Abstract
Plague meningitis is a serious and often fatal manifestation of Yersinia pestis infection. In the aftermath of a bioweapon attack with Y pestis, this typically rare manifestation may develop in a substantial number of patients, particularly if treatment delays occur. Risk factors, clinical evolution, and optimal treatment strategies for plague meningitis are not well understood. We searched PubMed Central and other databases for reports of plague meningitis in any language. Articles containing descriptions of patients with plague meningitis and their treatment and outcomes were included. Among 1,496 articles identified in our search, 56 articles describing 84 cases from 1898 to 2015 met inclusion criteria. The median age of patients was 16 years (range 6 weeks to 64 years); 68% were male. Most patients (n = 50, 60%) developed meningitis following primary bubonic plague. Common signs and symptoms included fever (n = 56, 66%), nuchal rigidity (n = 38, 45%), and headache (n = 33, 36%); 29% (n = 24) of patients had focal neurologic deficits such as cranial nerve abnormalities. Almost all (n = 23, 96%) of the 24 patients who did not receive antimicrobials died, and 42% (n = 25) of the 59 patients treated with antimicrobials died. The case fatality rate of patients grouped by antimicrobial received was 50% (1 out of 2) for fluoroquinolones, 19% (4 out of 21) for aminoglycosides, 14% (2 out of 14) for sulfonamides, 11% (2 out of 18) for chloramphenicol, and 0% (0 out of 13) for tetracyclines. Plague meningitis most often occurs as a complication of bubonic plague and can cause focal neurologic deficits. Survival is more likely in patients who receive antimicrobials; tetracyclines, aminoglycosides, and chloramphenicol had the lowest associated case fatality rates.
Keywords: Plague, Yersinia pestis, Meningitis, Public health preparedness/response, Surveillance
Introduction
Plague, caused by the gram-negative bacterium Yersinia pestis, has caused devastating historical epidemics1 and remains endemic in some regions of the world, including sub-Saharan Africa, the East Asian steppes, southern South America, and the western United States. Approximately 650 cases of human plague are reported worldwide each year.2 Y pestis also has the potential to be weaponized and is classified as a Tier 1 bioterrorism select agent, as it has a low infectious dose, can be aerosolized, and has potential for person-to-person transmission.3 A bioterrorism attack using Y pestis could cause victims to develop any clinical manifestation of plague, including meningitis, and could lead to thousands of deaths.4 Clinicians should be able to recognize plague meningitis and understand available treatment options following a bioterrorism attack.
Y pestis is most often transmitted to humans via the bite of an infected flea, inhalation of infectious droplets, or handling of contaminated tissues.5 The most common clinical forms are bubonic, pneumonic, and septicemic plague; additional rare manifestations include plague meningitis, ocular plague, and plague pharyngitis. Patients with plague may initially experience one clinical form (eg, bubonic), then develop a secondary manifestation (eg, meningitis) after dissemination of the bacteria, particularly if the patient is not treated promptly with effective antimicrobials.
Plague meningitis typically develops following delayed or inadequate treatment of another clinical form of plague, following the hematogenous spread of Y pestis and seeding of the meninges.6,7 Y pestis has been identified in the cerebrospinal fluid of patients with plague meningitis by direct visualization using Gram stain or other methods, direct fluorescent antibody testing, or culture.6 Similar to other types of meningitis, signs and symptoms of plague meningitis include nuchal rigidity, headache, and fever.8,9 Previous case reports and case series have reported that plague meningitis may be more common in children compared with adults,10,11 and that patients with axillary or cervical buboes may be at greater risk for plague meningitis than those with buboes in the inguinal region or other areas.8,12
Although plague meningitis is a rare clinical manifestation, it is important to recognize and treat promptly.13 The recommended firstline treatment is chloramphenicol plus either levofloxacin or moxifloxacin, since these antimicrobials penetrate the blood–brain barrier and have robust activity against Y pestis.3,8,13,14
Due to the rare and sporadic nature of human plague, and particularly plague meningitis, it is not feasible to conduct controlled studies on patients presenting with plague meningitis. To further the understanding of this condition, we conducted a systematic review of published cases of plague meningitis in the scientific literature.
Methods
Literature Review
This review included cases of plague meningitis identified in a 2020 systematic literature review, covering the years 1937 to 2019, on antimicrobial treatment of human plague.15 Medline, Embase, and other databases were searched for the literature review; methods are described in detail elsewhere.15 Cases of plague meningitis identified in that initial literature review were identified for further review and included in this analysis.
We conducted a separate literature search within PubMed Central (PMC) using the search terms [((plague) OR pestis) AND mening*] to identify reports of patients with plague meningitis. The title and abstract of each identified publication were reviewed by 2 team members, and articles that appeared to describe patients with plague meningitis were included for a full-text review by 2 team members. Articles that met inclusion criteria were included in this analysis.
We examined references of articles that met inclusion criteria to identify additional reports of plague meningitis that had not been revealed in the database searches. The references of those articles were further examined to identify additional articles for inclusion, for a total of 3 generations of reference examination.
Inclusion Criteria
Cases were included in this review if 1 or more of the following case definitions for plague meningitis were met: (1) presence of meningitis in a patient with plague, as demonstrated by either nuchal rigidity or at least 2 other clinical signs or symptoms of meningitis, including altered mental status, headache, seizures, or focal neurologic signs; (2) laboratory evidence of meningitis in a patient with plague, as demonstrated by Y pestis in the cerebrospinal fluid by culture, polymerase chain reaction, or Gram stain; or (3) author report of plague meningitis. Report of patient outcome, specifically whether the patient survived, was also required for inclusion.
We also created a subset category of cases with confirmed plague meningitis, defined as either laboratory evidence of Y pestis in cerebrospinal fluid (CSF) or evidence of Y pestis infection in other clinical samples (eg, bubo aspirate, blood) with concomitant CSF pleocytosis (>5 cells/mm3).
Data Abstraction and Analysis
Information from published reports was abstracted using Microsoft Access and Microsoft Excel. Data on patient demographics, exposure, signs and symptoms, diagnoses, treatment, laboratory testing, and outcome were recorded when available. The primary clinical form of plague for each patient was defined by the symptoms and signs that either the patient or clinician first noted, plus laboratory tests if available. Secondary clinical form(s) were defined by clinical manifestations that developed after the primary manifestation, as described in Nelson et al.15 This review included patients with either primary or secondary plague meningitis. The year of presentation for each patient was included, if available; the year the report was published was substituted if the year of clinical presentation was not available.
Descriptive analyses were conducted using Microsoft Excel. Antimicrobials were assigned to 2 groups: effective and minimally effective for plague. Antimicrobials deemed to be effective for plague treatment included aminoglycosides, chloramphenicol, fluoroquinolones, tetracyclines, and sulfonamides, although not all of these antimicrobials are considered firstline treatments for plague meningitis.13 All other classes of antimicrobials were defined as minimally effective for purposes of this analysis.
Results
The systematic literature review conducted by Nelson et al15 identified a total of 275 articles with information on cases of plague, of which 30 articles reported cases of either primary or secondary plague meningitis. Our literature search for plague meningitis within PMC uncovered 1,109 potentially eligible articles (Figure 1), plus 357 articles identified through manual reference analysis. Thus, combining articles found through the previous systematic review and references of eligible articles from the PMC review resulted in a total of 1,496 articles that were reviewed for this study. Of those, 449 met criteria for full-text review. In total, 56 articles reporting 84 distinct cases of plague meningitis met the inclusion criteria (28 cases were described in multiple publications). (For the complete list of cases see Table 3, located at the end of the Results section). All articles were case reports or case series except one, published in 1954, which summarized 29 published cases of plague meningitis.9
Figure 1.
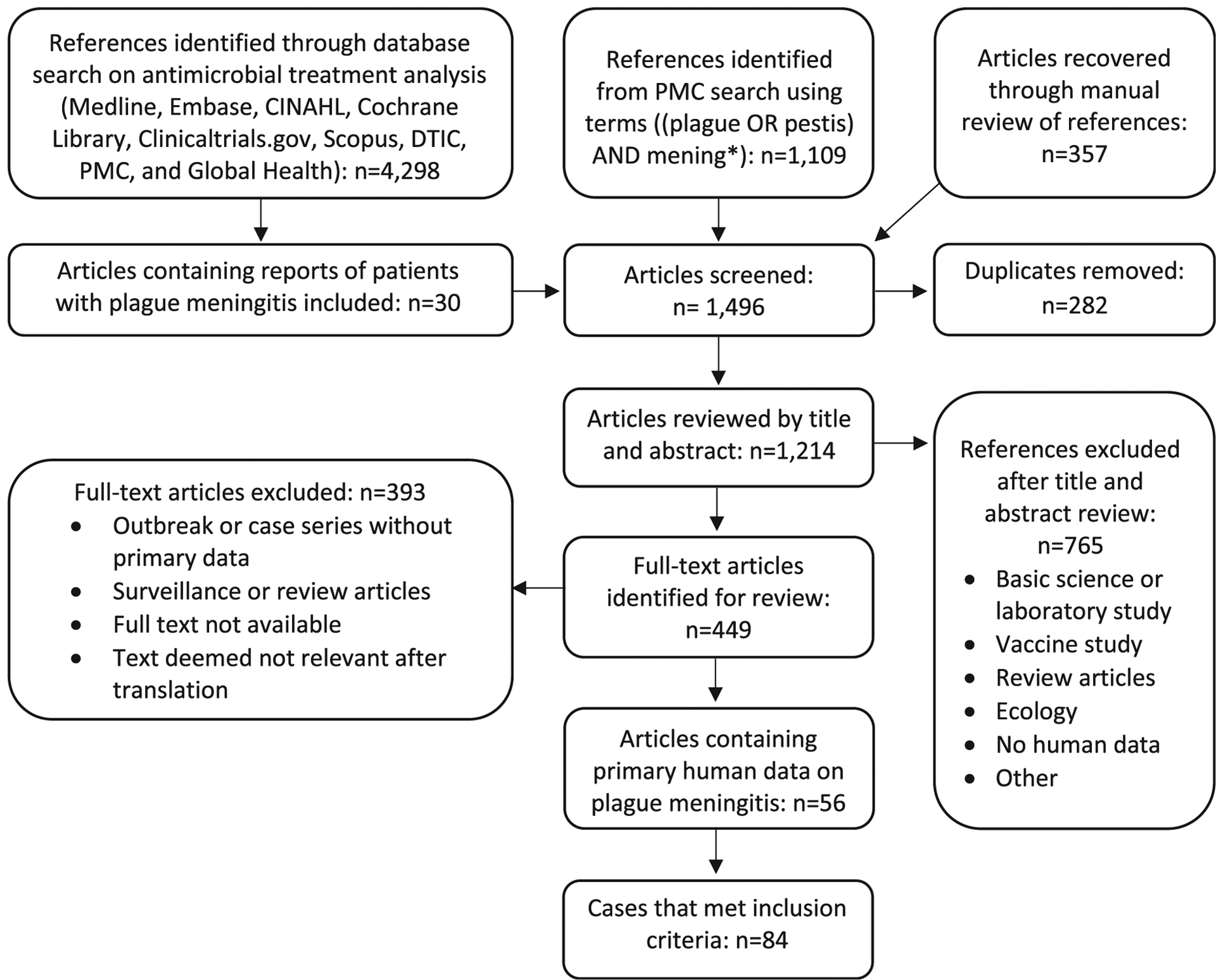
Flow diagram for systematic review of plague meningitis. Abbreviations: CINAHL, Cumulative Index to Nursing and Allied Health Literature; DTIC, Defense Technical Information Center; PMC, PubMed Central.
Table 3.
Distinct Cases of Plague Meningitis Reported, 1898–2015
| Year of Illness Occurrence | Country | Primary Clinical Form of Plague | Age in Years and (Sex) of Patient | Antimicrobial Treatment Received | Outcome (Recovered/Died) | Reference Number(s) |
|---|---|---|---|---|---|---|
| 1898a | India | Unclear | 35 (M) | n/a | Died | 32 |
| 1899a | India | Unclear | 16 (M) | n/a | Died | 32 |
| 1899a | India | Unclear | 30 (F) | n/a | Died | 32 |
| 1899a | India | Unclear | 13 (F) | n/a | Died | 32 |
| 1899a | India | Unclear | X (F) | n/a | Died | 32 |
| 1899a | Portugal | Bubonic | 25 (M) | n/a | Died | 32,50 |
| 1899a | Portugal | Bubonic | 7 (X) | n/a | Recovered | 32,50 |
| 1899 | United States | Bubonic | X (M) | n/a | Died | 50 |
| 1913 | Burma (now Myanmar) | Unclear | X (M) | n/a | Died | 44 |
| 1914 | Senegal | Septicemic | X (M) | n/a | Died | 40 |
| 1915a | Philippines | Bubonic | 18 (M) | n/a | Died | 32,41 |
| 1915a | Philippines | Bubonic | 19 (F) | n/a | Died | 32,41 |
| 1920a | United States | Bubonic | 25 (F) | n/a | Died | 53 |
| 1922a | China | Pneumonic | 24 (M) | n/a | Died | 30 |
| 1923a | Senegal | Pneumonic | 40 (M) | n/a | Died | 9,21 |
| 1924a | Argentina | Bubonic | X (M) | n/a | Died | 9,23 |
| 1925 | Argentina | Bubonic | X (X) | n/a | Died | 9 |
| 1933 | Uganda | Septicemic | X (M) | n/a | Died | 43 |
| 1934 | United States | Bubonic | 10 (M) | n/a | Died | 32 |
| 1935 | Senegal | Bubonic | 12 (F) | n/a | Died | 39 |
| 1940 | Belgian Congo (now DRC) | Meningitic | 9 (M) | n/a | Died | 42 |
| 1940 | Egypt | Bubonic | 20 (F) | S | Recovered | 34 |
| 1940 | Uganda | Septicemic | 3 (M) | S | Died | 35 |
| 1941 | Argentina | Bubonic | 14 (F) | S | Died | 24,25 |
| 1941 | Argentina | Bubonic | 9 (F) | S | Died | 25 |
| 1941 | Argentina | Bubonic | 8 (M) | S | Died | 25 |
| 1941 | Kenya | Unclear | X (M) | n/a | Died | 9,45 |
| 1942 | Argentina | Bubonic | 25 (M) | S | Died | 24,25 |
| 1942 | Argentina | Bubonic | 18 (M) | S | Recovered | 24,25 |
| 1942 | Argentina | Bubonic | 15 (M) | S | Recovered | 24,25 |
| 1942 | Argentina | Bubonic | 21 (M) | S | Recovered | 25 |
| 1942 | Argentina | Septicemic | 19 (M) | S | Recovered | 24,25 |
| 1942 | Argentina | Unclear | 17 (M) | S | Died | 25 |
| 1942a | Kenya | Unclear | X (M) | S | Died | 9,45 |
| 1942 | Kenya | Unclear | X (M) | n/a | Died | 9,45 |
| 1944a | Argentina | Bubonic | 7(F) | n/a | Died | 24 |
| 1944a | Argentina | Bubonic | 14 (F) | n/a | Died | 24 |
| 1944 | Argentina | Bubonic | 16 (M) | S | Died | 22 |
| 1944 | China | Bubonic | 28 (M) | S | Died | 12 |
| 1944 | China | Bubonic | 39 (F) | S | Died | 12 |
| 1945 | China | Bubonic | 40 (F) | S | Died | 12 |
| 1945 | China | Bubonic | 37 (M) | S | Died | 12 |
| 1945 | China | Bubonic | 16 (M) | S | Died | 12 |
| 1945 | China | Bubonic | 26 (M) | S | Died | 12 |
| 1945 | China | Meningitic | 0.08 (M) | S | Died | 12 |
| 1945 | India | Bubonic | 47 (F) | S | Died | 20 |
| 1945 | India | Bubonic | 7(F) | S | Died | 20 |
| 1946 | Argentina | Septicemic | 14 (M) | A, S | Recovered | 26 |
| 1947 | United States | Pneumonic | 22 (M) | A, T | Recovered | 56 |
| 1949 | China | Bubonic | 17 (M) | A, S | Recovered | 29 |
| 1949 | India | Pneumonic | 18 (M) | S | Died | 31 |
| 1951 | Belgian Congo (now DRC) | Unclear | 7 (X) | A, P, S | Recovered | 37 |
| 1951 | India | Meningitic | 25 (M) | A, S | Recovered | 33 |
| 1962 | Vietnam | Unclear | 0.5 (F) | A, C, P, S | Died | 54 |
| 1963 | Vietnam | Bubonic | 23 (M) | A, P, S, T | Recovered | 27 |
| 1965 | United States | Bubonic | 2.5 (M) | C, P, S | Recovered | 10,47 |
| 1965 | United States | Unclear | 3 (F) | C, P, S | Recovered | 10,47 |
| 1965 | United States | Unclear | 3.5 (M) | C, P, S | Recovered | 10 |
| 1966 | Vietnam | Meningitic | 0.3 (M) | A, C, P | Died | 54 |
| 1967 | United States | Bubonic | 60 (M) | C, P | Recovered | 19 |
| 1967 | South Africa | Bubonic | 11 (M) | C, P, S | Recovered | 36 |
| 1967 | Brazil | Bubonic | 8 (M) | A, S | Recovered | 38 |
| 1967 | Nepal | Bubonic | 13 (F) | A, C, P, T | Recovered | 55 |
| 1968 | Vietnam | Unclear | 0.1 (M) | A, C, P, S, T | Recovered | 54 |
| 1969 | United States | Bubonic | 3 (M) | A, C, P, T | Recovered | 48 |
| 1971 | United States | Bubonic | 39 (M) | A, P, T | Recovered | 46 |
| 1972 | South Africa | Bubonic | 58 (M) | A, C, S, T | Recovered | 17 |
| 1972 | Vietnam | Bubonic | X (F) | A, P | Died | 28 |
| 1972 | Vietnam | Bubonic | X (M) | A, P, T | Recovered | 28 |
| 1974 | United States | Unclear | 13 (F) | P | Died | 8 |
| 1974 | Vietnam | Bubonic | 38 (F) | Either A or S | Recovered | 14 |
| 1974 | Vietnam | Bubonic | 11 (M) | Either A or S | Died | 14 |
| 1975 | United States | Bubonic | 11 (M) | A, C, T, P | Recovered | 8 |
| 1975 | United States | Septicemic | 15 (M) | T, P | Recovered | 8 |
| 1975 | Vietnam | Bubonic | 18 (F) | S | Recovered | 14 |
| 1978 | United States | Bubonic | 10 (F) | C, Ce, P, T | Recovered | 8 |
| 1978 | United States | Septicemic | 35 (F) | C, T | Recovered | 8 |
| 1979 | United States | Unclear | 13 (F) | C, Ce, M, P | Recovered | 8 |
| 1984 | United States | Bubonic | 35 (M) | C, Ce | Recovered | 51 |
| 1988 | United States | Bubonic | 12 (M) | A, C, T | Recovered | 49 |
| 1995a | Vietnam | Bubonic | 63 (M) | A, C | Recovered | 16 |
| 1996 | United States | Bubonic | 16 (F) | Ce | Died | 52 |
| 2009 | China | Pneumonic | 64 (M) | A, Ce, F | Died | 18 |
| 2015 | United States | Meningitic | 14 (M) | A, Ce, F, G, S, | Recovered | 11 |
Year of illness occurrence not provided. Year of article publication was used for these cases.
Abbreviations: A, aminoglycosides; C, chloramphenicol; Ce, cephalosporins; DRC, Democratic Republic of the Congo; (F), female; F, fluoroquinolones; G, glycopeptide antibiotics; (M), male; M, macrolide antibiotics; P, penicillins; S, sulfonamides; T, tetracyclines; (X) or X, unknown or not reported.
Patient Demographics and Exposure History
Cases of plague meningitis included in this review occurred between 1898 and 2015. Of the 81 cases that reported the sex of the patient, most were male (n = 55, 68%). The age of patients ranged from 6 weeks to 64 years. Of the 71 cases that reported the ages of the patients, more than half (n = 41, 58%) were aged 18 years or younger. The largest proportion of patients by age and sex were adolescent boys 15 to 18 years of age (n = 10, 14%) and adolescent girls 10 to 14 years of age (n = 8, 11%). Only 8% (n = 6) of patients were aged 40 years or older (Figure 2).12,16–21 Of all included patients (n = 84), 24% (n = 20) resided in the United States, 18% (n = 15) in Argentina,9,22–26 12% (n = 10) in Vietnam,14,27,28 12% (n = 10) in China,12,18,29,30 11% (n = 9) in India,20,31–33 and 24% (n = 20) in other countries.17,21,32,34–45 Of the 20 patients residing in the United States, most lived in New Mexico (n = 11, 55%),8,10,46–49 while others lived in California (n = 4, 20%),11,32,50,51 Colorado (n = 2, 10%),19,52 Arizona (n = 1, 5%),8 and Texas (n = 1, 5%).53 One case of laboratory-acquired plague occurred in a patient who lived in Maryland.17
Figure 2.

Age distribution, sex, and outcome of patients with plague meningitis reported in the scientific literature. Note: Twelve patients did not have reported age; 2 patients did not have reported sex. These 14 patients were not included in the graph.
More than a quarter (n = 22, 26%) of included patients contracted plague meningitis between 1898 and 1940, before effective antimicrobial therapy was available.9,21,23,30,32,39–41,43,44,50,53 Most (n = 56, 67%) cases occurred between 1940 and 19808,10,12,14,17,19,20,22,24–29,31,33,35–38,42,45–48,54–56; only 6 cases (7%) of plague meningitis have been reported since 198111,16,18,49,51,52(Figure 3).
Figure 3.

Number of cases and outcome of patients with plague meningitis reported in the scientific literature, by time period.
Almost a quarter (n = 20, 24%) of included case reports described the patient’s exposure history to Y pestis. Of these cases, 55% (n = 11) were likely exposed to Y pestis by an infected animal, including cats (n = 5, 45%),8,17,51,52 prairie dogs (n = 2, 18%),10,48 a coyote (n = 1, 9%),8 and a fox (n = 1, 9%).22 Other patients with reported exposure likely contracted Y pestis from the bite of an infected flea (n = 6, 30%),8,32,55 via respiratory droplets from an infected person (n = 2, 10%),18,31 or in a laboratory (n = 1, 5%).56
Clinical Findings and Laboratory Testing
Primary plague meningitis occurred in 6% (n = 5) of all 84 patients.11,12,33,42,54 More than half (n = 50, 60%) of patients developed plague meningitis secondary to bubonic plague.8,12,14,16,17,19,20,22–25,27–29,32,34,36,38,39,41,46–53,55 Plague meningitis developed secondary to primary septicemic plague in 8% (n = 7)8,25,26,35,40,43 or primary pneumonic plague in 6% (n = 5)18,21,30,31,56 of patients. The primary clinical syndrome was unclear or not reported for 20% (n = 17)8,10,25,32,37,44,45,54 of patients. For the 50 patients with primary bubonic plague, the location of the bubo(es) was reported for 90% (n = 45). Of those 45 patients, buboes were reported in the axillary areas (n = 25, 56%),8,12,14,16,17,19,20,22,24,25,29,32,39,47,48,51–53 the inguinal areas (n = 16, 36%),12,14,20,25,27,32,36,45–48 and/or the cervical area (n = 7, 16%)8,24,34,38,41,50,55 (Figure 4). Additional bubo locations included the epitrochlear (n = 4, 9%),12,14,22 crural (n = 3, 7%),24,25 retropectoral (n = 1, 2%),25 suprascapular (n = 1, 2%),48 subpectoral (n = 1, 2%),24 retropharyngeal (n = 1, 2%),41 and supratrochlear (n = 1, 2%)25 areas.
Figure 4.

Bubo locations among 45 patients with primary bubonic plague who developed secondary plague meningitis. Additional bubo locations reported included retropectoral/subpectoral (n = 2, 4%), suprascapular (n = 1, 2%), and retropharyngeal (n = 1, 2%).
The most commonly reported signs and symptoms of plague meningitis in all 84 patients were fever (n = 56, 66%), nuchal rigidity (n = 38, 45%), and headache (n = 33, 39%). Generalized neurologic signs were noted in 24 (29%) patients12,14,20,25,26,30,32,38,44–47,52,54 and included delirium or clouded mentality (n = 10, 42%),12,14,20,32,52 convulsions or seizures (n = 9, 38%),12,30,32,44,45,50 and opisthotonos (n = 4, 17%).12,20,25,32 Of the 84 total patients, 10 (12%)11,12,14,25,30,32,38,40,50 had reported focal neurologic deficits, including slow pupillary response or eye movement dissociation (n = 4),11,25,40 diplopia (n = 2),11,32 vertigo (n = 1),12 tinnitus (n = 1),12 deafness (n = 1),12 and abnormal gait (n = 1).38 Neuroimaging was reported for only 1 patient, aged 14 years, whose MRI showed enhancement in the left subcutaneous parietal and occipital scalp tissues but no nervous system abnormalities.11
Laboratory test results were available for 83% (n = 70) of included patients. Four patients had negative culture results for either blood or CSF.10,14,18 Of the 66 patients with a positive laboratory result for Y pestis, bacteria was demonstrated in the CSF (by a stain or by culture) in 79% (n = 52). Of the 52 cases, the CSF isolate was identified as Y pestis by phage lysis in 10% (n = 5)8,27,48 or by polymerase chain reaction in 2% (n = 1).11
Antimicrobial Treatment and Patient Outcomes
The overall case fatality rate (CFR) of the 84 patients with plague meningitis was 58% (n = 49). The CFR was 91% (n = 21) in the 23 cases that occurred between 1898 and 1940, before the widespread availability of effective antimicrobial therapy; 47% (n = 26) in the 55 cases that occurred between 1941 and 1980; and 33% (n = 2) in the 6 cases that occurred since 1981.
A majority (n = 59, 70%) of all patients were treated with antimicrobials. Of those 59 patients, 42% (n = 25) died. A median of 3 days (range 0 to 14 days) passed between onset of first plague symptoms and start of treatment, with 67% (n = 37) receiving treatment 3 or more days after illness onset. The CFR was similar between patients who received an antimicrobial within 2 days versus 3 or more days following symptom onset (n = 5, 42% of 12 vs n = 15, 41% of 37; P = .95). However, timing between symptom onset and antimicrobial treatment was not clear for 17% (n = 10) of the 59 treated patients, which limits interpretation. Antimicrobials used to treat plague meningitis were sulfonamides (n = 40, 68%), aminoglycosides (n = 21, 36%), penicillins (n = 20, 34%), chloramphenicol (n = 18, 31%), and tetracyclines (n = 13, 22%). Cephalosporins were used for 6 (10%) patients,8,11,18,51,52 and erythromycin and vancomycin were given to 1 patient each (Figure 5).8,11
Figure 5.

Antimicrobial treatment and outcome among patients with plague meningitis reported in the scientific literature. A total of 59 patients received antimicrobial treatment. Of these, 31 patients received treatment with 2 or more antimicrobial classes; those patients are included in multiple categories based on all antimicrobials received.
Monotherapy was provided to 47% (n = 28) of treated patients.8,9,12,14,20,22,25,27,31,34,35,52 Of those, 86% (n = 24) received a sulfonamide and had an associated CFR of 75% (n = 18).9,12,14,20,22,25,31,34,35 Two patients, 1 of whom died, received either an aminoglycoside or a sulfonamide, but it was unclear which antimicrobial was given.14 The remaining 2 patients died after receiving monotherapy with either ceftriaxone or penicillin (Table 1).8,52
Table 1.
Antimicrobial Monotherapy Treatment and Case Fatality Rate of Patients With Plague Meningitis
| Antimicrobial Class/Antimicrobial | Patients Who Received Antimicrobial as Monotherapya | Case Fatality Rate |
|---|---|---|
| Sulfonamides | 24 | 75% |
| Sulfathiazole/Cibazol | 13 | 69% |
| Sulfapyridine (Dagenan) | 3 | 67% |
| Sulfadiazine | 2 | 100% |
| Trimethoprim-sulfamethoxazole | 1 | 0% |
| Unspecified Sulfonamide(s) | 5 | 100% |
| Cephalosporins | 1 | 100% |
| Ceftriaxone | 1 | 100% |
| Penicillins | 1 | 100% |
| Penicillin | 1 | 100% |
| No antimicrobial treatment | 25 | 96% |
Two patients received either trimethoprim-sulfamethoxazole or streptomycin and were not included in this table, since it was unclear which antibiotic was given.
Therapy with 2 or more antimicrobial classes was provided to 53% (n = 31) of the 59 treated patients.8,10,11,16–19,26–29,33,36–38,46–49,51,54–56 The overall CFR of patients treated with 2 or more antimicrobial classes was 13% (n = 4) (Table 2, Figure 5).
Table 2.
Antimicrobial Treatment and Case Fatality Rate of Patients With Plague Meningitis Who Received 2 or More Antimicrobial Classesa
| First Antimicrobial Class Received | Second Antimicrobial Class Received | Third Antimicrobial Class Received | Fourth Antimicrobial Class Received | Fifth Antimicrobial Class Received | Patients Who Received Regimen n | CFR of Patients Who Received Regimen |
|---|---|---|---|---|---|---|
| Aminoglycosides | Chloramphenicol | - | - | - | 1 | 0% |
| Aminoglycosides | Penicillins | - | - | - | 1 | 100% |
| Aminoglycosides | Sulfonamides | - | - | - | 4 | 0% |
| Aminoglycosides | Tetracyclines | - | - | - | 1 | 0% |
| Cephalosporins | Chloramphenicol | - | - | - | 1 | 0% |
| Chloramphenicol | Penicillins | - | - | - | 1 | 0% |
| Chloramphenicol | Tetracyclines | - | - | - | 1 | 0% |
| Penicillins | Tetracyclines | - | - | - | 1 | 0% |
| Aminoglycosides | Chloramphenicol | Penicillins | - | - | 1 | 100% |
| Aminoglycosides | Chloramphenicol | Tetracyclines | - | - | 1 | 0% |
| Aminoglycosides | Penicillins | Sulfonamides | - | - | 1 | 0% |
| Aminoglycosides | Penicillins | Tetracyclines | - | - | 2 | 0% |
| Aminoglycosides | Cephalosporins | Fluoroquinolones | - | - | 1 | 100% |
| Chloramphenicol | Penicillins | Sulfonamides | - | - | 4 | 0% |
| Aminoglycosides | Chloramphenicol | Penicillins | Sulfonamides | - | 1 | 100% |
| Aminoglycosides | Chloramphenicol | Sulfonamides | Tetracyclines | - | 1 | 0% |
| Aminoglycosides | Chloramphenicol | Penicillins | Tetracyclines | - | 3 | 0% |
| Aminoglycosides | Penicillins | Sulfonamides | Tetracyclines | - | 1 | 0% |
| Cephalosporins | Chloramphenicol | Penicillins | Tetracyclines | - | 1 | 0% |
| Cephalosporins | Chloramphenicol | Macrolides | Penicillins | - | 1 | 0% |
| Aminoglycosides | Cephalosporins | Fluoroquinolones | Glycopeptides | Sulfonamides | 1 | 0% |
| Aminoglycosides | Chloramphenicol | Penicillins | Sulfonamides | Tetracyclines | 1 | 0% |
Antimicrobials were not necessarily provided to patients in the order listed in this table. Abbreviation: CFR, case fatality rate.
Among the 25 patients who did not receive antimicrobial treatment, nearly all (n = 24, 96%) died.9,21,23,24,30,32,39–45,50,53 The patient who survived without antimicrobial treatment was a 7-year-old child who became ill in 1899 with bubonic plague that progressed to plague meningitis. The child was treated with plague antiserum; after a prolonged illness, the buboes were incised and drained and the patient recovered fully.18
Patients with Confirmed Plague Meningitis
Over half (n = 46, 55%) of the 84 included patients had confirmed plague meningitis.8,10–12,14,16,17,19,20,24–28,32,35,36, 39–43,47,51,52,54 Patients in this group most commonly experienced fever (n = 38, 79%), nuchal rigidity (n = 25, 54%), and headache (n = 21, 44%). Y pestis was cultured from the CSF in 54% (n = 25) of patients.8,11,12,14,17,19,20,24,25,27,28,35,39–43,47,52,54 The CSF leukocyte count was recorded in 46% (n = 21) of patients and ranged from 5 cells/mm3 to 27,000 cells/mm3 with a median of 210 cells/mm3.10–12,14,16,24,25,27,32,36,39,47,51 CSF glucose levels were recorded for 5 patients and ranged from 7 mg/100 ml to 73 mg/100 ml with a median of 20 mg/100 ml.10,27,36,47 Protein levels in the CSF were recorded for 4 patients and ranged from 72 mg/100 ml to 210 mg/100 ml with a mean of 146 mg/100 ml.10,16,36,47
Eight patients with confirmed plague meningitis did not receive antimicrobial treatment, all of whom died.24,32,39–43 Among the 38 patients who received antimicrobial treatment and had confirmed plague meningitis, 45% (n = 17) died.8,12,14,20,24,25,35,52,54 The most common antimicrobials used for treatment in this group were sulfonamides (n = 25, 66%), chloramphenicol (n = 15, 39%), penicillins (n = 15, 39%), aminoglycosides (n = 10, 26%), and tetracyclines (n = 8, 21%). Twenty patients from this group were treated with 2 or more antimicrobial classes, 10% (n = 2) of whom died.54
Eighteen patients received monotherapy.8,12,14,20,24,25,35,52 Of those patients, 78% (n = 14) received sulfonamides, 86% (n = 12) of whom died.12,20,25,35 Two patients received monotherapy with either a cephalosporin or a penicillin, and both died.8,52 The remaining 2 patients who received monotherapy were treated with either an aminoglycoside or a sulfonamide, but it was unclear which antimicrobial they received; 1 died.14
Discussion
Plague meningitis is most commonly reported as a complication of primary bubonic plague, particularly for patients with an axillary bubo. Adolescents and males comprised the highest proportion of patients reported in the literature. Plague meningitis is a serious clinical manifestation of Y pestis infection, which is nearly always fatal if untreated. In this review, we found that CFR improved with antimicrobial treatment; however, nearly half (42%) of patients succumbed to the infection despite treatment. No significant difference in survival rate was noted for patients who received treatment 3 or more days after symptom onset.
Although plague meningitis is rare, if Y pestis were to be used as a biological weapon, thousands of people could become infected4 and hundreds could develop plague meningitis. Thus, improved understanding of this manifestation can inform clinician recognition and treatment of patients with naturally acquired infections and preparedness efforts for a potential bioweapon attack. In this review, tetracyclines, chloramphenicol, and aminoglycosides showed the lowest associated CFRs. Patients treated with tetracyclines had the lowest CFR among these, although none of the included patients received tetracycline monotherapy. Patients treated with sulfonamide monotherapy had a high CFR, although this was likely confounded by time period bias since most patients received sulfonamide monotherapy prior to the 1950s. Medical advances and increased availability of antimicrobial treatment have markedly improved survival rates for all plague manifestations including plague meningitis.15,57
Currently, the recommended firstline treatment for plague meningitis in the United States is chloramphenicol plus either levofloxacin or moxifloxacin.13 Chloramphenicol has demonstrated efficacy for patients with plague meningitis.8,14 Levofloxacin and moxifloxacin have robust central nervous system penetration and demonstrated activity against Y pestis; however, human data on the use of these drugs specifically for plague meningitis is limited.58 Chloramphenicol demonstrated high efficacy in this review, although interpretation is limited by low numbers and combination therapy. Fluoroquinolones, however, were used for treatment in only 2 patients. In both instances the fluoroquinolone was used in combination with an aminoglycoside and a cephalosporin, limiting interpretability. One of those patients had primary pneumonic plague and received streptomycin and ceftriaxone in addition to ciprofloxacin but died.18 The other patient had apparent primary plague meningitis and received gentamicin, trimethoprim-sulfamethoxazole, and ceftriaxone in addition to levofloxacin and recovered.11
The majority of patients with plague meningitis in this review were children, similar to previous observations.7,10 There is not a clear reason for this association, although there are several potential explanations. One possible reason that children experience a greater burden of plague meningitis may be that they have trouble communicating their symptoms, thus clinical illness is not recognized and treated promptly. Children may also be more likely to touch an infected animal and contract Y pestis through that exposure, which some researchers have suggested may be more likely to cause plague meningitis.6,8,59 Finally, it is possible that Y pestis passes through the blood–brain barrier more easily in children.
Some experts have noted that plague meningitis often develops following delayed, ineffective, or inadequate treatment of bubonic plague,9,10,60 and it has been suggested that meningitis develops only after the illness is prolonged in treated patients who would have otherwise died rapidly.9,12,20 In this review, the majority of patients with plague meningitis either never received antimicrobial treatment or received an antimicrobial 3 or more days after initial symptom onset. Additionally, some included patients with bubonic plague were treated with an antimicrobial briefly, then developed meningitis after their treatment had stopped.14 Surprisingly, delay in receiving treatment was not found to have a significant impact on patient outcome in this analysis, although this is limited by small case numbers and incomplete treatment information. Clinicians treating patients with bubonic plague should be mindful of the potential for plague meningitis development, particularly for patients with bubonic plague who delayed seeking care and who may have had a prolonged illness that was not recognized until the manifestation was severe.
It has been hypothesized that plague meningitis only develops secondary to other plague clinical manifestations, and that primary plague meningitis does not exist.8–10,12 In contrast, our review included 5 patients with plague meningitis for whom meningitis was the first reported clinical manifestation; these patients were considered cases of primary plague meningitis by their treating physicians.11,12,33,42,54 However, 2 of those cases were in infants and it is possible those patients had earlier unrecognized symptoms of bubonic or septicemic plague.12,54 Two other patients with reported primary plague meningitis were children who were examined by clinicians more than 3 days after illness onset and may have had unnoticed clinical symptoms of septicemic or bubonic plague.11,42 The final patient with reported primary plague meningitis was an adult male who presented initially with fever, chills, severe headache, and back pain who developed neck rigidity and a positive Kernig’s sign the following day.33 Further research is needed to determine if patients can develop primary plague meningitis and, if so, whether those patients have different characteristics than patients with secondary plague meningitis.
Previous studies have indicated a possible association between the occurrence of an axillary bubo and the development of plague meningitis,6,14 including a summary by Butler,6 which indicated that of 32 patients with plague meningitis, 20 (63.1%) had axillary buboes. A recent review of patients treated for any form of plague found that only 24% of patients with bubonic plague had an axillary bubo,15 less than half the percentage of patients with an axillary bubo in this review. Based on these findings, the presence of axillary buboes should increase clinical suspicion for potential development of plague meningitis. Butler hypothesized that this could be due to the proximity of the axillary lymph nodes to the meninges.6 However, there are alternative explanations for this association; for example, axillary buboes may be more likely in patients who are younger or who develop plague after handling an infected animal.5
This review is subject to several limitations. First, articles included in this study were case reports or case series, which are observational in nature and lack the rigor of controlled trials. Second, time period bias impacted these data, as the number of effective therapeutic options has increased and medical advancements have improved the quality of supportive care. The different standards in medical care in earlier time periods may have contributed to the high CFR associated with sulfonamides, and possibly that of aminoglycosides, as these were primarily used in patients during earlier time periods. Finally, numerous articles included only brief descriptions of the patients with plague meningitis, which limited the available data for this review. For example, information on exposure route was missing for the majority of included patients.
Conclusion
Additional research on the pathophysiology of plague meningitis would improve our understanding of this clinical condition. A better understanding of how and why plague meningitis develops in certain patients could help to prevent cases of plague meningitis. Furthermore, additional information on the clinical presentation and clinical course of plague meningitis may allow clinicians to recognize plague meningitis and treat patients effectively sooner. Research on antimicrobial treatment regimens for patients with plague meningitis, including randomized controlled trials, would also be valuable, although such trials would be impractical given the rarity of plague meningitis.
Plague meningitis is associated with a high CFR, but survival is more likely in patients who receive effective antimicrobial therapy. Development of meningeal signs in persons undergoing therapy for bubonic or other forms of plague should prompt a rapid and thorough workup for plague meningitis, including lumbar puncture and alteration of antimicrobial therapy to include chloramphenicol and moxifloxacin or levofloxacin.13
Acknowledgments
The authors thank Joanna Taliano, librarian at the US Centers for Disease Control and Prevention (CDC), for her assistance with the systematic literature review. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
References
- 1.Cunha CB, Cunha BA. Impact of plague on human history. Infect Dis Clin North Am. 2006;20(2):253–272. [DOI] [PubMed] [Google Scholar]
- 2.Bertherat E Plague around the world in 2019. Wkly Epidemiol Rec. 2019;94(25):289–292. [Google Scholar]
- 3.Inglesby TV, Dennis DT, Henderson DA, et al. Plague as a biological weapon: medical and public health management. JAMA. 2000;283(17):2281–2290. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO). Health Aspects of Chemical and Biological Weapons; Report of a WHO Group of Consultants. Geneva: WHO; 1970. https://apps.who.int/iris/handle/10665/39444 [Google Scholar]
- 5.Poland JD, Dennis DT. Diagnosis and clinical manifestations. In: Dennis DT, Gage KL, Gratz NG, Poland LD, Tikhomirov, eds. Plague Manual: Epidemiology, Distribution, Surveillance and Control. Geneva: World Health Organization; 1999:43–53. https://apps.who.int/iris/handle/10665/66010 [Google Scholar]
- 6.Butler T Clinical syndromes and pathology in humans. In: Butler T Plague and Other Yersinia Infections. New York: Springer; 1983:73–108. [Google Scholar]
- 7.McEntire CRS, Song KW, McInnis RP, et al. Neurologic manifestations of the World Health Organization’s list of pandemic and epidemic diseases. Front Neurol. 2021;12: 634827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker TM, Poland JD, Quan TJ, White ME, Mann JM, Barnes AM. Plague meningitis–a retrospective analysis of cases reported in the United States, 1970–1979. West J Med. 1987;147(5):554–557. [PMC free article] [PubMed] [Google Scholar]
- 9.Plague Pollitzer R.. World Health Organization Monograph Series. No. 22. Geneva: World Health Organization; 1954. https://apps.who.int/iris/handle/10665/41628 [Google Scholar]
- 10.Martin AR, Hurtado FP, Plessala RA, et al. Plague meningitis. A report of three cases in children and review of the problem. Pediatrics. 1967;40(4):610–616. [PubMed] [Google Scholar]
- 11.Padua LT, Kamali A, Kim H, et al. Unique case of disseminated plague with multifocal osteomyelitis. J Pediatr Infect Dis Soc. 2017;6(3):e165–e168. [DOI] [PubMed] [Google Scholar]
- 12.Landsborough D, Tunnell N. Observations on plague meningitis. Br Med J. 1947;1(4487):4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson CA, Meaney-Delman D, Fleck-Derderian S, Cooley KM, Yu PA, Mead PS. Antimicrobial treatment and prophylaxis of plague: recommendations for naturally acquired infections and bioterrorism response. MMWR Recomm Rep. 2021;70(3):1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler T, Levin J, Linh NN, Chau DM, Adickman M, Arnold K. Yersinia pestis infection in Vietnam. II. Quantitative blood cultures and detection of endotoxin in the cerebrospinal fluid of patients with meningitis. J Infect Dis. 1976; 133(5):493–499. [DOI] [PubMed] [Google Scholar]
- 15.Nelson CA, Fleck-Derderian S, Cooley KM, et al. Antimicrobial treatment of human plague: a systematic review of the literature on individual cases, 1937–2019. Clin Infect Dis. 2020;70(suppl 1):S3–S10. [DOI] [PubMed] [Google Scholar]
- 16.Pokrovskii VI, Maleev VV, Shcherbak IuF. The clinical, diagnostic, and treatment characteristics of plague today. Ter Arkh. 1995;67(11):3–5. [PubMed] [Google Scholar]
- 17.Thornton DJ, Tustin RC, Pienaar BJ, Pienaar WN, Bubb HD. Cat bite transmission of Yersinia pestis infection to man. J S Afr Vet Assoc. 1975;46(2):165–169. [PubMed] [Google Scholar]
- 18.Wang H, Cui Y, Wang Z, et al. A dog-associated primary pneumonic plague in Qinghai Province, China. Clin Infect Dis. 2011;52(2):185–190. [DOI] [PubMed] [Google Scholar]
- 19.Mollohan CS. Human plague - Colorado. MMWR Morb Mortal Wkly Rep. 1967;16(50):420. [Google Scholar]
- 20.Koenigsfeld EGH, Nambiar KPS. Plague-meningitis. Ind Med Gaz. 1946;81(11):474–475. [PMC free article] [PubMed] [Google Scholar]
- 21.Nogue M Un cas de meningite pesteuse. Bull Soc Pathol Exot Filiales. 1923:378–384. [Google Scholar]
- 22.Miyara S, Conte D, et al. Rural plague in the province of Mendoza: clinical epidemiological study. Rev Asoc Med Argent. 1947;61(601–602):161–182. [PubMed] [Google Scholar]
- 23.Uriarte L Persistance du bacille de la peste dans l’organisme infecté. C R Seances Soc Biol Fil. 1924;91(2):1039–1040. [Google Scholar]
- 24.de Villafane Lastra T Meningitis pestosa. Rev Med Cordoba. 1943;31:99–105. [Google Scholar]
- 25.de Villafane Lastra T, Goobar JK, Rodiero MSV. Tratamiento de la peste de Oriente. Boletín mensual Córdoba. 1942;2(15):3. [Google Scholar]
- 26.Videla CA. Treatment of plague. Dia Med. 1947;19(1):1–6. [Google Scholar]
- 27.Feeley EJ, Kriz JJ. Plague meningitis in an American serviceman. JAMA. 1965;191(5):412–413. [DOI] [PubMed] [Google Scholar]
- 28.Cantey JR. Plague in Vietnam: clinical observations and treatment with kanamycin. Arch Intern Med. 1974;133(2):280–283. [DOI] [PubMed] [Google Scholar]
- 29.Chen RTS. Streptomycin in bubonic plague. Chin Med J. 1949;67(8):442–443. [PubMed] [Google Scholar]
- 30.Teh WL, Chun JWH, Pollitzer R. Clinical observations upon the Manchurian plague epidemic, 1920–21. J Hyg (Lond). 1923;21(3):289–306. [PMC free article] [PubMed] [Google Scholar]
- 31.Seal SC. Pneumonic plague cases in Calcutta and Gaya. Ind Med Gaz. 1949;84(4):162–170. [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer KF, Connor CL, Smyth FS, Eddie BB. Chronic relapsing latent meningeal plague. Arch Intern Med (Chic). 1937;59(6):967–980. [Google Scholar]
- 33.Singh A A case of plague meningitis. Indian Med J. 1951; 45(10):242–243. [PubMed] [Google Scholar]
- 34.Kamal AM, Gayed I, Anwar M. On the epidemiology and treatment of plague in Egypt. “The 1940 Epidemic.” J Egypt Public Health Assoc. 1941:31–103. [Google Scholar]
- 35.Burton E, Hennessey RSF. An unusual case of plague with meningitis. East Afr Med J. 1940;17(7):266–270. [Google Scholar]
- 36.Isaacson M, Levy D, Pienaar BJ, et al. Unusual cases of human plague in Southern Africa. S Afr Med J. 1973;47(44): 2109–13. [PubMed] [Google Scholar]
- 37.Fain A, Schoetter M, Ampe R. Cure of three cases of human plague in the plague-endemic area of Lake Albert. Ann Soc Belg Med Trop. 1951;31(5):541–546. [PubMed] [Google Scholar]
- 38.Coura JR, Rodrigues Da Silva J, De Oliveira Z, Lopes PFA. Persistent foci of plague in Brazil. Rev Soc Bras Med Trop. 1967;1(6):293–310. [Google Scholar]
- 39.Montagne M, Rivoalen A. A case of plague meningitis. (French) Bull Soc Pathol Exot Filiales. 1936;29(1):21–23. [Google Scholar]
- 40.Lafont A, Lecomte A, Heckenroth F. An observation of cerebrospinal meningitis in Dakar, caused by the Yersin bacillus. (French) Bull Soc Pathol Exot Filiales. 1915;8(3):92–96. [Google Scholar]
- 41.Crowell BC. Pathologic anatomy of bubonic plague. Philippine J Sci. Sect B Trop Med. 1915;10(4):249–307. [Google Scholar]
- 42.Lewillon R, Devignat R, Schoetter M. A case of primary plague meningitis. Ann Soc Belg Med Trop. 1940;20(1):79–82. [Google Scholar]
- 43.Williams AW. Some unusual forms of plague. East Afr Med J. 1934;11(7):229–232. [Google Scholar]
- 44.Sheldon JH, Lond MB. A note on plague Bacilli in an unusual situation in the body. Lancet. 1915;185(4790):1294. [Google Scholar]
- 45.Wright FJ. Some unusual clinical manifestations of plague. East Afr Med J. 1941;18(8):226–235. [Google Scholar]
- 46.Palmer DL, Kisch AL, Williams RC, Reed WP. Clinical features of plague in the United States: the 1969–1970 epidemic. J Infect Dis. 1971;124(4):367–371. [DOI] [PubMed] [Google Scholar]
- 47.Doran HG, Brutsché RL, Bourne J. Plague in New Mexico. MMWR Morb Mortal Wkly Rep. 1965;14(30):257–258. [Google Scholar]
- 48.Storrs B, Weber N, Johnson D. Epidemiologic note and reports: plague - New Mexico. MMWR Morb Mortal Wkly Rep. 1969;18(27):233. [Google Scholar]
- 49.Doll J, Englender SJ, Werner SB, et al. Human plague- United States, 1988. MMWR Morb Mortal Wkly Rep. 1988; 37(42):653–656. [PubMed] [Google Scholar]
- 50.Calmette A, Salimbeni A-T. La peste bubonique;étude de l’épidémie d’Oporto en 1899. Ann Inst Pasteur. 1899;13(12): 865–936. [Google Scholar]
- 51.Johnson B, Almas J, Salkin M, et al. Plague pneumonia -California. MMWR Morb Mortal Wkly Rep. 1984;33(34): 481–482. [PubMed] [Google Scholar]
- 52.Tenborg M, Davis B, Smith D, et al. Fatal human plague -Arizona and Colorado, 1996. MMWR Morb Mortal Wkly Rep. 1997;46(27):617–620. [PubMed] [Google Scholar]
- 53.Levy MD, McMicken D. Bubonic plague. Tex State J Med. 1920;16(5):195–200. [Google Scholar]
- 54.Tuan PD, Dai VQ, Ha TT, Trinh V. Plague meningitis in infants. Southeast Asian J Trop Med Public Health. 1971;2(3): 403–405. [PubMed] [Google Scholar]
- 55.Laforce FM, Acharya IL, Stott G, et al. Clinical and epidemiological observations on an outbreak of plague in Nepal. Bull World Health Organ. 1971;45(6):693–706. [PMC free article] [PubMed] [Google Scholar]
- 56.Burmeister RW Tigertt WD, Overholt EL. Laboratory-acquired pneumonic plague. Report of a case and review of previous cases. Ann Intern Med. 1962;56(5);789–800. [DOI] [PubMed] [Google Scholar]
- 57.Kugeler KJ, Staples JE, Hinckley AF, Gage KL, Mead PS. Epidemiology of human plague in the United States, 1900–2012. Emerg Infect Dis. 2015;21(1):16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nau R, Sorgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Von Reyn CF, Weber NS, Tempest B, et al. Epidemiologic and clinical features of an outbreak of bubonic plague in New Mexico. J Infect Dis. 1977;136(4):489–494. [DOI] [PubMed] [Google Scholar]
- 60.Butler T The black death past and present. 1. Plague in the 1980s. Trans R Soc Trop Med Hyg. 1989;83(4):458–460. [DOI] [PubMed] [Google Scholar]


