Visual Abstract
Key Words: BHB, heart failure, histone methylation, ischemia, mitochondria, myocardial infarction, β-hydroxybutyrate
Highlights
-
•
We provide a comprehensive set of assays using human cardiac specimens from patients with postischemic HF and healthy control subjects, a murine model of HF, and mechanistic studies ex vivo and in vitro.
-
•
We identified a specific epigenetic modification of the chromatine, at the level of histone 3, namely a double methylation of lysine 27 and a single methylation of lysine 36 (H3_K27me2K36me1), which is consistently induced by ischemic injury in all the above-mentioned settings.
-
•
H3_K27me2K36me1 mediates the ischemia-induced transcriptional repression of PGC1α, master regulator of mitochondrial function and biogenesis.
-
•
Both the augmented H3_K27me2K36me1 and the mitochondrial dysfunction caused by the down-regulation of PGC1α are significantly attenuated by the treatment with the ketone body BHB.
Summary
Ischemic cardiac disease is a major cause of mortality worldwide. However, the exact molecular processes underlying this disorder are not fully known. This study includes a comprehensive and coordinated set of in vivo and in vitro experiments using human cardiac specimens from patients with postischemic heart failure (HF) and healthy control subjects, a murine model of HF, and cellular systems. These approaches identified for the first time a specific pattern of maladaptive chromatin remodeling, namely a double methylation of histone 3 at lysine 27 and a single methylation at lysine 36 (H3_K27me2K36me1) consistently induced by ischemic injury in all these settings: human HF; murine HF; and in vitro models. Mechanistically, this work demonstrates that this histone modification mediates the ischemia-induced transcriptional repression of PPARG coactivator 1α (PGC1α), master regulator of mitochondrial function and biogenesis. Intriguingly, both the augmented H3_K27me2K36me1 and the mitochondrial dysfunction ensued by PGC1α down-regulation were significantly attenuated by the treatment with β-hydroxybutyrate, the most abundant ketone body in humans, revealing a novel pathway coupling metabolism to gene expression. Taken together, these findings establish maladaptive chromatin remodeling as a key mechanism in postischemic heart injury, functionally modulated by ketone bodies.
The number of patients with postischemic heart failure (HF) has recently reached epidemic proportions.1 Unfortunately, HF prognosis remains poor despite the current availability of several pharmacologic and nonpharmacologic approaches.1,2 Understanding the cellular and molecular mechanisms underlying HF development and progression is therefore critical. Specifically, the identification of maladaptive mechanisms to be inhibited and adaptive mechanisms to be reinforced by harnessing their biological mediators, is a fundamental goal of translational research and a promising alternative to currently available therapeutic strategies.
Since HF pathophysiology is governed in part by epigenetic events, representing the molecular transducers of environmental stimuli for gene expression control,3 we sought to explore the histone modifications triggered by an ischemic cardiac insult in 3 different models: in left ventricles from patients with postischemic HF; in a mouse model of myocardial infarction (MI); and in a cellular setting in vitro. To this aim, we analyzed by mass spectrometry a wide spectrum of histone modifications, including acetylation and methylations, in our models.
Equally important, mitochondrial dysfunction and the resulting metabolic disarrangement have been shown to substantially contribute to the pathobiology of HF.4 However, a precise mechanism linking epigenetic modifications and mitochondrial dysfunction is missing.
Methods
An extended version of the Methods section, including ex vivo and in vitro experiments, is reported in the Supplemental Appendix 1.
Human cardiac specimens and ethics statement
Samples of left ventricles from patients with a confirmed diagnosis of postischemic HF and age-matched control donors who had no history of macroscopic or laboratory signs of cardiac diseases were obtained in compliance with the protocol on tissue collection and use approved by our Einstein/Montefiore Institutional Review Board (no. 2021-12729; Principal Investigator: Santulli). Relevant clinical characteristics of these individuals are reported in Supplemental Table 1. All investigations followed the principles outlined in the Declaration of Helsinki. A written consent was waived by the Institutional Review Board because subjects were deceased and samples were deidentified.
In vivo model
Experiments were carried out in 20-week-old male C57BL/6N mice housed under a standard 12-hour light-dark cycle under pathogen-free conditions with ad libitum access to food and water. All protocols were approved by the Einstein IACUC committee (no. 00001302; Principal Investigator: Santulli). MI was induced by occlusion of the proximal left anterior descending coronary artery.5 Immediately after surgery, rodents were randomly divided in 2 groups: one was fed standard diet, the other group was fed β-hydroxybutyrate (BHB) diet for 4 weeks. For dietary BHB supplementation, 125 g of BHB was added to each kilogram of standard diet.
In vitro assays
Cardiomyocyte isolation, contractility assessment, and calcium (Ca2+) imaging were carried out as we described5 (see the Supplemental Appendix 1 for details on all the experimental procedures). Real-time reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) was performed as we previously reported5; primer sequences are in Supplemental Table 2.
Statistical analysis
All results are presented as mean ± SD or mean ± SEM, as indicated. Statistical analysis was performed using Prism software (version 9.0, GraphPad by Dotmatics). For all analyses, normal distribution was tested using the Shapiro-Wilk normality test. For comparisons of 2 groups, the unpaired 2-tailed Student’s t-test using (when appropriate) Welch correction for unequal variances was performed. The nonparametric Mann-Whitney U test was used for comparisons between 2 groups that did not show a Gaussian distribution. For comparisons of multiple groups, one-way analysis of variance was performed followed by Tukey-Kramer post hoc test for multiple pairwise comparisons. The Kruskal-Wallis test, followed by Dunn test was used when 1 or more groups did not show a Gaussian distribution. Kaplan-Meier methods were used to compare survival curves (log-rank test). Significant differences were established at a P value <0.05. For RNA-sequencing assays, the P values were adjusted for multiple comparisons by false discovery rate by applying the Benjamini-Hochberg method. Statistical analysis in liquid chromatography with tandem mass spectrometry was carried out using heteroscedastic Student’s t-test (if P < 0.05 for unequal variance test).
Results
Cardiac ischemic injury leads to a specific histone modification in patients, mice, and cells
We analyzed the chromatin signature in 3 different experimental models—in patients with postischemic HF, in mice, and in vitro—to evaluate the presence of histone modifications elicited by an ischemic injury. Our in vivo model was obtained via permanent ligation of the left anterior descending coronary artery, whereas for the in vitro assays, we exposed H9c2 cardiac cells to a buffer capable of simulating an ischemic status in vitro (see also the Methods section in the Supplemental Appendix 1). Untargeted mass spectrometry was performed to characterize the pattern of histone acetylation and methylation in the different samples, revealing several chromatin modifications induced by the ischemic injury (as shown in the heat-maps in Figures 1A to 1C).
Figure 1.
Evaluation of Chromatin Remodeling in Different Experimental Settings
Histone modifications were assessed to determine common epigenetic modifications in vitro (A), in a mouse model of postischemic heart failure (HF) (B), and in human left ventricular samples (C). Mass spectrometry analysis was performed to assess a wide spectrum of histone modifications, including acetylation and methylations. Heat-maps showing the relative abundance of the analyzed histone modifications. A single combination of histone modifications was affected both in vitro (A) and in vivo, in cardiomyocytes (CMs) isolated from murine failing hearts (B) with the same trend: a double methylation of histone H3 at lysine 27 and one methylation at lysine 36 (H3_K27me2K36me1), which was consistently up-regulated under ischemic conditions but not after β-hydroxybutyrate (BHB) treatment; H3_K27me2K36me1 was also increased in human left ventricular tissue from patients with postischemic HF (C). The data (mean ± SEM) are representative of at least 3 independent experiments. ∗P < 0.05 versus vehicle (A), analysis of variance; versus sham (B), analysis of variance; versus sham (C), Mann-Whitney U test. MI = myocardial infarction.
Ischemic insult leads to apoptosis and mitochondrial dysfunction in vitro
To identify novel therapeutic strategies in postischemic HF, we first verified in vitro the effects of a bona fide ischemic injury, examining apoptosis and mitochondrial fitness in H9c2 cells. We observed that ischemia significantly increased apoptosis, assessed via caspase 3 activation (Figures 2A and 2B), annexin V (Figure 2C), and terminal deoxynucleotidyl transferase dUTP nick end labeling (Figure 2D).
Figure 2.
The Ketone Body BHB Significantly Attenuates Ischemia-Induced Cell Death
H9c2 cells were pre-treated with β-hydroxybutyrate (BHB) or vehicle for 8 hours, then exposed to a buffer simulating ischemic conditions (ischemia) for 12 hours and collected. Immunoblot analysis revealed the increase of cleaved-caspase-3 in the cells exposed to ischemia, whereas BHB inhibited the ischemia-induced caspase-activation (A and B). Apoptosis was assessed via annexin-V staining, analyzing the fluorescence by fluorescent activated cell sorter (C) and via terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) (D). The percentage of apoptotic cells (annexin-V–positive) increased in response to ischemia; on the other end, BHB exposure reduced the amount of ischemia-induced apoptosis. Cytosolic fraction was obtained from the cells and the levels of cytochrome C (Cyt C) evaluated by immunoblot. Ischemia induced Cyt C release in the cytosol, whilst BHB treatment inhibited mitochondria permeabilization to Cyt C induced by ischemia (E and F). All images are representative of triplicate independent experiments; bar = 75 μm; mean ± SEM; ∗P < 0.05 versus vehicle; #P < 0.05 versus ischemia, Kruskal-Wallis test (B and F), analysis of variance (C and D). DAPI = 4′,6-diamidino-2-phenylindole; GAPDH = glyceraldehyde-3-phosphate dehydrogenase.
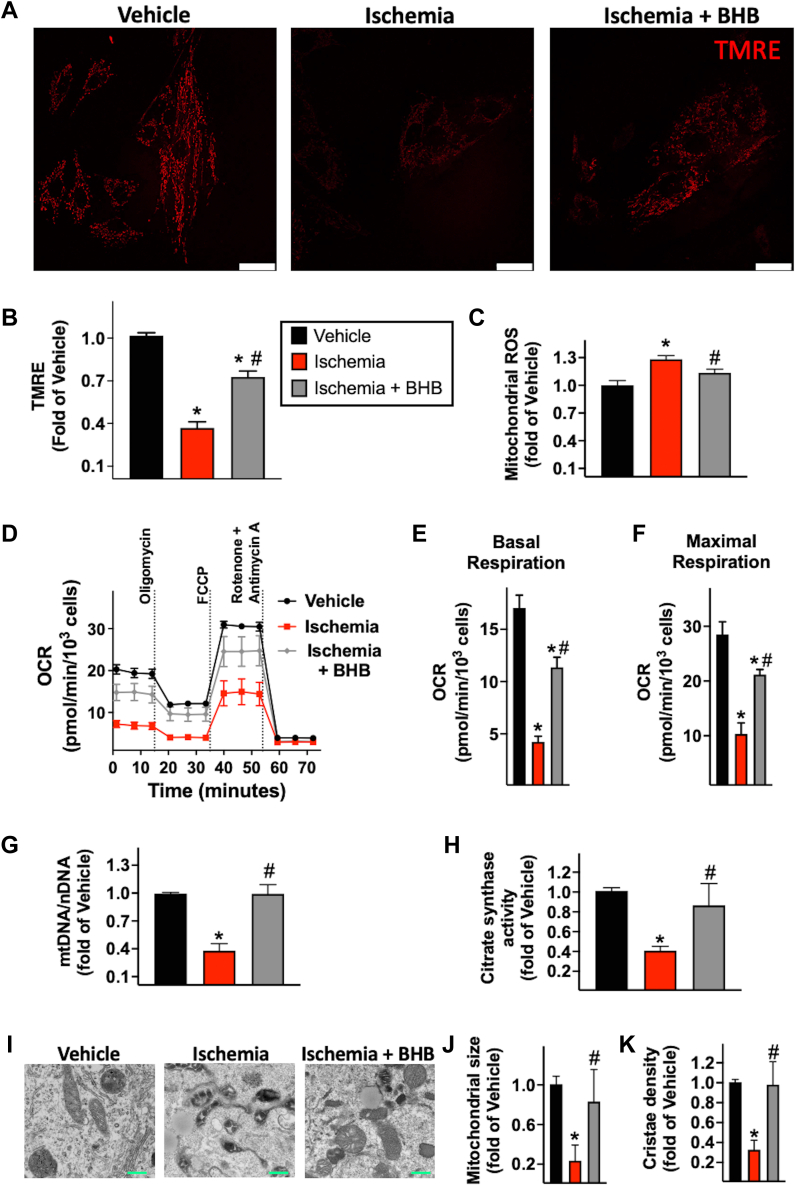
The loss of mitochondrial integrity resulting in cytochrome C (Cyt C) release is among the main mechanisms underlying apoptosis in ischemic conditions.6 Accordingly, in our in vitro model, the ischemic insult ensued increased Cyt C levels in cytosolic extracts (Figures 2E and 2F). We then focused on other vital aspects of mitochondrial health, including membrane potential, reactive oxygen species (ROS) production, and respiration. High-resolution imaging on live cells was used to assess mitochondrial potential by tetramethyl rhodamine ethyl ester staining. Simulated ischemia promoted mitochondrial depolarization (Figures 3A and 3B) and ROS generation (Figure 3C), decreased basal and maximal oxygen consumption rate (OCR) (Figures 3D to 3F), mitochondrial DNA (Figure 3G), and citrate synthase activity (Figure 3H); these observations were mirrored by morphologic modifications evidenced by electron microscopy (Figures 3I to 3K). Consistently, ischemia induced mitochondrial fragmentation (Figures 4A and 4B) and reduced mitochondrial biogenesis (Figures 4C to 4F).
Figure 3.
Effects of Ischemia and BHB on Mitochondrial Function and Morphology
H9c2 cells were pretreated with β-hydroxybutyrate (BHB) or vehicle for 8 hours, then exposed to a buffer simulating ischemic conditions (ischemia) for 12 hours and stained to evaluate mitochondrial membrane potential and reactive oxygen species (ROS) production. Mitochondrial membrane potential was assessed by confocal microscopy staining the cells with tetramethyl rhodamine ethyl ester (TMRE) (red); ischemia evoked a loss of mitochondrial membrane potential, whereas BHB treatment preserved mitochondrial membrane potential under ischemia condition (representative pictures from quadruplicate independent assays are shown [A] and quantified [B]). MitoSOX (Thermo Fisher Scientific) was used to measure mitochondrial ROS generation, and the signal was detected by plate-reader. Ischemia induced mitochondrial ROS hyperproduction, but the response was inhibited in presence of BHB (C). Mitochondrial respiration was assessed by measuring oxygen consumption rate (OCR) at baseline and after sequential injection of oligomycin, carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone (FCCP), and rotenone/antimycin A (D to F). The ratio of mitochondrial DNA (mtDNA) to nuclear DNA (nDNA) was calculated (G) and the citrate synthase activity was measured (H). Representative electron microscopy pictures (I) and quantification (J and K) at the indicated conditions. Bars = 25 μm (white) or 500 μm (green). Data (mean ± SEM) are representative of at least 3 independent experiments; ∗P < 0.05 versus vehicle; #P < 0.05 versus ischemia (analysis of variance).
Figure 4.
BHB Alleviates the Detrimental Effects of Ischemia on Mitochondrial Dynamics and Biogenesis
H9c2 cells were pretreated with BHB or vehicle for 8 hours and then exposed to a buffer simulating ischemic conditions (ischemia) for 12 hours; mitochondrial morphology was assessed by high-resolution imaging using MitoTracker (green) for mitochondrial staining. The elongation factor was used as index of elongated versus fragmented mitochondria. The ischemic injury induced mitochondria fragmentation and damage, decreasing the elongation factor. With BHB treatment, mitochondrial shape was preserved under ischemic conditions: representative pictures of triplicate biological experiments are shown (A) (bar = 10 μm) and quantified (B). Mitochondrial biogenesis was assessed by immunoblot by measuring the expression levels of the subunit A of the succinate dehydrogenase complex (SDH-A) levels: ischemia reduced SDH-A expression, but BHB counteracted this phenomenon (representative blots from quadruplicate independent assays are shown [C] and quantified [D]). In whole cell lysates, PPARG coactivator 1α (PGC1α) levels were detected by immunoblot. Ischemia induced the down-regulation of PGC1α, whereas BHB treatment preserved PGC1α in the ischemic cells (representative blots from triplicate biological experiments are shown [E] and quantified [F]). Data (mean ± SEM) are representative of at least 3 independent experiments.∗P < 0.05 versus vehicle; #P < 0.05 versus ischemia (analysis of variance [B], Kruskal-Wallis test [D and F]). Abbreviations as in Figures 1 and 2.
BHB counteracts the histone modifications induced by ischemia, both in vitro and in vivo
During HF, in addition to reducing fatty acid use, cardiomyocytes (CMs) shift their metabolism toward ketone bodies (BHB, acetoacetate, and acetone).7 In CMs, ketone bodies are oxidized in mitochondria producing acetyl-coenzyme A as a substrate for the tricarboxylic acid cycle and for adenosine triphosphate production.
On these grounds, we sought to determine the effects of BHB, the most abundant ketone body in mammals, in cardiac ischemia, focusing on chromatin remodeling and mitochondrial dysfunction. Specifically, mass spectrometry analysis allowed us to identify a single modification, namely an increased double methylation at lysine 27 and a single methylation at lysine 36 of histone H3 (H3_K27me2K36me1), displaying a similar response to ischemic conditions reversed by BHB treatment, both in H9c2 cells (Figure 1A) and in post-MI murine CMs (Figure 1B). Of note, H3_K27me2K36me1 was also augmented in human left ventricles obtained from patients with postischemic HF (Figure 1C). These data unveil an unprecedented role of BHB in counteracting the maladaptive epigenetic remodeling under ischemic stress conditions.
Moreover, the transcriptomic profiles of H9c2 cells analyzed via RNA-sequencing revealed PPARG coactivator 1α (PGC1α, also PPARGC1a), the master regulator of mitochondrial biogenesis, as the top differentially expressed gene to be decreased by ischemia and increased after BHB treatment (Supplemental Figures 1A and 1B, Supplemental Tables 3 and 4).
BHB protects against ischemia-induced mitochondrial apoptosis
We then explored the effects of BHB on ischemia-induced cellular damage and apoptosis in vitro. Ischemia-induced caspase 3 activation was significantly attenuated by BHB (Figures 2A and 2B). We also confirmed the protective effects of BHB on ischemia-induced apoptosis (Figures 2C and 2D). To verify whether the beneficial effects of BHB were mediated by actions directed toward mitochondria, we measured Cyt C levels in cytosolic extracts, and we found that BHB attenuated the release of Cyt C induced by ischemia (Figures 2E and 2F). In line with these results, BHB preserved mitochondrial membrane potential (Figures 3A and 3B), alleviated oxidative stress (Figure 3C), mitigated the impairment in mitochondrial respiration (Figure 3D to 3F) and organelle fragmentation (Figures 4A and 4B) induced by ischemia, and rescued mitochondrial biogenesis (Figures 4C to 4F).
Consistent with previous reports,8,9 we did not observe any significant effect of BHB in nonischemic settings (not shown). These data indicate that BHB protects cardiac cells from ischemia-induced apoptosis, at least in part by preserving mitochondrial integrity.
BHB treatment in vivo protects against postischemic HF
To translate our findings in vivo, we used an established murine model of HF induced by MI, obtained by permanent ligation of the left anterior descending coronary artery, recapitulating the pathophysiology of post-MI HF observed in patients. After surgery, the mice were fed standard diet or BHB-supplemented diet for 1 month. The homogeneity between groups in terms of myocardial damage induced by coronary ligation was verified 24 hours after surgery by measuring circulating troponin I levels in all mice, as a reliable surrogate of the extension of the infarct (Supplemental Figure 2A), as well as by determining the actual acute infarct area in some mice randomly selected from each group (Supplemental Figure 2B). To determine the effectiveness of BHB diet in inducing a chronic increase in BHB availability, we measured plasmatic BHB levels 1 month after the beginning of this diet regimen (Supplemental Figure 3A); we did not observe major differences in food intake between groups (Supplemental Figure 3B). BHB supplementation significantly increases survival after MI (Figure 5A). Cardiac remodeling and dysfunction induced by MI were significantly attenuated in mice exposed to BHB (Supplemental Table 5). Indeed, ejection fraction and left ventricular diameter were preserved in post-MI mice treated with BHB (Figures 5B and 5C). Interstitial cardiac fibrosis induced by MI was also prevented by BHB supplementation (Figures 5D and 5E). Accordingly, atrial natriuretic peptide levels, a marker of pathological hypertrophy and remodeling,10 were reduced in MI mice treated with BHB then in MI untreated mice (Figure 5F). As observed in vitro, we did not detect any significant effect of BHB on sham mice in vivo (not shown). The protective effects of BHB were also confirmed at the cellular level, on primary isolated CMs. We verified the contractile profile of CMs and the excitation-contraction coupling by single-cell analysis using the IonOptix system. BHB supplementation prevented the hypercontractile phenotype (Supplemental Figures 4A to 4F). Hence, our in vivo data confirmed that BHB counteracts the maladaptive morphologic and functional cardiac remodeling toward HF development after an ischemic injury such as MI. We also observed that BHB significantly decreased the activity of S-adenosylhomocysteine hydrolase (also adenosylhomocysteinase) (Figure 5G), a rate-limiting enzyme of the methionine cycle, thereby augmenting the levels of S-adenosylhomocysteine, a potent inhibitor of histone methyltransferases,11 in left ventricular tissue (Figure 5H); this finding was substantiated by similar results obtained in H9c2 cardiac cells (Supplemental Figure 5).
Figure 5.
In Vivo Effects of BHB in a Murine Model of Myocardial Infarction
Survival curves obtained in 20 animals per group starting 24 hours after coronary ligation (A); ∗P < 0.05, log rank test. One month after MI, cardiac phenotype was assessed by echocardiography. Left ventricular ejection fraction (EF) was impaired in MI rodents compared to sham littermates, whereas BHB diet significantly attenuated cardiac dysfunction (B). Left ventricular end-diastolic diameter (LVEDD) increased after MI; conversely BHB supplementation markedly reduced MI-induced maladaptive remodeling (C). Cardiac fibrosis was determined by picrosirius red staining; representative pictures (bar = 150 μm) and relative quantification showing the increase in interstitial fibrosis in MI left ventricles compared to sham, but not in the left ventricles from MI-mice fed BHB diet (D and E). Atrial natriuretic peptide (ANP) levels were determined by reverse transcriptase quantitative polymerase chain reaction on left ventricles, and its levels were augmented in post-MI left ventricles but not in post-MI samples from mice fed BHB diet (F). S-adenosylhomocysteine hydrolase (SAHH) enzymatic activity (G) and S-adenosylhomocysteine (SAH) levels (H) were measured in left ventricular tissue. n = 8 mice per group; mean ± SEM; ∗P < 0.05 versus sham; #P < 0.05 versus MI (analysis of variance). mRNA = messenger RNA; other abbreviations as in Figure 1.
BHB protects cardiac mitochondria from MI-induced organelle dysfunction preventing PGC1α down-regulation
To verify ex vivo whether BHB could prevent mitochondrial dysfunction in the postischemic heart, we isolated CMs from sham, MI, and MI+BHB left ventricles to assess mitochondrial function. We have previously shown that cardiac ischemic injury induces mitochondrial Ca2+ overload and damage.5 In CMs isolated from MI mice treated with BHB, we observed a significant attenuation of mitochondrial Ca2+ overload, implying a reduced mitochondrial stress and workload (Figures 6A and 6B). Therefore, we evaluated mitochondrial performance by quantifying OCR; CMs primarily isolated from post-MI left ventricles displayed an impaired OCR, especially in terms of maximal respiration rate. Interestingly, BHB improved the respiration profile of MI CMs (Figures 6C to 6E). To determine whether in vivo the effect of BHB in supporting mitochondrial health could be mediated by the modulation of PGC1α, we assessed cardiac PGC1α levels in isolated CMs: as we hypothesized, chronic BHB supplementation was able to preserve PGC1α expression after MI (Figures 6F and 6G). These findings validate the ability of BHB in improving mitochondrial fitness in vivo, most likely modulating PGC1α expression under ischemic conditions.
Figure 6.
Effects of BHB on Mitochondrial Fitness Determined Ex Vivo in Isolated Cardiomyocytes
Mitochondrial phenotype assessed in adult cardiomyocytes (CMs) isolated from the indicated groups of mice 1 month after MI. Mitochondrial calcium (Ca2+) was assessed via Rhod-2 dynamic recording (A) (representative traces). BHB diet reduced mitochondrial Ca2+ overload induced by MI (B). Mitochondrial OCR was impaired after MI and BHB administration improved mitochondrial respiration in MI-CMs (C to E). PGC1α levels were determined by immunoblot analysis on isolated CMs; PGC1α was significantly reduced post-MI, whereas BHB exposure preserved PGC1α levels (F and G). n = 8 mice per group. Data are representative of at least 3 independent experiments; mean ± SEM; ∗P < 0.05 versus sham; #P < 0.05 versus MI (Student’s t-test [B], analysis of variance [D and E], Kruskal-Wallis test [G]). Abbreviations as in Figures 1, 2, 3, and 4.
H3_K27me2K36me1 mediates the maladaptive transcriptional repression of PGC1α during ischemia
H3K27me2 has been shown to be implicated in transcriptional repression, whereas H3K36me1 is associated with gene repression when co-occupying genes with H3K27me2/3-competent chromatin.12 Thus, we hypothesized that during ischemic stress, H3_K27me2K36me1 could occur in the proximity of the PGC1α promoter, inducing a maladaptive repression of PGC1α expression (Figure 7A). To validate this hypothesis, we evaluated by chromatin immunoprecipitation (ChIP) whether in ischemic H9c2 cells and in post-MI CMs, H3_K27me2K36me1 occurred at the level of PGC1α promoter (Figure 7B). Using specific antibodies for H3_K27me2 and H3_K36me1, we specifically immunoprecipitated the fragments of chromatin-DNA complexes where both these histone modifications were present. RT-qPCR analyses on DNA obtained from ChIP revealed the presence of the PGC1α promoter in both ischemic H9c2 cells (Figures 7C and 7D) and post-MI murine CMs (Figures 7E and 7F). These data indicate that PGC1α is a target of H3_K27me2K36me1 histone modification.
Figure 7.
The Double Methylation at Lysine 27 and the Single Methylation at Lysine 36 of Histone H3 Reduce PGC1α Transcription
K27-K36 H3 methylation targets the promoter of PGC1α, producing the transcriptional repression of the gene (A). Schematic workflow of the chromatin immunoprecipitation (ChIP) analysis performed in ischemic H9c2 cells and adult CMs isolated from post-MI left ventricles. Chromatin-DNA crosslinking was performed, followed by chromatin-DNA fragmentation. Using antibodies (Ab) specific for the investigated methylations, chromatin fragments with K27me2 or K36me1 were immunoprecipitated. A decrosslinking step allowed to separate chromatin from the immunoprecipitated DNA. DNA was used to perform polymerase chain reaction (PCR) using primers detecting PGC1α promoter (B). Reverse transcriptase quantitative PCR was conducted on DNA obtained from ChIP using K27me2- or K36me1 H3-specific antibodies. DNA from ChIP performed using immunoglobulin G antibodies was used to measure nonspecific binding (negative control [Neg CTRL]); we detected the PGC1α promoter for both ChIP-antibodies (ie, K27me2 and K36me1 H3) in H9c2 exposed to ischemia (C). The PCR product was separated by electrophoresis to further verify the presence of the product and its size. Representative agarose gel picture from triplicate independent biological replicates (D) indicating the presence of the product at the expected size of 230 bp, with the same pattern obtained from the PCR conducted on whole DNA genome (input) using the same primers for the PGC1α promoter; data are normalized to the input. The same conditions were used for the ChIP experiment conducted on post-MI murine CMs. The chromatin associated with the PGC1α promoter is characterized by K27me2-K36me1H3 in post-MI CMs as well (E). Representative picture from triplicate experiments showing the expected 230 bp product corresponding to the PGC1α promoter (F). All data (mean ± SEM) are representative of at least 3 independent experiments. RAb = rabbit antibody; other abbreviations as in Figures 1, 4, and 6.
To further verify that the histone methylation is mechanistically able to affect PGC1α expression during ischemia, we performed in vitro experiments using an established modulator of histone methyl-transferase, BIX01294 (Figure 8A), which inhibits G9a, a lysine-preferring mammalian histone methyltransferase13 with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. We observed that BIX01294 preserved PGC1α expression, blocking its down-regulation induced by ischemia (Figures 8B and 8C). Then, to compare the relative abundance of the PGC1α promoter targeted by H3_K27me2K36me1 histone modification among the different experimental groups, we performed quantitative double-ChIP experiments (for H3_K27me2 and H3_K36me1) in control, ischemic cells, and ischemic cells treated with BHB or BIX01294. The RT-qPCR was conducted on DNA collected after the decrosslinking of the immunoprecipitated H3_K27me2K36me1 chromatin. The differences in the relative abundance of PGC1α promoter among samples were determined by normalizing for the levels of the PGC1α promoter detected in the respective input samples. We observed that the targeting of PGC1α promoter by both histone modifications (H3_K27me2 and H3_K36me1) was significantly higher in ischemic conditions, and BHB exposure significantly tempered this phenomenon, reducing the levels of the PGC1α promoter under the H3_K27me2K36me1 chromatin modification induced by ischemia; BHB effects were similar to the ones induced by BIX01294, which, as expected, reduced H3_K27me2K36me1 on the PGC1α promoter as well (Supplemental Figure 6). To evaluate the role of BHB in absence of PGC1α, we knocked-down PGC1α in H9c2 cells, and we assessed the effects of BHB on ischemia-induced apoptosis. Strikingly, the down-regulation of PGC1α abrogated the protective effect of BHB on ischemia-induced caspase activation (Supplemental Figure 7), mechanistically confirming that the beneficial actions of BHB are strongly dependent on PGC1α.
Figure 8.
Inhibiting Histone HR Methylation Preserves PGC1α Transcription
Schematic representation of our mechanistic hypothesis showing how BIX01294, an inhibitor of G9a (a lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3) blocks H3 methylation preserving PGC1α transcription (A). H9c2 cells underwent ischemia and were treated with BIX01294 or vehicle. Immunoblot analysis was conducted to evaluate PGC1α levels. Ischemia caused a down-regulation of PGC1α. The administration of BIX01294 preserved PGC1α levels in response to ischemia, as shown in blots, representative of 3 assays (B) and quantified (C); mean ± SEM; ∗P < 0.05 versus vehicle; #P < 0.05 versus ischemia (Kruskal-Wallis test). Cartoon depicting the main results of our study (D). Abbreviations as in Figures 2 and 4.
Taken together, our observations unveil a pivotal pathogenic role for H3_K27me2K36me1 in HF, mediating the maladaptive transcriptional repression of key genes such as PGC1α. In this scenario, the reduction of H3_K27me2K36me1 induced by BHB in ischemic conditions, most likely mediated by S-adenosylhomocysteine hydrolase inhibition and the ensuing increased expression of S-adenosylhomocysteine, which inhibits histone methyltransferases, represents a mechanism by which BHB preserves PGC1α expression (Figure 8D). Further dedicated studies are warranted to explore in detail these pathways.
Discussion
Ketone bodies are bioactive metabolites produced in the liver from fatty acid–derived acetyl–coenzyme A, during fasting or carbohydrate deprivation; once released, they are exported to brain, heart, and muscles to be used as an alternative fuel. Recent reports have proposed a protective role for the metabolic intermediates produced via ketogenesis in several pathological conditions.14,15 Similarly, other approaches aiming at increasing circulating levels of ketone bodies have been shown to advance cognitive performance in Alzheimer’s disease.16,17
The effects of ketone bodies are not exclusively dependent on their activity as energetic metabolites; in fact, ketone bodies may orchestrate a variety of signaling functions.18,19 Specifically, a role as a signaling molecule is increasingly acknowledged for BHB. Nevertheless, therapeutic applications of BHB have been mainly considered in neurologic disorders and cancer.14,20,21 Several investigators have propounded that the shift toward ketone bodies’ metabolism could be adaptive and beneficial in cardiac ischemia/reperfusion injury.7,22,23 Yet the studies currently available have focused on BHB as a mere carrier of energy, whilst its signaling pathways have been definitely underinvestigated. With the present research we aimed to explore the signaling properties of BHB in the failing heart, examining its potential therapeutic effects on mitochondria. Understanding the mechanisms linking ketone metabolism and HF may provide new opportunities for therapeutic intervention.
Ketone bodies represent natural compounds that our body produces to cope with stress conditions. In particular, ischemic events, such as MI, enhance BHB release and use.7,24
To the best of our knowledge, our study is the first one focusing on the signaling effects of BHB in postischemic HF, providing a novel molecular mechanism of its therapeutic potential. Through in vitro and in vivo assays, we unveil that by modulating a maladaptive chromatin remodeling, BHB preserves PGC1α expression after ischemia, thus supporting mitochondrial function and preventing the progression toward HF. In this context, it should be noted that the crucial role of PGC1α in BHB-mediated effects was also confirmed by an unbiased RNA-sequencing assay. In agreement with our results, mounting evidence implicates that the metabolic milieu may affect the epigenetic landscape of the myocardium;25 additionally, if some metabolites are able to modify epigenetic marks in the nucleus, these epigenetic modifications may in turn affect metabolism by regulating the expression of mitochondrial proteins. Consistent with this view, Oka et al26 have shown that ketone bodies confer resistance against oxidative stress.
Herein, we identify BHB as a metabolic mediator of epigenetic remodeling in the ischemic heart and PGC1α as an essential target of its epigenetic action. In agreement with our data, the selective cardiac overexpression of D-β-hydroxybutyrate dehydrogenase I has been shown to improve cardiac function in HF,27 whereas an impaired ketone oxidation promotes pathologic myocardial remodeling.24 We unveil H3_K27me2K36me1 as a specific histone modification, which is sensitive to BHB levels. The abundance of this histone marker in ischemic conditions is significantly decreased in response to BHB treatment, in vitro as well as in vivo. This aspect is a central point of our study: H3_K27me2K36me1 abundance exhibited the exact same trend when comparing the in vitro and in vivo models, as well as the data from human specimens. Furthermore, our results highlight the importance of analyzing not only single histone modifications, but also their coexistence on chromatin. Our study buttresses the view that combinations of histone modifications can lead to differential DNA readouts compared to respective single modifications.
Beside the effects on H3_K27me2K36me1, our data identify this histone marker as an unprecedented mechanism of PGC1α epigenetic regulation. Indeed, our ChIP experiments demonstrate that the PGC1α promoter is targeted by H3_K27me2K36me1, which is responsible for PGC1α down-regulation during ischemia, when this histone marker significantly increases. Such a phenomenon is in line with the established contribution of H3_K36me1 to transcriptional repression12 and with the emerging role in epigenetic regulation of PGC1α, for instance in response to environmental stimuli.28
PGC1α is a master regulator of mitochondrial health that is capable of activating cellular remodeling by finely tuning global oxidative metabolism.29 This scenario denotes PGC1α as an attractive target for HF therapy. Herein, we demonstrated that BHB could be a potent tool to reactivate PGC1α during ischemia. Remarkably, BHB could be very useful as it acts upstream, reducing PGC1α epigenetic silencing during ischemia. It is not our intention to present PGC1α as the sole target of BHB epigenetic action(s), but we show that the regulation of PGC1α is among the main events induced by BHB, mediating its global beneficial effect on cardiac mitochondria. In all of our models of ischemic injury, we detected a better mitochondrial performance after BHB treatment, including in terms of OCR profile and potential; the reduction in mitochondrial ROS generation and Ca2+ overload indicates that mitochondrial workload did not occur. These phenotypes, alongside organelle renewal and repairing, have PGC1α as a common denominator. Henceforth, BHB supplementation is able to sustain mitochondrial function in ischemic conditions by reducing H3_K27me2K36me1 and preserving PGC1α expression.
Study limitations
Our study is not exempt from limitations, including the small sample size in the assays using human specimens. We also reckon that the model used in some of our in vitro experiments (H9c2 cells) does not fully recapitulate mature CMs, especially in terms of contractile apparatus; nevertheless, H9c2 cells are commonly used to study mitochondrial fitness; ideally our findings should be confirmed in human primary or induced pluripotent stem cell–derived CMs. Finally, our data refer to postischemic HF and should not be generalized to ischemia/reperfusion injury or nonischemic HF.
Conclusions
In summary, our experiments establish maladaptive chromatin remodeling as a fundamental mechanism in postischemic heart injury, functionally modulated by BHB. We believe that the novel mechanism shown here represents only 1 of the pleiotropic actions of BHB; for instance, other pathways, including lysine β-hydroxybutyrylation, could be involved in the modulation of postischemic vascularization and/or interstitial fibrosis. The increased circulating levels of ketone bodies observed in patients with HF who are treated with SGLT2 inhibitors enrolled in the DAPA-HF (Study to Evaluate the Effect of Dapagliflozin on the Incidences of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure) trial is particularly fascinating30 and lends further support to our findings while inspiring future research exploring the effects of BHB supplementation in HF and other cardiovascular disorders.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: HF remains a major cause of mortality worldwide. By improving mitochondrial function and cardiac bioenergetics, ketone bodies and BHB could represent novel tools to tackle HF in the clinical scenario.
TRANSLATIONAL OUTLOOK: The exact mechanisms underlying ischemic cardiac disease are not fully known. This study presents a comprehensive characterization of the chromatin maladaptive remodeling caused by an ischemic insult, highlighting the beneficial effects of BHB on mitochondrial dysfunction. Based on our combination of consistent results from in vitro, in vivo, and human settings, it is tempting to speculate that BHB-based therapies could have the potential to rescue HF.
Funding Support and Author Disclosures
Prof Santulli has received support in part from the National Institutes of Health (NIH): National Heart, Lung, and Blood Institute (NHLBI: R01-HL164772, R01-HL159062, R01-HL146691, T32-HL144456), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK: R01-DK123259, R01-DK033823), National Center for Advancing Translational Sciences (NCATS: UL1-TR002556-06, UM1-TR004400) to Dr Santulli, from the Diabetes Action Research and Education Foundation (to Dr Santulli), and from the Monique Weill-Caulier and Irma T. Hirschl Trusts (to Dr Santulli). Dr Gambardella has received support from a postdoctoral fellowship of the American Heart Association (AHA-20POST35211151). Dr Jankauskas has received support from a postdoctoral fellowship of the American Heart Association (AHA-21POST836407). Dr Kansakar has received support from a postdoctoral fellowship of the American Heart Association (AHA-23POST1026190). Dr Varzideh has received support from a postdoctoral fellowship of the American Heart Association (AHA-22POST915561). Dr Sidoli has received support from the Leukemia Research Foundation (Hollis Brownstein New Investigator Research Grant), Relay Therapeutics, Deerfield (Xseed award), Merck, AFAR (Sagol Network GerOmics award), and the NIH Office of the Director (1S10-OD030286-01). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank F. Macaluso, M. Morelli, J. Pessin, and E. Zaccaro for their assistance.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental methods, figures, tables, and references, please see the online version of this paper.
Appendix
References
- 1.Santulli G., Wang X., Mone P. Updated ACC/AHA/HFSA 2022 guidelines on heart failure: what is new? From epidemiology to clinical management. Eur Heart J Cardiovasc Pharmacother. 2022;8:e23–e24. doi: 10.1093/ehjcvp/pvac029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mascolo A., di Mauro G., Cappetta D., et al. Current and future therapeutic perspective in chronic heart failure. Pharmacol Res. 2022;175 doi: 10.1016/j.phrs.2021.106035. [DOI] [PubMed] [Google Scholar]
- 3.Feng Y., Cai L., Hong W., et al. Rewiring of 3D chromatin topology orchestrates transcriptional reprogramming and the development of human dilated cardiomyopathy. Circulation. 2022;145(22):1663–1683. doi: 10.1161/CIRCULATIONAHA.121.055781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu C., Zhang Z., Zhang W., Liu X. Mitochondrial dysfunction and mitochondrial therapies in heart failure. Pharmacol Res. 2022;175 doi: 10.1016/j.phrs.2021.106038. [DOI] [PubMed] [Google Scholar]
- 5.Santulli G., Xie W., Reiken S.R., Marks A.R. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci U S A. 2015;112(36):11389–11394. doi: 10.1073/pnas.1513047112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narula J., Pandey P., Arbustini E., et al. Apoptosis in heart failure: release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc Natl Acad Sci U S A. 1999;96(14):8144–8149. doi: 10.1073/pnas.96.14.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manolis A.S., Manolis T.A., Manolis A.A. Ketone bodies and cardiovascular disease: an alternate fuel source to the rescue. Int J Mol Sci. 2023;24(4):3534. doi: 10.3390/ijms24043534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shippy D.C., Wilhelm C., Viharkumar P.A., Raife T.J., Ulland T.K. β-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer's disease pathology. J Neuroinflammation. 2020;17(1):280. doi: 10.1186/s12974-020-01948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu Y., Zhang C., Xie M. Beta-hydroxybutyrate, friend or foe for stressed hearts. Front Aging. 2021;2 doi: 10.3389/fragi.2021.681513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holtwick R., van Eickels M., Skryabin B.V., et al. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest. 2003;111(9):1399–1407. doi: 10.1172/JCI17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson M.I., Cao J., Zeng H., Uthus E., Combs G.F., Jr. S-adenosylmethionine-dependent protein methylation is required for expression of selenoprotein P and gluconeogenic enzymes in HepG2 human hepatocytes. J Biol Chem. 2012;287(43):36455–36464. doi: 10.1074/jbc.M112.412932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bicocca V.T., Ormsby T., Adhvaryu K.K., Honda S., Selker E.U. ASH1-catalyzed H3K36 methylation drives gene repression and marks H3K27me2/3-competent chromatin. Elife. 2018;7 doi: 10.7554/eLife.41497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ligresti G., Caporarello N., Meridew J.A., et al. CBX5/G9a/H3K9me-mediated gene repression is essential to fibroblast activation during lung fibrosis. JCI Insight. 2019;5(12) doi: 10.1172/jci.insight.127111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dmitrieva-Posocco O., Wong A.C., Lundgren P., et al. β-Hydroxybutyrate suppresses colorectal cancer. Nature. 2022;605(7908):160–165. doi: 10.1038/s41586-022-04649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman J.C., Covarrubias A.J., Zhao M., et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 2017;26(3):547–557e8. doi: 10.1016/j.cmet.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dynka D., Kowalcze K., Paziewska A. The role of ketogenic diet in the treatment of neurological diseases. Nutrients. 2022;14(23):5003. doi: 10.3390/nu14235003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dewsbury L.S., Lim C.K., Steiner G.Z. The efficacy of ketogenic therapies in the clinical management of people with neurodegenerative disease: a systematic review. Adv Nutr. 2021;12(4):1571–1593. doi: 10.1093/advances/nmaa180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puchalska P., Crawford P.A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017;25(2):262–284. doi: 10.1016/j.cmet.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C., Wang J., Liu H., et al. Ketone body beta-hydroxybutyrate ameliorates colitis by promoting M2 macrophage polarization through the STAT6-dependent signaling pathway. BMC Med. 2022;20(1):148. doi: 10.1186/s12916-022-02352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayashankar S.S., Tajul Arifin K., Nasaruddin M.L. β-Hydroxybutyrate regulates activated microglia to alleviate neurodegenerative processes in neurological diseases: a scoping review. Nutrients. 2023;15(3):524. doi: 10.3390/nu15030524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woolf E.C., Syed N., Scheck A.C. Tumor metabolism, the ketogenic diet and β-hydroxybutyrate: novel approaches to adjuvant brain tumor therapy. Front Mol Neurosci. 2016;9:122. doi: 10.3389/fnmol.2016.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen R., Moller N., Gormsen L.C., et al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation. 2019;139(18):2129–2141. doi: 10.1161/CIRCULATIONAHA.118.036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Y., Yu Y., Zhang Y., Zhang Z., An W., Zhao X. Treatment with D-beta-hydroxybutyrate protects heart from ischemia/reperfusion injury in mice. Eur J Pharmacol. 2018;829:121–128. doi: 10.1016/j.ejphar.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Schugar R.C., Moll A.R., d'Avignon D.A., Weinheimer C.J., Kovacs A., Crawford P.A. Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Mol Metab. 2014;3(7):754–769. doi: 10.1016/j.molmet.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinsey T.A., Foo R., Anene-Nzelu C.G., et al. Emerging epigenetic therapies of cardiac fibrosis and remodelling in heart failure: from basic mechanisms to early clinical development. Cardiovasc Res. 2023;118(18):3482–3498. doi: 10.1093/cvr/cvac142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oka S.I., Tang F., Chin A., et al. β-Hydroxybutyrate, a ketone body, potentiates the antioxidant defense via thioredoxin 1 upregulation in cardiomyocytes. Antioxidants (Basel) 2021;10(7):1153. doi: 10.3390/antiox10071153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uchihashi M., Hoshino A., Okawa Y., et al. Cardiac-specific Bdh1 overexpression ameliorates oxidative stress and cardiac remodeling in pressure overload-induced heart failure. Circ Heart Fail. 2017;10(12) doi: 10.1161/CIRCHEARTFAILURE.117.004417. [DOI] [PubMed] [Google Scholar]
- 28.Gill J.A., La Merrill M.A. An emerging role for epigenetic regulation of Pgc-1alpha expression in environmentally stimulated brown adipose thermogenesis. Environ Epigenet. 2017;3(2):dvx009. doi: 10.1093/eep/dvx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oka S.I., Sabry A.D., Cawley K.M., Warren J.S. Multiple levels of PGC-1alpha dysregulation in heart failure. Front Cardiovasc Med. 2020;7:2. doi: 10.3389/fcvm.2020.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varzideh F., Kansakar U., Santulli G. SGLT2 inhibitors in cardiovascular medicine. Eur Heart J Cardiovasc Pharmacother. 2021;7(4):e67–e68. doi: 10.1093/ehjcvp/pvab039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.