Abstract
The synergy between amoxicillin and cefotaxime against two strains of Enterococcus faecalis (JH2-2 and 6370) in vitro and in rabbit endocarditis was investigated. In vitro synergy was obtained only when amoxicillin concentrations were below the MBC and when cefotaxime concentrations were above 1 μg/ml. No synergy was observed in vivo, because of the short period of time during which these pharmacologic requirements were achieved.
Recently, an in vitro synergistic effect between amoxicillin and cefotaxime was demonstrated against 50 clinical Enterococcus faecalis strains (9). This combination could be an attractive therapeutic alternative to the therapy of infections due to E. faecalis strains, particularly in case of high-level resistance to aminoglycosides. Nevertheless, the in vivo relevance of this in vitro synergistic combination remains to be assessed.
The study strains were E. faecalis JH2-2 and 6370, which had low and high levels of resistance to aminoglycosides, respectively (9). MICs and MBCs were determined by the macrodilution method in brain heart infusion broth at 106 CFU/ml for amoxicillin alone and in combination with cefotaxime and for cefotaxime alone and in combination with amoxicillin at 0.12 μg/ml (10). In addition, MICs and MBCs were determined with a medium containing 50% complement-inactivated rabbit plasma. The in vitro bactericidal killing rates were determined by the macrodilution method in brain heart infusion broth at 107 CFU/ml for amoxicillin used at 0.12 and 2 μg/ml, alone or in combination with cefotaxime (4 μg/ml), and for cefotaxime at 4 μg/ml (8).
Aortic endocarditis was produced in rabbits, as previously described (1). Forty-eight hours after the injection of 108 E. faecalis CFU, a 5-day treatment protocol of intramuscular (i.m.) injections was initiated with one of the following regimens: amoxicillin at 50 mg/kg of body weight four times a day (q.i.d.), amoxicillin at 150 mg/kg q.i.d., cefotaxime at 50 mg/kg q.i.d., gentamicin at 1 mg/kg q.i.d., amoxicillin at 50 mg/kg q.i.d. plus cefotaxime at 50 mg/kg q.i.d., amoxicillin at 50 mg/kg q.i.d. plus cefotaxime at 150 mg/kg q.i.d., amoxicillin at 150 mg/kg q.i.d. plus cefotaxime at 150 mg/kg q.i.d., and amoxicillin at 50 mg/kg q.i.d. plus gentamicin at 1 mg/kg q.i.d. A control group was left untreated. Six hours after the last antibiotic injection, animals were sacrificed and colony counts in vegetation were determined as previously described (1).
Pharmacokinetic studies were performed with uninfected animals after a single i.m. injection of amoxicillin (50 or 150 mg/kg), cefotaxime (50 or 150 mg/kg), gentamicin (1 mg/kg), and amoxicillin (50 mg/kg) in combination with cefotaxime (50 or 150 mg/kg). Drug concentrations in plasma and vegetation were measured as previously described (7, 11). The binding of amoxicillin and cefotaxime to proteins in rabbit and human plasma was measured according to the ultrafiltration method as described by Craig and Suh (2). Plasma pharmacokinetics of the antibiotics were determined by using a two-compartment open model, with the 1.1 version of Siphar/Win software (Simed, Creteil, France) (6). Bacterial concentrations in vegetation from the different treatment groups were compared by analysis of variance followed by multiple comparisons tests (1).
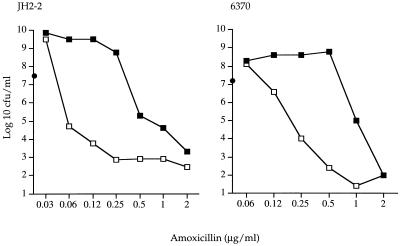
The in vitro susceptibilities of the study strains are shown in Table 1. Additional studies showed that the cefotaxime minimum concentration required to obtain in vitro synergy was 1 μg/ml, which corresponded to the MIC at which 50% of the isolates are inhibited of cefotaxime in the presence of 0.06 μg of amoxicillin per ml, as previously reported for 50 clinical strains of E. faecalis (9). The killing curves showed a synergistic and bactericidal effect of the combination of a low concentration of amoxicillin (0.12 μg/ml) with 4 μg of cefotaxime per ml, which was observed for both strains (data not shown). It should be noted that the bactericidal effect of this combination was comparable but not superior to that of amoxicillin alone at 2 μg/ml after 24 h of incubation. However, synergy was not obtained when this higher concentration of amoxicillin (2 μg/ml) [i.e., superior or equal to the MBC]) was used, as shown in Fig. 1. Protein binding of cefotaxime was 79% ± 6% in rabbit plasma but 30% ± 10% in human plasma. In contrast, protein binding of amoxicillin was 24% ± 7% in rabbit plasma, comparable to the 17% reported in humans (2).
TABLE 1.
MICs and MBCs of amoxicillin and cefotaxime alone and in combination for E. faecalis JH2-2 and 6370
| Drug | MIC or MBC (μg/ml) for strain:
|
|||
|---|---|---|---|---|
| JH2-2
|
6370
|
|||
| MIC | MBC | MIC | MBC | |
| Amoxicillin | 0.5 | 1 | 1 | 2 |
| Amoxicillin + cefotaxime (4 μg/ml) | 0.06 | 0.12 | 0.25 | 0.25 |
| Cefotaxime | 256 | 1,024 | 256 | 256 |
| Cefotaxime + amoxicillin (0.12 μg/ml) | 1 | 512 | 8 | 256 |
FIG. 1.
Bactericidal effect on E. faecalis JH2-2 and 6370 after 24 h of incubation with amoxicillin alone (▪) or combined with cefotaxime at 4 μg/ml (□). •, the initial inoculum.
Antibiotic pharmacokinetics determined in uninfected animals are shown in Table 2. In rabbits with aortic endocarditis, bactericidal concentrations of amoxicillin were achieved at the peak level in vegetation (6.7 ± 3.5 μg/g), and trough levels of cefotaxime and amoxicillin were at the level of the threshold of detection in most animals after 5 days of therapy (1.5 ± 1.5 and 0.5 ± 0.2 μg/ml, respectively).
TABLE 2.
Pharmacokinetic parameters of amoxicillin and cefotaxime (alone and in combination) in uninfected rabbits after a single i.m. injectiona
| Regimen (mg/kg) | Concn in plasma (μg/ml)
|
t1/2βb (min) | AUC0–∞c (min · mg/liter) | Vβd (liters/kg) | |
|---|---|---|---|---|---|
| Peak (Cmax)e | Trough | ||||
| Amoxicillin (50) | 28 ± 10 | 0.8 ± 0.5 | 70 ± 20 | 2,500 ± 500 | 2.1 ± 0.7 |
| Amoxicillin (50) + cefotaximef (50) | 29 ± 9 | 0.4 ± 0.4 | 80 ± 33 | 2,100 ± 400 | 2.7 ± 1.0 |
| Amoxicillin (150) | 68 ± 32 | 5.5 ± 2.9 | 130 ± 38 | 9,500 ± 3,200 | 4.1 ± 2.3 |
| Cefotaxime (50) | 56 ± 26 | 0.9 ± 1.3 | 45 ± 14 | 4,600 ± 800 | 0.7 ± 0.2 |
| Cefotaxime (50) + amoxicillinf (50) | 56 ± 22 | 1.4 ± 0.7 | 74 ± 33 | 5,200 ± 1,700 | 1.2 ± 0.8 |
| Cefotaxime (150) | 102 ± 19 | 6.9 ± 4.0 | 158 ± 57 | 13,800 ± 2,900 | 2.6 ± 1.0 |
| Gentamicin (1) | 2.1 ± 1 | <0.27 | 61 ± 14 | 311 ± 103 | 0.4 ± 0.2 |
Each value is the mean (± standard deviation) of measurements made for at least six animals.
t1/2, half-life.
AUC, area under the concentration-time curve.
V, volume of distribution.
Cmax, maximum concentration of drug in serum.
The drug indicated was coadministered, but its pharmacokinetic parameters were not determined.
As shown in Table 3, the low dose of amoxicillin had activity against both strains in experimental endocarditis. Cefotaxime alone was not active (12). The reference therapies (i.e., low-dose amoxicillin plus gentamicin against JH2-2 and high-dose amoxicillin against 6370) had significant and comparable bactericidal effects in vivo and were the most effective regimens. Against both strains, the in vivo effect of the combination of low-dose cefotaxime and low-dose amoxicillin was comparable to that of amoxicillin alone. Against 6370, the combination of a high dose of cefotaxime and either a low or high dose of amoxicillin did not produce any advantage over amoxicillin alone. Survival was not statistically different among treatment groups. No valve was found to be sterilized with any regimen. No resistant strain selected by combined amoxicillin-cefotaxime treatments was detected.
TABLE 3.
Results of different 5-day treatment regimens in rabbits infected with E. faecalis strains
| Regimen (mg/kg q.i.d.) | Mean ± SD log10 CFU/g of vegetation (n) in strain:
|
|
|---|---|---|
| JH2-2 | 6370 | |
| Control | 9.9 ± 1.1 (10) | 10.7 ± 1.1 (6) |
| Cefotaxime (50) | 9.7 ± 0.8 (8) | NDc |
| Gentamicin (1) | 10.1 ± 0.9 (6) | ND |
| Amoxicillin (50) | 7.7 ± 0.8a (8) | 7.1 ± 0.9a (8) |
| Amoxicillin (50) + cefotaxime (50) | 7.9 ± 0.6a (11) | 7.9 ± 0.8a (9) |
| Amoxicillin (50) + cefotaxime (150) | ND | 7.8 ± 1.2a (11) |
| Amoxicillin (150) | ND | 6.5 ± 1.2a (6) |
| Amoxicillin (150) + cefotaxime (150) | ND | 6.9 ± 0.8a,b (9) |
| Amoxicillin (50) + gentamicin (1) | 6.9 ± 0.4a,b (6) | ND |
P < 0.0001 versus control.
P < 0.05 versus amoxicillin (50 mg/kg) plus cefotaxime (50 mg/kg).
ND, not done.
As shown in Fig. 1, the synergistic effect of the amoxicillin-cefotaxime combination was obtained for amoxicillin levels ranging from the MIC of the combination to the MBC of amoxicillin alone. For JH2-2, this interval was approximately 0.06 to 1 μg/ml, whereas for 6370 it was 0.25 to 2 μg/ml. In addition, the lowest concentration of cefotaxime required for synergism was approximately 1 μg/ml. These specific pharmacological conditions are in agreement with the mechanism of amoxicillin-cefotaxime synergy that was previously described (9): the synergistic effect of cefotaxime combined with nonbactericidal concentrations of amoxicillin for E. faecalis JH2-2 was explained by the complementary saturation of penicillin-binding protein 2 (PBP 2) and PBP 3 by cefotaxime and PBP 4 and PBP 5 by amoxicillin. With bactericidal levels of amoxicillin alone, all PBPs were saturated.
The level of susceptibility of strains 6370 and JH2-2 to amoxicillin (i.e., MIC of <4 μg/ml) was comparable to those of the majority of E. faecalis strains observed in clinical practice (3, 13). In vitro, a bactericidal effect was obtained with 2 μg of amoxicillin per ml. Therefore, because bactericidal concentrations of amoxicillin were achieved in plasma even with the low-dose regimen, a 5-day treatment resulted in a significant and almost optimal bacterial reduction. A different result might have been obtained against E. faecalis strains that are more resistant or tolerant to amoxicillin, as was recently reported for the ampicillin-ceftriaxone combination in an experimental model of endocarditis caused by an E. faecalis strain tolerant to ampicillin (MIC of 0.5 μg/ml, MBC of 32 μg/ml) (5).
The lack of in vivo synergy may be explained by pharmacokinetic studies of uninfected animals showing that antibiotic concentrations in plasma adequate for synergy were ideally obtained with the regimen of amoxicillin at 50 mg/kg and cefotaxime at 150 mg/kg only 4 h after a given injection. In addition, the high protein binding of cefotaxime in rabbits and a decrease of cefotaxime trough levels over time during therapy in infected animals (4) may also account for this result.
In conclusion, the discrepancy observed between in vitro and in vivo results for both study strains may be explained by different limiting factors. (i) Specific concentrations of amoxicillin (i.e., below the MBC) and cefotaxime (i.e., >1 μg/ml) were required to obtain synergy. (ii) Amoxicillin alone, even at the lowest dose, was significantly bactericidal in vivo. (iii) The period of time during which both antibiotics were simultaneously present and at adequate concentrations to obtain in vivo synergy was limited.
REFERENCES
- 1.Aslangul E, Baptista M, Fantin B, Depardieu F, Arthur M, Courvalin P, Carbon C. Selection of glycopeptide-resistant mutants of VanB-type Enterococcus faecalis BM4281 in vitro and in experimental endocarditis. J Infect Dis. 1997;175:598–605. doi: 10.1093/infdis/175.3.598. [DOI] [PubMed] [Google Scholar]
- 2.Craig W A, Suh B. Protein binding and the antimicrobial effects: methods for the determination of protein binding. In: Lorian V, editor. Antibiotics in laboratory medicine. 2nd ed. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 265–293. [Google Scholar]
- 3.Fontana R, Grossato A, Ligozzi M, Tonin E A. In vitro response to bactericidal activity of cell wall-active antibiotics does not support the general opinion that enterococci are naturally tolerant to these antibiotics. Antimicrob Agents Chemother. 1990;34:1518–1522. doi: 10.1128/aac.34.8.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganzinger U, Haslberger A. Pharmacokinetics of cephalosporins in normal and septicemic rabbits. Antimicrob Agents Chemother. 1985;28:473–477. doi: 10.1128/aac.28.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gavalda J, Capdevila J A, Torres C, Laguarda M, Tenorio C, de Otero J, Pahissa A. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Efficacy of ampicillin (A) and ceftriaxone (C) in the treatment of experimental endocarditis due to Enterococcus faecalis highly-resistant to aminoglycosides, abstr. B4; p. 22. [Google Scholar]
- 6.Gomeni R. An interactive programme for individual and population parameter estimation. In: Van Bemmel J H, Ball N, Wigertz N, editors. Medinfo 83. Amsterdam, The Netherlands: North Holland; 1983. pp. 1022–1025. [Google Scholar]
- 7.Jehl F, Caillon C, Martone W J. High performance liquid chromatography of antibiotics. J Chromatogr. 1990;531:509–548. doi: 10.1016/s0378-4347(00)82293-8. [DOI] [PubMed] [Google Scholar]
- 8.Krogstad D J, Moellering R C. Antimicrobial combinations. In: Lorian V, editor. Antibiotics in laboratory medicine. 2nd ed. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 537–595. [Google Scholar]
- 9.Mainardi J-L, Gutmann L, Acar J F, Goldstein F W. Synergistic effect of amoxicillin and cefotaxime against Enterococcus faecalis. Antimicrob Agents Chemother. 1995;39:1984–1987. doi: 10.1128/aac.39.9.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearson R D, Steigbigel R T, Davis H T, Chapman S W. Method for a reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980;18:699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Provost Y, Farinotti R. Dosage immunologique par polarisation de fluorescence: application aux médicaments. J Pharmacol Clin. 1984;3:199–216. [Google Scholar]
- 12.Sullam P M, Drake T A, Täuber M G, Hackbarth C J, Sande M A. Influence of the developmental state of valvular lesions on the antimicrobial activity of cefotaxime in experimental enterococcal infections. Antimicrob Agents Chemother. 1985;27:320–323. doi: 10.1128/aac.27.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toala P, McDonald A, Wilcox C, Finland M. Susceptibility of group D Streptococcus (enterococcus) to 21 different antibiotics in vitro, with special reference to species differences. Am J Med Sci. 1969;258:416–430. doi: 10.1097/00000441-196912000-00006. [DOI] [PubMed] [Google Scholar]