., Mykula R., Stanley D. Complex fractures of the distal humerus in the elderly: the role of total elbow replacement as primary treatment. Bone Jt J. 2002;84:812–816. doi: 10.1302/0301-620x.84b6.12911. [DOI] [PubMed] [Google Scholar]
- 22.Mansat P., Nouaille Degorce H., Bonnevialle N., Demezon H., Fabre T. Total elbow arthroplasty for acute distal humeral fractures in patients over 65 years old – results of a multicenter study in 87 patients. Orthop Traumatol Surg Res. 2013;99(7):779–784. doi: 10.1016/j.otsr.2013.08.003. d. [DOI] [PubMed] [Google Scholar]
- 23.Barco R., Streubel P.N., Morrey B.F., Sanchez-Sotelo J. Total elbow arthroplasty for distal humeral fractures: a ten-year-minimum follow-up study. J Bone Jt Surg Am. 2017;99(18):1524–1531. doi: 10.2106/JBJS.16.01222. [DOI] [PubMed] [Google Scholar]
- 24.Prasad N., Dent C. Outcome of total elbow replacement for distal humeral fractures in the elderly: a comparison of primary surgery and surgery after failed internal fixation or conservative treatment. J Bone Jt Surg Br. 2008;90(3):343–348. doi: 10.1302/0301-620X.90B3.18971. [DOI] [PubMed] [Google Scholar]
- 25.Varecka T.F., Myeroff C. Distal humerus fractures in the elderly population. J Am Acad Orthop Surg. 2017;25(10):673–683. doi: 10.5435/JAAOS-D-15-00683. [DOI] [PubMed] [Google Scholar]
- 26.Ellwein A., Lill H., Smith T., Voigt C., Imrecke J., Katthagen J.C. Primary vs. secondary total elbow arthroplasty for distal humerus fractures: clinical results of a retrospective case–control study. Obere Extrem. 2019;14(4):256–262. [Google Scholar]
- 27.Logli A.L., Shannon S.F., Boe C.C., Morrey M.E., O’Driscoll S.W., Sanchez-Sotelo J. Total elbow arthroplasty for distal humerus fractures provided similar outcomes when performed as a primary procedure or after failed internal fixation. J Orthop Trauma. 2020;34(2):95–101. doi: 10.1097/BOT.0000000000001631. [DOI] [PubMed] [Google Scholar]
- 28.Cheng H., Novak C.B., Veillette C., von Schroeder H.P. Influence of psychological factors on patient-reported upper extremity disability. J Hand Surg Eur Vol. 2020;45(1):71–76. doi: 10.1177/1753193419859373. [DOI] [PubMed] [Google Scholar]
- 29.Crijns T.J., Bernstein D.N., Teunis T., et al. The association between symptoms of depression and office visits in patients with nontraumatic upper-extremity illness. J Hand Surg Am. 2020;45(2):159.e1–159.e8. doi: 10.1016/j.jhsa.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Crijns T.J., Bernstein D.N., Ring D., Gonzalez R.M., Wilbur D.M., Hammert W.C. Depression and Pain interference correlate with physical function in patients recovering from hand surgery. Hand. 2019;14(6):830–835. doi: 10.1177/1558944718777814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenkins P.J., Watts A.C., Norwood T., Duckworth A.D., Rymaszewski L.A., McEachan J.E. Total elbow replacement: outcome of 1,146 arthroplasties from the Scottish Arthroplasty Project. Acta Orthop. 2013;84(2):119–123. doi: 10.3109/17453674.2013.784658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welsink C.L., Lambers K.T.A., Van Deurzen D.F.P., Eygendaal D., Van Den Bekerom M.P.J. Total Elbow Arthroplasty. JBJS Rev. 2017;5(7):1–10. doi: 10.2106/JBJS.RVW.16.00089. [DOI] [PubMed] [Google Scholar]
- 33.Fevang B.T.S., Lie S.A., Havelin L.I., Skredderstuen A., Furnes O. Results after 562 total elbow replacements: a report from the Norwegian Arthroplasty Register. J Shoulder Elb Surg. 2009;18(3):449–456. doi: 10.1016/j.jse.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 34.Prkić A., van Bergen C.J.A., The B., Eygendaal D. Total elbow arthroplasty is moving forward: Review on past, present and future. World J Orthop. 2016;7(1):44–49. doi: 10.5312/wjo.v7.i1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallo J., Goodman S.B., Konttinen YT R.M. Particle disease: biologic mechanisms of periprosthetic osteolysis in total hip arthroplasty. Innate Immun. 2013;19:213–224. doi: 10.1177/1753425912451779. [DOI] [PMC free article] [PubMed] [Google Scholar]