Abstract
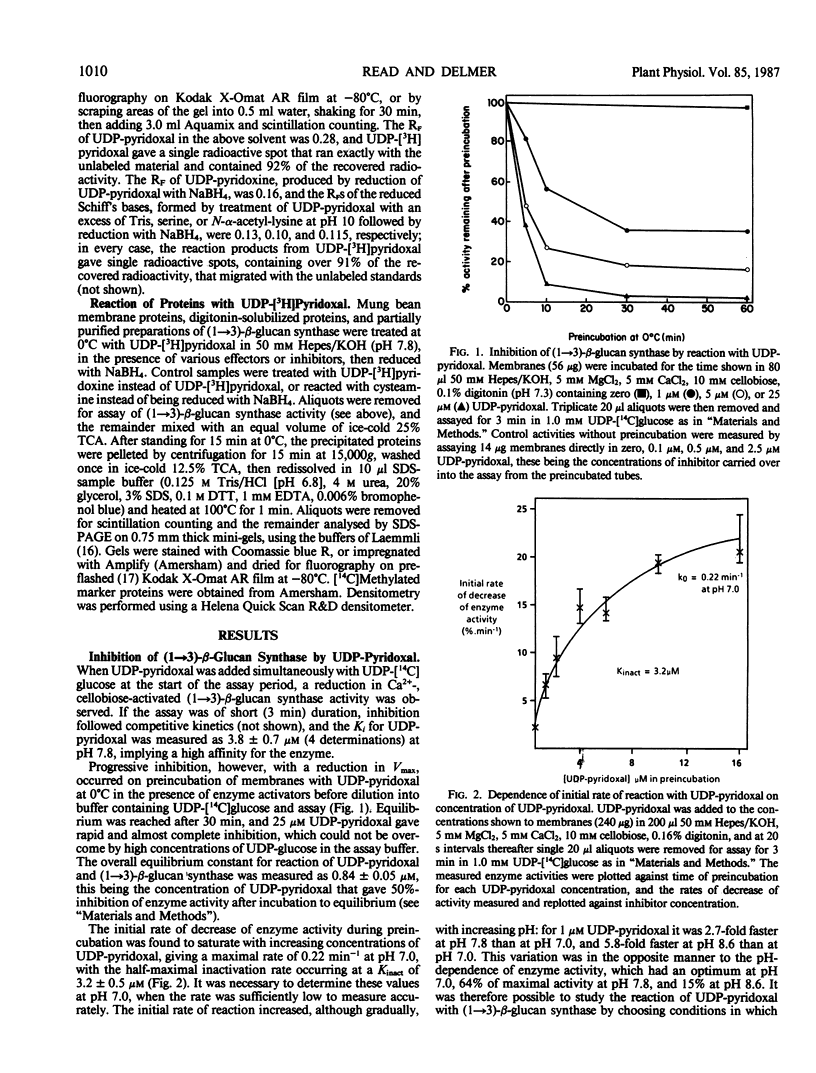
UDP-pyridoxal competitively inhibits the Ca2+-, cellobiose-activated (1→3)-β-glucan synthase activity of unfractionated mung bean (Vigna radiata) membranes, with a Ki of 3.8 ± 0.7 micromolar, when added simultaneously with the substrate UDP-glucose in brief (3 minute) assays. Preincubation of membranes with UDP-pyridoxal and no UDP-glucose, however, causes progressive reduction of the Vmax of subsequently assayed enzyme and, after equilibrium is reached, 50% inhibition occurs with 0.84 ± 0.05 micromolar UDP-pyridoxal. This progressive inhibition is reversible provided that the UDP-pyridoxylated membranes are not treated with borohydride, indicating formation of a Schiff's base between the inhibitor and an enzyme amino group. Consistent with this, UDP-pyridoxine is not an inhibitor. The reaction of (1→3)-β-glucan synthase with UDP-pyridoxal is stimulated strongly by Ca2+ and, less effectively, by cellobiose or sucrose, and the enzyme is protected against UDP-pyridoxal by UDP-glucose or by other competitive inhibitors, implying that modification is occurring at the active site. Pyridoxal phosphate is a less potent and less specific inhibitor. Latent (1→3)-β-glucan synthase activity inside membrane vesicles can be unmasked and rendered sensitive to UDP-pyridoxal by the addition of digitonin. Treatment of membrane proteins with UDP-[3H]pyridoxal and borohydride labels a number of polypeptides but labeling of none of these specifically requires Ca2+ and sucrose; however, a polypeptide of molecular weight 42,000 is labeled by UDP-[3H]pyridoxal in the presence of Mg2+ and copurifies with (1→3)-β-glucan synthase activity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cornish-Bowden A., Eisenthal R. Estimation of Michaelis constant and maximum velocity from the direct linear plot. Biochim Biophys Acta. 1978 Mar 14;523(1):268–272. doi: 10.1016/0005-2744(78)90030-x. [DOI] [PubMed] [Google Scholar]
- Eiberger L. L., Wasserman B. P. Partial Purification of Digitonin-Solubilized beta-Glucan Synthase from Red Beet Root. Plant Physiol. 1987 Apr;83(4):982–987. doi: 10.1104/pp.83.4.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Maclachlan G. Biosynthesis of pentosyl lipids by pea membranes. Biochem J. 1984 Feb 1;217(3):791–803. doi: 10.1042/bj2170791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Read S. M., Bussell J., Thelen M., Lin F. C., Brown R. M., Delmer D. P. UDP-Glucose: (1-->3)-beta-Glucan Synthases from Mung Bean and Cotton: Differential Effects of Ca and Mg on Enzyme Properties and on Macromolecular Structure of the Glucan Product. Plant Physiol. 1987 Apr;83(4):1054–1062. doi: 10.1104/pp.83.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R. J., Stone B. A. Solubilization of beta-glucan synthases from the membranes of cultured ryegrass endosperm cells. Biochem J. 1982 Jun 1;203(3):629–636. doi: 10.1042/bj2030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe M. J., Leopold A. C. Callose deposition during gravitropism of Zea mays and Pisum sativum and its inhibition by 2-deoxy-D-glucose. Planta. 1984 May;161(1):20–26. doi: 10.1007/BF00951455. [DOI] [PubMed] [Google Scholar]
- Kauss H., Jeblick W. Influence of Free Fatty Acids, Lysophosphatidylcholine, Platelet-Activating Factor, Acylcarnitine, and Echinocandin B on 1,3-beta-d-Glucan Synthase and Callose Synthesis. Plant Physiol. 1986 Jan;80(1):7–13. doi: 10.1104/pp.80.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga P. G., Cross R. L. Pyridoxylation of essential lysine residues of mitochondrial adenosine triphosphatase. Biochim Biophys Acta. 1982 Feb 17;679(2):269–278. doi: 10.1016/0005-2728(82)90297-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Moreno S., Cardini C. E., Tandecarz J. S. alpha-Glucan synthesis on a protein primer, uridine diphosphoglucose: protein transglucosylase I. Separation from starch synthetase and phosphorylase and a study of its properties. Eur J Biochem. 1986 Jun 16;157(3):539–545. doi: 10.1111/j.1432-1033.1986.tb09700.x. [DOI] [PubMed] [Google Scholar]
- Morrow D. L., Lucas W. J. (1-->3)-beta-d-Glucan Synthase from Sugar Beet : I. Isolation and Solubilization. Plant Physiol. 1986 May;81(1):171–176. doi: 10.1104/pp.81.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa I., Webster R. E. The orientation of the major coat protein of bacteriophage f1 in the cytoplasmic membrane of Escherichia coli. J Biol Chem. 1981 Oct 10;256(19):9951–9958. [PubMed] [Google Scholar]
- Parsons T. F., Preiss J. Biosynthesis of bacterial glycogen. Isolation and characterization of the pyridoxal-P allosteric activator site and the ADP-glucose-protected pyridoxal-P binding site of Escherichia coli B ADP-glucose synthase. J Biol Chem. 1978 Nov 10;253(21):7638–7645. [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Stock A., Ortanderl F., Pfleiderer G. Darstellung von radioaktiv markiertem Pyridoxal-5'-phosphat. Biochem Z. 1966 Jun 7;344(4):353–360. [PubMed] [Google Scholar]
- Tagaya M., Nakano K., Fukui T. A new affinity labeling reagent for the active site of glycogen synthase. Uridine diphosphopyridoxal. J Biol Chem. 1985 Jun 10;260(11):6670–6676. [PubMed] [Google Scholar]
- Tamura J. K., Rakov R. D., Cross R. L. Affinity labeling of nucleotide-binding sites on kinases and dehydrogenases by pyridoxal 5'-diphospho-5'-adenosine. J Biol Chem. 1986 Mar 25;261(9):4126–4133. [PubMed] [Google Scholar]
- Thelen M. P., Delmer D. P. Gel-Electrophoretic Separation, Detection, and Characterization of Plant and Bacterial UDP-Glucose Glucosyltransferases. Plant Physiol. 1986 Jul;81(3):913–918. doi: 10.1104/pp.81.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman B. P., McCarthy K. J. Regulation of Plasma Membrane beta-Glucan Synthase from Red Beet Root by Phospholipids : Reactivation of Triton X-100 Extracted Glucan Synthase by Phospholipids. Plant Physiol. 1986 Oct;82(2):396–400. doi: 10.1104/pp.82.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]