Abstract
Introducing dental polymers has accelerated biotechnological research, advancing tissue engineering, biomaterials development, and drug delivery. Polymers have been utilized effectively in dentistry to build dentures and orthodontic equipment and are key components in the composition of numerous restorative materials. Furthermore, dental polymers have the potential to be employed for medication administration and tissue regeneration. To analyze the influence of polymer-based investigations on practical medical trials, it is required to evaluate the research undertaken in this sector. The present review aims to gather evidence on polymer applications in dental, oral, and maxillofacial reconstruction.
Keywords: polymers, regeneration, oral, dental, maxillofacial
Introduction
Numerous conditions, including infections, genetic disorders, malignancies, and trauma, can lead to dental, oral, and maxillofacial tissue defects. The Global Burden of Diseases, Injuries, and Risk Factors Study (GBD 2017) demonstrated that oral problems had the most significant occurrence and frequency rates on a global scale.1 In this context and thanks to recent advancements in biomaterials and production technologies, natural and synthetic polymeric biomaterials have been introduced as viable therapeutic alternatives for various defects and abnormalities in dental, oral, and maxillofacial structures.2 The therapeutic application of polymeric scaffolds has been extensively studied in regenerative therapies for several tissues. Numerous tissue engineering methods and surgical interventions have been introduced with an emphasis on comprehending the unique features of a biomaterial at cellular contact. Accordingly, this review provides an overview of the most important polymeric scaffolds utilized for therapeutic, and regenerative purposes in dental, oral and maxillofacial tissues, as well as information on their characteristics, formulations, manufacturing techniques, advantages, disadvantages, and clinical uses.
Overview of Biomaterials in Dental Applications
Polymers are macromolecules made up of repeated monomeric subunits that have outstanding characteristics. Innovation in biomaterials research encompasses applications in tissue engineering, nanotechnology, and the administration of bioactive molecules for the healing and regeneration of diverse tissues. The new generation of polymers was eminently distinguished by the development of resorbable, biodegradable polymers that displayed controlled degradation of the polymer chain3.Various biomaterials have been implemented in the dental field (Table 1).4 Naturally, materials (eg, collagen) attracted attention; synthetically derived polymeric biomaterials were later introduced. Chitosan and collagen are naturally occurring polymers with high biocompatibility, bone conductivity, and minimal immunological reactions.5 Nevertheless, disadvantages include a slow degradation rate and inferior mechanical qualities.
Table 1.
Various Biomaterials Employed in the Dental Field
| Biomaterials | Examples |
|---|---|
| Polymers | Chitosan, cellulose, hyaluronic acid etc. |
| Metals | Collagen, gelatin, albumin, etc. |
| Lipid-based | Polyethylene glycol (PEG), etc. |
| Ceramics | Chitosan/ hydroxyapatite scaffolds |
| Carbon-based biomaterials | Graphene, nanodiamonds, etc. |
Breakthroughs in the composition of synthetic biopolymers have paved the way for the development of further novel materials with excellent tissue responsiveness and integration, and capable of being degraded and removed from the system, permitting correct tissue integration as the material breaks down, without exerting any harmful influence on the biological systems.7 Further investigations are underway to develop scaffold formulations appropriate for biological systems with little or no negative impact on living systems.
Polymers are macromolecules made up of repeated monomeric subunits that are covalently bound. They can be amorphous or crystalline, having linear, branching, or cross-linked chains. Their mechanical characteristics could be extremely well regulated, allowing for the acquisition of desirable features based on application requirements. Polymers have lately been used to construct microneedles (MNs), hydrogels, microcapsules, microspheres, and fibers. These frameworks can further be designed to be biocompatible and biodegradable, eliminating possible immune reactions and allowing for simple body disposal (Figure 1 shows the advantages of polymeric scaffolds).6 Furthermore, their hydrophilicity may give them biologically advantageous properties, especially as reservoirs for biological compounds in various medical applications.6
Figure 1.
Advantages of polymeric scaffolds.
Biological Requirements
The essential component in applying biomaterial in situ tissue engineering frameworks is biocompatibility. The scaffold is biocompatible, does not elicit an immune response, generates no hazardous byproducts, and permits cells to attach, proliferate, reproduce, and survive on its surface.8,9
Structural Features
Scaffolds must have high porosity to allow cell growth and movement, essential micronutrients, angiogenesis, and spatial layout. They should be customized to fit the regenerated tissue. They should be strong enough to endure biomechanical loads until restored tissue can withstand forces.10 Another critical factor is architecture, which could be altered by adjusting artificial ECM and/or biomolecules to be administered in the microenvironment.11
Biomaterial Composition
Depending on their architecture and intended use, they can be injectable or rigid.12 Polymers may be both natural and synthetic. Chitosan and collagen are naturally occurring polymers with high biocompatibility, bone conductivity, and minimal immunological reactions. Nevertheless, disadvantages include a slow degradation rate and restricted mechanical qualities.5
Polymeric-Based Biomaterials
Nowadays, polymer-based methods can be used as a tactic to enhance tissue regeneration. Examples of polymeric materials implemented in tissue engineering applications, as represented in Table 2.10,13–15 Figure 2 represents natural and synthetic polymers in dental applications. Many polymeric formulations, including dendrimers, nano gels, microneedles, and nanocapsules, have been studied as prospective approaches for dental applications.
Table 2.
Polymeric Biomaterials, Benefits, and Limitations in Biomedical Applications
| Types | Polymers | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Natural polymer based scaffold |
|
|
|
[10,13] |
| Synthetic polymer based Scaffold |
|
|
|
[14] |
| Hybrid Scaffolds |
|
|
|
[15] |
Figure 2.
Natural and synthetic polymers in dental applications.
Natural Polymers
Natural polymers originate from natural substances and guaranteed natural restructure biomimetic character and biocompatibility. Table 3 shows the advantages, and disadvantages of some natural biopolymers.16 Polysaccharide and protein-based polymers are roughly classified based on their monomeric units and structure.17
Table 3.
Advantages and Limitations of Some Natural Polymers
| Polymer | Advantages | Limitations | References |
|---|---|---|---|
| Alginate |
|
|
[18] |
| Cellulose |
|
|
[19] |
| Chitosan |
|
|
[20] |
| Silk |
|
|
[21] |
| Protein-based polymers |
|
|
[22,23] |
| Hyaluronic acid |
|
|
[34] |
Polysaccharide-Based Polymers
Thanks to their resemblance to the extracellular matrix, polysaccharides have great degradability, bioactivity, ease of chemical modification, and low manufacturing costs.25 Even with their numerous advantages, natural polymers have some downfalls related to their branching, dispersion, pattern, and molecular mass, which results in the preparation of unreliable scaffolds, which harms biological recognition events and rheology.26
Chitosan
Chitosan is the world’s second most common natural polysaccharide, following cellulose. Several studies have investigated the biological applications of chitin and chitosan.27 Chitosan is principally a chitin metabolite that has been deacetylated.28 Because of its unique features of bioactivity, good biocompatibility, and non-immunogenicity, chitosan has become increasingly popular in the biomedical field.20 Chitosan-derived metabolites have been employed in several applications.24
Dextran
Scheible in 1874 created the term Dextran.27 It is an extracellular microbial carbohydrate derived from sucrose-derived bacterial lactic acid.29 Because of their nontoxicity, hydration, bio-compatibility, and degradability, dextran polymers are frequently employed in the food sector, personal care products, wastewater treatment, and biomedical purposes.30,31
Hyaluronic Acid
HA contributes significantly to the environment’s extracellular matrix and synovial fluids.32 The molecular weight of HA governs numerous biomedical actions. High molecular weight HA has been discovered to have anti-inflammatory and immune-modulatory properties.33 A few high molecular weight HA metabolites have also been proven to show anti-angiogenic characteristics and the capability to suppress cell growth.34 Low molecular weight HA promotes cell motility and vasculogenesis.35 HA is classified as a naturally existing negatively charged polysaccharide because of a carboxyl group in its backbone.36 This feature allows HA to maintain a substantial amount of water, leading to bulging behavior of HA up to 1000 times its solid dimensions, which several investigators have used to develop HA pharmacological carriers,37 and numerous biological implementations.38,39
Heparin
While researching cephalin in 1916, Jay McLean observed anticoagulant phosphatide fractions independent from it. Emmett Holt and William Henry Howell later called Heparin after another anti-coagulant molecule obtained from the liver.40 Heparin is a natural glycosaminoglycan produced by mast cells in the Golgi apparatus and the endoplasmic reticulum. It is derived from various origins for pharmaceutical and industrial use.41 Heparin has been employed as a transporter of growth factors.42 Heparin is also anticoagulant since its penta saccharide specifically reacts with anti-thrombin.43 These features emphasize the ubiquitous utilization of heparin-based carriers, like micelles and nano gels, as drug delivery vehicles.44
Alginate
E. C. C. Stanford revealed alginic acid in 1881, detailing the isolation of alginate with sodium carbonate and its deposition in an acidic solution.45 Species of brown seaweeds like Laminaria and nereocystis, along with bacterial strains like Azotobacter and Pseudomonas, are significant sources of alginates.46 Because of its unique characteristics, alginate-based biomaterials are utilized in many biomedical applications.18 The thickness of polymeric alginate impression materials should be reduced into minimal as it greatly influences the tear strength of the polymer after setting.47
Pullulan
Pullulan is a structurally intermediate polysaccharide containing matotriose particles between amylose and dextran.48 Pullulan is hydrophilic and moderately soluble in alkaline solutions, whereas inorganic solvents, including formamide and dimethyl sulphoxide, are completely insoluble (DMSO).49 Pullulan is an intriguing material for the nutraceutical and pharmaceutical sectors, as well as medication delivery, biomedical engineering, gene therapy, and so on, due to its biodegradability, non-toxicity, and non-mutagenicity.50
Carrageenan
Carrageenan is an anionic, sulfated polygalacton discovered in the cell walls of red algae that is comparable to ECM-derived glycosaminoglycans. Carrageenan’s structure promotes osteoblastic growth and attachment.51 It increases osteoblastic activity when combined with hydroxyapatite.52 Carrageenan was mixed with other chemicals in bone regeneration tissue engineering investigations to generate hybridized bio-scaffolds.53
Chondroitin Sulphate
Chondroitin Sulphate (CoS) is an essential cartilage component connected with compression resistance. CoS originated from chondrocytes and has a crucial influence in preserving the physiological functions of cartilages.54 CoS absorbs water and has a cushion-like effect on cartilage.55 CoS’s polyanionic nature offers a foundation for electrostatic interactions with positively charged moieties, which could be employed to deliver medications and growth nutrients. CoS is anti-inflammatory, anti-apoptotic, antioxidant, and anti-cancer.56
Cellulose
Cellulose is often recognized as the most widely recognized polysaccharide on the planet.57 Cellulose derived from bacteria, termed bacterial cellulose (BC), is highly pure, but cellulose derived from plants is documented to include trace levels of contaminants such as lignin, pectin, and hemicellulose.58 Because of its nontoxicity, degradability, biocompatibility, wide availability, and remarkable mechanical qualities, cellulose, and its variants are valuable materials for drug administration, wound healing, and other biological purposes.19 Using oral thin film made from a blend of hydroxyethyl-cellulose (HEC) and cellulose nanofibers (CNF), loaded with nepheline fluorapatite glass powder as a remineralizing agent for prevention of carious lesion by remineralization was tested. Significant quantities of calcium and fluoride ions discharged and the pH values were practically neutral. The arrangement of glass powder particles was consistent, according to SEM. In comparison to the demineralized samples, the findings of enamel micro-hardness (VHN) and ultra-morphology showed a considerable rise in mean VHN following remineralization for 15 and 30 days.59
Agar
Agar is another polysaccharide biopolymer that is commonly recognized for its strong bioactivity and biocompatibility.36 It can generate a gel that resembles the physical properties of the ECM and tissues essential for transferring growth factors to wounds.60 Previous studies proved its non-toxicity and successful cell proliferation. Consequently, it can be employed in healing and skin regeneration.61
Starch
Due to starch’s degradability, non-cytotoxicity, and processability, it is an excellent example of a storing polysaccharide.62 Polymers made from starch have shown promise in a variety of biological applications. Starch hydrogels fortified with zeolite NPs and chamomile preparations successfully treated chronic ulcers with no hypersensitivity reaction to the scaffold.61
Gum Tragacanth
This is a polysaccharide originating from the Astragalus plant.63 Gum Tragacanth is widely utilized in tissue engineering to create biodegradable frameworks and medicine administration mechanisms. This scaffold has demonstrated encouraging outcomes in terms of osseous regeneration.64
Natural Proteins
Collagen
Collagen is one of the most widely utilized protein biopolymers. It possesses a significant conjunction effectiveness with other compounds, which may be changed to achieve improved biological and mechanical qualities.22 Over 28 isomers of collagen have been extracted, each with a different helix length and the type and dimensions of the non-helical parts.65 Type I collagen is the most prevalent kind that constitutes the ECM of several oral tissues, such as periodontal ligament, enamel, etc.66–70 As an ECM structural element, it has strong biocompatibility, low immunogenicity, and significant mechanical integrity, and it assists in growth, differentiation, cell attachment, and ECM formation.71 It is an attractive natural resource due to the ease with which it may be modified.72 Tannic acid cross-linked collagen frameworks made by casting have been used to improve wound healing qualities.
Fibronectin
Fibronectin is a glycoprotein with a high molecular mass that is extracted from plasma. Its major purpose as a scaffold is to facilitate cell attachment and motility, influencing cell division and growth.73 Fibronectin nano fibers produced by rotary jet spinning demonstrated faster wound closure.74
Gelatin
Gelatin is an organic protein generated from collagen commonly employed in biomedicine thanks to its poor immunogenicity, bioactivity, and good biocompatibility.75 Because of its gelation properties and simplicity of processing, it may be processed into numerous formulations such as injectable hydrogels, sponges, and microspheres.76 Gelatin has also been combined with copper-activated faujasite to generate composites with high anti-microbial action using the freeze-drying process.77 Li et al established a straightforward strategy for creating gelatin-based scaffolds with hollow alginate fibers to create a vascular network.78
Elastin
Elastin is a hydrophobic and insoluble extracellular matrix (ECM) protein. Collagen-elastin frameworks have been used to treat full-thickness skin abnormalities such as burn wounds as dermal replacement.79
Keratin
Keratin is a protein in the skin, fingernails, and hair.80 This protein is found in the skin, nails, and hair. It is biocompatible and capable of building frameworks that coordinate cellular activities.61 By lyophilization, Keratin has also been used with chitosan to create a porous keratin/chitosan scaffold. The antimicrobial activity and cell proliferation rates of keratin/-chitosan scaffolds used for wound dressings were increased.81
Fibrin
Fibrin is a naturally degradable matrix composed of fibrinogen, created soon after trauma.82 The fibrin-based scaffolds promote growth by allowing the attachment of various biologically active biomolecules, promoting specific cell-matrix interactions and boosting tissue regeneration.83 They allow enough time to form neo matrix and progressive re-assimilation via protease activity.84 Fibrin can be employed as a biological framework with other elements to regenerate primary cells, skin, bones, and so on.85 Nevertheless, it has the disadvantage of quick degradation, which makes it unsuitable for creating particular scaffolds.86
Soy
Soy protein extract is a sustainable and readily accessible protein with more than 90% polypeptide. It has a macromolecular structure equal to the naturally generated proteins in bone.23 Wu et al employed a comparable bi-component scaffold for bone tissue creation in experiment.23
Silk Fibroin
Silk fibroin is a significant constituent with a unique amino acid composition that encapsulates sericin obtained from Bombyx mori cocoons.87 Because the protein resembles the stroma of bone, it is synthesized into frameworks for regenerating bone tissue.21 The addition of fibroin enhances the hydrophilicity and mechanical properties of the scaffolds, allowing human osteoblast-like cells to survive.88 A two-stage functionalization procedure generated silk fibroin nano-fibers containing hydroxyapatite particles.89
Poly Glutamic Acid
Polyglutamic acid is a biopolymer made up of carboxy-linked glutamate residues released by Bacillus subtilis.90 This polymer is edible and has great biocompatibility, nontoxicity, and biodegradability. These characteristics make it a desirable material for tissue engineering,91 biodegradable wrappers,92 wound bandages,92 and pharmaceutical administration.93
Polydopamine (PDA)
PDA is a darkish, opaque polymer generated by dopamine autoxidation.94 Thanks to its intriguing features and simplicity of manufacture, PDA has recently attracted significant attention in various biological applications, including medication delivery, photodynamic therapy, skeletal tissue engineering, cell attachment and imprinting, and antibacterial use.95
Lignin (LIG)
LIG is a recyclable, sustainable, safe, economical, and natural biomaterial that has grabbed the spotlight of research scientists thanks to its distinctive characteristics and environmentally friendly nature.96 LIG is antibacterial, antioxidant, and antiviral, with UV protection, biocompatibility, and minimal cytotoxicity.97 LIG has been poorly investigated in the biomedical area until now, with just a few research reporting on its use in healthcare applications.98
Synthetic Polymers
In the research laboratories, synthetic polymers are created using hydrocarbon-building components.99 They have the benefit of infinite shapes and well-established structures. They are mechanically stable and free of contaminants.100 They enable convenience and administration of manufacturing via polymerization, interlinkage, and productivity due to their molecular weight, molecular weight, and physicochemical characteristics. These polymers are classified according to their hydrophobicity and hydrophilicity101 and can also be categorized according to degradability.102 They are instrumental since their features may be easily modified for various and specialized purposes.14 However, their fundamental drawback is the lack of cell attachment sites and the need for chemical functionalization for cellular growth.103
Degradable Polymers
As the term indicates, biodegradable polymers may be rapidly decomposed or converted into simpler molecules or by-products.104
Polyethylene Glycol
Polyethylene glycol (PEG) is a commercially important polyether among degradable polymers (PEG). It is an ethylene oxide polymer called polyethylene oxide (PEO) or polyoxyethylene.105 It is non-ionic, biocompatible, and has excellent biological and physicochemical qualities. The cross-linking techniques used to create hydrophilic PEG hydrogels can impact scaffolds’ physicochemical characteristics and degradation rates.106 Architecture’s ease of manipulation and chemical makeup makes it an appealing scaffold material.107
Polyethylene Oxide (PEO)
PEO is a hydrophilic polymer with minimal pathogenicity, cell attachment, immunogenicity, and the ability for protein binding.108 PEG’s smaller molecular structure became well-known when its ability to block protein absorption was discovered.109 More research is needed to modify these gels to include places for cell attachment, which will help cells penetrate the scaffolds.110 When combined with electro-spun scaffolds made from other biodegradable polymers, PEO microparticles generated utilizing electro spraying approach enhanced the porosity and cellular penetration of scaffolds.111
Poly-β-Hydroxybutyrate (PHB)
Poly-β-hydroxybutyrate (PHB) is a linear biodegradable homopolymer of -hydroxybutyric acid that is found in many bacteria as an energy storage molecule.112
PPF (Poly (Propylene) Fumarate)
PPF (poly (propylene) fumarate) is a linear polyester with fumaric acid as the repeating unit. It is biodegradable, cross-linkable, and powerful.113 Cross-linking of fumarate double bonds results in creating a polymeric network.114 Because of its high mechanical strength, it is predominantly employed in orthopedic surgery.115 It is also employed with other polymers116,117 to improve their hydrophobicity. To study bone formation, pre-treated PPF scaffolds were placed into the craniums of rabbits with cranial abnormalities.118 The PPF scaffold covered with rh TGF- (PPF-TGF-) performed better regarding bone area%, bone surface area, and gap-filling percentage.61
Polycaprolactone (PCL)
Polycaprolactone (PCL) is a robust aliphatic polymer with good biocompatibility.119 It is a polyester with remarkable elasticity, physical qualities, minimal breakdown levels, non-toxicity, and low price.120 The capacity of PCL as a biologically friendly material has been demonstrated for several biomedical purposes.121 The fundamental drawback of PCL is its weak bioactivity and excessive hydrophobicity, which leads to poorer cell adhesion and restricted tissue regeneration.122 Polylactic acid is mixed with PCL to produce a less hydrophobic structure with increased processability and mechanical behavior.123
Polyglycolic Acid
Sekiya et al used polyglycolic acid to build a nano-fiber with collagen and test it’s in vivo potential to induce neovascularization and granulation histology. The study’s outcomes revealed strong cellular migration with excellent neovascularization.124
Polyvinyl Alcohol (PVA)
Polyvinyl alcohol (PVA) is a commonly utilized polymer employed in numerous biomedical applications thanks to its favorable features such as biodegradability, biocompatibility, and non-toxicity.125 It is frequently used with other polymers to create nano-fibers for wound healing and other tissue engineering applications.126
Poly (Lactic-Co-Glycolic Acid) (PLGA)
PLGA is an artificial, biodegradable, and FDA-approved polymer.127 Because of its degradability, simplicity of modification, superior mechanical properties, potential for continuous drug delivery, and sustained clinical applicability, PLGA has been extensively researched in the realm of nanomaterials for creating pharmaceutical delivery systems.128
Polyethylenimine (PEI)
PEI is an artificial, water-soluble, positively charged, linear, or branching polymer amino group. PEI has been employed in various biomedical applications such as medicine administration, gene transfer, antimicrobials, and multimodal imaging. Due to its amino groups, PEI has been extensively utilized to fabricate various organic/inorganic hybrid nano materials for various biological purposes.129
Poly (Vinylpyrrolidone) (PVP)
PVP is an FDA-approved, artificial, neutral, water-soluble polymer widely used in biologics, personal care products, and nutritional supplements. Because of its advantageous properties, including bioactivity, biodegradability, and environmental friendliness, PVP is frequently employed as a protective coating.130
Non-Degradable Polymers
Non-biodegradable polymers are generally utilized in wound dressings because they are neither destroyed nor dissolved into smaller molecules by biochemical processes.131
Polyurethane (PU)
Polyurethane (PU) is characterized by its high flexibility and capacity to degrade into degradable forms such as poly(ester-urethane) urea.132 It is used in various preparations, such as mixes with olive oil that contain antioxidant properties and a photoprotective mechanism.133 Dextran fibers electro-spun with PU showed intense angiogenic activity and significant anti-inflammatory activity, accelerating dermatological lesion repair.134
Poly Methyl Methacrylate (PMMA)
PMMA is a common biocompatible polymer that is used alone or in composites to produce frameworks for biomedical applications.135
Polystyrene (PS)
Polystyrene (PS) is a crystalline, colorless polymer with excellent water vapor permeability and high electrical resistance.136 In one study, combining PS with chamomile extract and poly (-caprolactone) expedited wound healing by increasing collagen fiber build-up, improving re-epithelialization, and increasing granulation tissue development.137 Porous PS scaffolds were created to allow fibroblast cells to construct a network of indigenous extracellular proteins.138 This enables the establishment of a sub-epithelial 3D microenvironment rich in chemical and mechanical signals, which promotes epithelial cell functionalization.139
Polyethylene Terephthalate (PET)
PET is a robust synthetic fiber that is extensively utilized in biomedical engineering as an implanted biomaterial.140 PET nano-fibers made with honey have shown astonishing outcomes as a functional wound dressing, with enhanced ability of the mats to absorb water, which is essential for wound healing.141
Polyether Sulfone (PES)
PES is a polymer with high porosity and a large surface area.142 The electro-spun nano-fibers generated from PES displayed enhanced adsorption efficiency, which is crucial for optimal wound healing.143
Poly (Di (Ethylene Glycol) Methyl Ether Methacrylate) (PDEGMA)
Nanofibers composed of PDEGMA and poly (l-lactic acid-co-caprolactone (PLLA-CL) have been utilized for controlled drug delivery, specifically for the antimicrobial ciprofloxacin.144 Because they are thermo-sensitive, they facilitate cell dissociation and ciprofloxacin distribution to wound-infected regions when the temperature is reduced. In vivo, testing on mice with excision wounds revealed that the composite scaffold had superior healing properties to commercial gauzes.145
Zwitterionic Polymers
Zwitterionic Polymers gained so much interest thanks to their remarkable features. Despite their excellent biocompatibility and stimulus reactivity, zwitterionic hydrogels need mechanical properties to be suitable tissue-promoting material in direct blood contact. This issue was overcome in a 2020 study by adding Electro spun fiber scaffold to zwitterionic hydrogels for biocompatibility and mechanical strength, allowing long-term blood contact devices to be developed.146 Over the past ten years, the combination of zwitterionic polymers’ increased hemocompatibility, outstanding non-fouling characteristics, charge-switching qualities, and resistance to protein adsorption has made them appealing in biomedical applications, particularly in tissue engineering.146 Other zwitterion therapeutic applications include implantable medical devices, tissue repair bandages and tissue frameworks, drug delivery vehicles, and biosensors.147
Hybrid Scaffolds
Hybrid scaffolds are one of the most exciting topics of study due to improved characteristics that are more acceptable for diverse tissue engineering applications resulting from combining numerous materials.15
Different Polymeric Formulations
Different formulations of polymeric biomaterials are currently employed in different biomedical applications, as shown in Figure 3.
Figure 3.
Different polymeric formulations.
Micro Needles
MNs are collections of tiny needles up to 1 mm in length that enable medicinal materials to pass through without triggering tissue injury or patient pain.148 Cells are among the therapeutic materials given through MN-based medication carriers,149 tiny substances,150 and macromolecules.151–153 MNs might be hard, disintegrating, porous, covered, or hydrogel-forming.154 Although no MN-based medicines are commercially available, various clinical studies using MN-based pharmaceutical delivery methods have already been accomplished.155 Moreover, MN-based drug delivery systems are feasible for meeting the demands of vulnerable groups receiving biological therapy, including older people and children.156 Due to favorable characteristics, including improved bioactivity, better drug loading potential, a divalent affinity for their specialized biochemical implementations, versatility, wettability, and low cost of production, polymer-based MN materials are the most notable topic for researchers in drug delivery systems.157
Microrobots
Micro robots have shown enormous promise for doing micro-scale activities such as medicine delivery, cell handling, micro construction, and bio detection 158–160 using manual control. Microrobots might be employed for minimally invasive procedures as well.161 A micro-robot may perform surgical procedures such as vein or channel opening, electrocautery, hyperthermia treatments, diagnostics, electrotherapy, chopping, drilling, or biomaterial elimination.162
Nano Fibers
Nanofibers have sizes ranging from 1 to 1000 nm and give advantages, including a large surface area, permeability that provides effective mechanical action, pore connectivity, and flow properties.163,164 Nanofibers are used in medical services such as inserts for longer drug release, pharmaceutical items, healing dressings, and tissue engineering scaffolding.165 Table 4 shows the characteristics of the various production processes of nanofibers.
Table 4.
Features of Nanofiber Manufacturing Strategies
| Strategy | Self-Assembly | Phase Separation | Electrospinning |
|---|---|---|---|
| Status | Semi-solid | Solid | Solid |
| Physical shape | Hydrogel | Scaffold | Scaffold |
| Size (nm) | 5–20 | 50–500 | 50–2000 |
| Benefits |
|
|
|
| Limitations |
|
|
|
Micelles
They are amphipathic particles with a hydrophobic tail that faces the core and an external hydrophilic spike that links to the fluids. An amphoteric chemical can also form an inverted micelle in a neutral medium, with the spike towards the core and the tail pointing outward. Two copolymers mix with chemicals in some solvents to form polymeric micelles. The solvent dissolves one copolymer but not the others. The inner component comprises insoluble copolymers, whereas the outside part comprises soluble copolymers, from which the micellar assembly is made. This polymeric micelle is helpful in a variety of applications.166
Polymersome
Polymersomes are synthetic vesicles with an aqueous cavity formed by amphiphilic copolymer self-assembly, as shown in Figure 4.167 Furthermore, vesicular membrane improvement can aid in the adaptation of polymersomes for several purposes, such as drug delivery systems168 or artificial organelles.169 Polymersomes are excellent antibiotic delivery vehicles for drugs that do not ordinarily enter host cells.170
Figure 4.
Polymersome.
Dendrimers
D.A.Tomalia coined the name Dendrimer from two Greek words: dendrons (trees) denotes their morphology, and meros (parts) indicate their chemical structure, which is made up of continuous monomers, as represented in Figure 5. Even though dendrimers are not polymerized, they are classified as polymers due to their repetitive pattern.171 Dendrimers can function as biomimetic materials because of their globular design with less than 10nm dimensions. This results in constant dimensions and morphologies that can be changed to develop new dendrimer populations. When employed as scaffolding, their low polydispersity ensures polymeric agent cellular absorption repeatability.172 Dendrimers can be utilized for several functions, including target-specific medication delivery, neovascularization, and gene delivery.173–175
Figure 5.
Structure of Dendrimer.
Nano Capsules
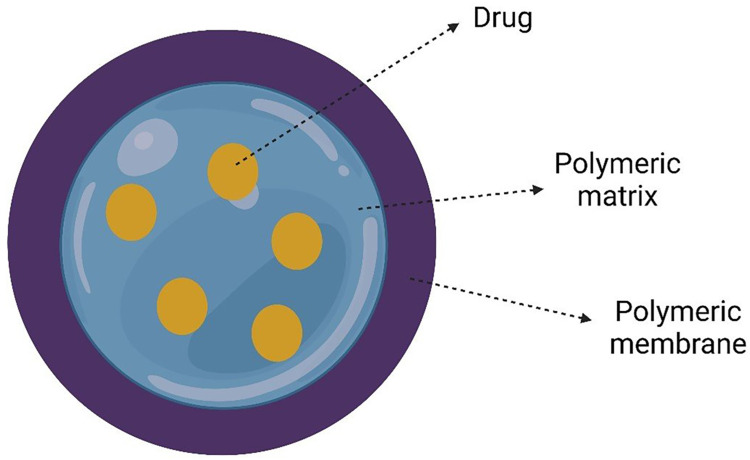
Polymeric nanocapsules have recently received much attention as a possible medication delivery mechanism. They are nanoparticles with a distinct architecture comprising a liquid/solid core surrounded by a polymeric shell, as represented in Figure 6. They can increase payload bioavailability and provide prolonged and localized distribution as medication delivery devices. Similarly, they can substantially decrease the adverse effects of payload and tissue conditions. By encapsulating the medicine in them, it is possible to protect the drug against breakdown or disintegration induced by the biological microenvironment. Furthermore, it can help lessen drugs’ adverse effects on healthy tissue.176
Figure 6.
Structure of drug loaded nano capsule.
Aerogels
Aerogels are polymeric materials with high porosity, large surface area, a large pore volume, and varied chemical structures. They are frequently used in aircraft, chemical engineering, building, electronics, and bioengineering.177 In the past few years, there has been a significant increase in the production of innovative aerogels with unique physicochemical features and functionalities. However, native aerogels with a single element typically suffer from substantial issues like poor structural rigidity and a shortage of functionalities. Building hybrid aerogels is one solution to the challenges. The initial aerogel was produced by Kistler in 1931, and he characterized aerogels as “gels where the liquid has been substituted by air, with the modest shrinking of the solid network”. There is, nevertheless, no commonly recognized description of porous materials. The most widely recognized report is that “aerogels are materials in which the usual pore patterns and connections are amazingly preserved when the liquid of a gel is displaced by air”, as stated by Hüsing and Schubert.178
Nano Gels
Nano gels are created by cross-linkers with hydrophilic or amphiphilic groups, resulting in a three-dimensional architecture with particle sizes ranging from 1–200 nm, shared by hydrogels and nanoparticles.179 Nano gels are now recognized as a new class of sophisticated delivery vehicles. This unique capacity also allows Nano gels can absorb bioactive compounds into their network, reducing bioactive component degradation due to external factors.180 Furthermore, nano gels have smaller particle sizes and a larger specific surface area, allowing for greater bio-coupling. Nano gels have the potential to significantly increase the beneficial in vivo content of bioactive compounds, making clinical or daily usage of a wide range of bioactive substances more convenient.181 Moreover, it is reported that addition of nanogel reduce the polymerization shrinkage.182
Photopolymerizable Polymers
Photopolymerizable and biodegradable polymers are increasingly used in dental applications, as shown in Figure 7. Photo-triggered polymerization technique has enabled the development of injectable photopolymerizable biomaterials for dentistry and other purposes.183 Consequently, the procedure leads to the formation of cells and a conductive microenvironment for development, which aids the manufacture of scaffold materials and, thus, the formation of complex systems. The ingredients produced by this technique vary from totally synthetic goods like polyethylene glycol to those formed entirely of natural polymers like hyaluronic acid.184 Photopolymerizable biomaterials are utilized to create injectable biomaterials that may be utilized to create multifunctional scaffolding and distribute growth factors, cells, chemicals and pharmaceuticals.
Figure 7.
Polymeric biomaterials in oral, dental, and maxillofacial reconstruction.
Hydrogels
Hydrogels are three-dimensional frameworks of different polymers, such as chitosan, alginate, casein, and others, with increased wettability and excellent permeability for several molecules. They have features that are pretty useful for a variety of purposes. Furthermore, the hydrogel architecture and characteristics may be dramatically altered by a reversible sol-gel phase transformation induced by temperature variations or the existence of certain chemicals reacting with the polymer network and causing their collapse or swelling.185,186
Membranes
Barrier membranes are utilised in dentistry to enhance the chances of successful periodontal tissue regeneration, particularly in the bifurcation area and during implant therapy.187 The treatment of periodontal and peri-implant abnormalities has been made possible by the development of a number of resorbable membranes that have proven clinically successful. The resorbable nature of these membranes eliminates the need for additional surgery. Patients favour them over non-resorbable membranes as a result. Depending on the epithelial exclusion concept, commercially accessible resorbable membranes have been employed over the past ten years to address periodontal and peri-implant abnormalities by GTR.188
Films
Thanks to their simple manufacturing process, polymer-based films were originally brought to the field of tissue engineering. In the realm of wound treatment, the polymeric films have gradually proven to be effective for application as occlusive wound dressings. Wound dressing materials made of natural and synthetic polymer-based films have been widely researched.189 Natural polymer-based films have been demonstrated for possessing lower durability and more readily degradable characteristics. Chemical cross-linkers like glutaraldehyde were first utilised to enhance their physical qualities. Researchers have begun to look towards non-cytotoxic cross-linkers since chemical cross-linkers have shown to be toxic to growing cells in recent years.190 A polymeric film was prepared for biological purposes using several cross-linking techniques, including physical cross-linking and ionic cross-linking.191 There have been reports of increased antibacterial and healing characteristics in polymeric films loaded with bioactive molecules.192–194
Micro-Particles
Microspheres and are other names for microparticles. They are tiny, irregularly shaped polymeric particles having a range of sizes. Micrometres, generally between 1 and 1000, are the range in which microparticle sizes vary. Typically, a polymer matrix where a lower quantity of an active ingredient may be immobilised forms polymeric microparticles.195 Natural or synthetic polymers, that might be biodegradable or nondegradable, can be used to create micro-spheres. Emulsification-solvent evaporation, coacervation, spray drying, electrospraying, and phase separation are a few techniques for producing the micro-particles.196 Interleukin-1 receptor antagonist (IL-1ra) was added to dextran/poly (d,l-lactide-co-glycolide) microspheres in an experiment conducted by Lu et al, and the system was then tested for its physicochemical and anti-inflammatory capabilities.197
Methods of Scaffold Fabrication
Common approaches for creating polymeric scaffolds involve solvent casting/salt leaching, phase separation, gel casting, precipitation, and emulsion freeze-drying.198,199 Despite the idea that traditional fabrication methods can integrate pores of the necessitated surface topography by influencing numerous variables, the frameworks generated from these strategies can be made from a single polymer. They could result in inaccurate and unexpected amorphous morphological features.200 Furthermore, these procedures need an organic solvent purification phase, which is time-consuming and difficult for quick adoption.
Electro Spinning
The electrospinning technology was utilized to develop 3D nanofibrous scaffolds. This technology is essential to build a supporting scaffold for cell proliferation by mimicking the precise nanoarchitecture of an extracellular matrix. Electrospun scaffolds have a controlled fiber diameter and are ideal for constructions constructed in sheets and layers.201 Despite the small number of in vivo uses, multiple research has demonstrated the bioactivity of electro-spun scaffolds in regenerative endodontics, demonstrating the diversity of scaffold synthesis for pulpal regeneration.202,203
Supercritical Fluid-Gassing
Maspero et al proposed a unique approach for rapidly fabricating a net-shaped porous scaffold.200 This method, which comprised the rapid centralization of PLGA particles in a mould using sub-critical CO2, enabled the rapid manufacture of a precise porous copy of a tooth root without chemicals. In this method, a mould was created using sterile polyvinylsiloxane, recreating the meticulous architecture of the tooth by inserting the dental root into the polyvinylsiloxane polymer. After the imprint had been set, the root was drained, and the mould was filled with sterile PLGA particles ranging in size from 700 to 1400 m, resulting in an indiscernible structure. Open porous scaffolds with the required form were made using the demonstrated moulding procedure.200 Tai et al found limitations and improvement information.204 They revealed that PLGA structures are brittle and that their pore size decreases as glycolic acid concentration increases. A nonporous layer may emerge when the depressurization phase is finished too quickly. They pushed for a lesser speed of pressure loss to develop more uniform and broader pores. This technique is slower; it may take an hour or more instead of a few minutes, but it may provide better outcomes.
Three-Dimensional Bio Printing
The most recent advancement in tissue engineering methodologies is the incremental pouring of a cell-containing hydrogel using inkjet equipment and CAD/CAM technology.205 The exact placement of various cells in 3D printing is useful, like the implantation of odontoblasts in the scaffold’s perimeter and angiogenic fibroblasts in the core, which will preserve a fundamental matrix of blood vessels and nerves in the substituted pulp tissue.206 Bioprinting techniques can result in readily customizable cell orientation inside a scaffold, improving cell adhesion and regeneration. It can also generate functioning small-diameter blood arteries local to the pulp chamber.207 Nevertheless, in vivo, models continue to have constraints.
Self-Assembling
Moreover, innovative regenerative approaches were developed using a tissue regeneration technique that utilized a hydrogel scaffold implanted with SHED and DPSCs in addition to peptide-amphiphile (PA). Cell-matrix relationships can then be carried out by including the cell adhesion sequence, RGD, and an enzyme-cleavable region. SHED and DPSCs implanted in PA hydrogels were grown for four weeks under varied osteogenic induction settings. With the hydrogel scaffolds, these cells differentiated and proliferated typically. This technology might also be used to create 3D PAs self-assembly structures of Nanofibers and tissues. Moreover, due to the hydrogels’ physicochemical qualities, they may be inserted into microscopic and randomized faults. The suggested approach is ideal for creating soft and hard mineralized dental/pulp tissue regeneration frameworks.208
Clinical Applications
Polymeric biomaterials are extensively utilized in oral, dental, and maxillofacial reconstruction,209 as depicted in Figure 7.
Alveolar Ridge Preservation
The goal of alveolar ridge maintenance following tooth extraction is to prevent or eliminate resorptive bone remodeling and to optimize tissue distribution before fitting the final prosthetic replacement; Table 5 represents the application of biopolymers in alveolar ridge preservation.210–213 Socket preservation treatments employ biomaterials and barrier materials to refill the socket. Salamanca et al found that hydroxyapatite/b-TCP ceramic combined with homogeneous collagen solution had slightly greater effectiveness in osteogenesis and similar osteoconductivity to bovine-derived bone (Bio-Oss®) with collagen membrane in a canine investigation.214
Table 5.
Implementation of Polymeric Materials in Ridge Preservation
| Scaffold | Outcome | Reference |
|---|---|---|
| N-carboxyethyl chitosan (CEC) hydrogel loaded with nano-hydroxyapatite |
|
[210] |
| BMP-2 loaded PLGA microspheres with gelatin/ hydroxyapatite/b-tricalcium phosphate cryogel |
|
[211] |
| Chitosan (CS) gel loaded with berberine (BBR) |
|
[212] |
| Polyphosphate-cross-linked collagen scaffolds |
|
[213] |
Vertical and Horizontal Ridge Augmentation
Guided bone regeneration (GBR) utilizing a membrane is the most often used surgical approach to regenerate atrophic residual ridges. GBR depends on three primary criteria: (1) cell occlusivity, (2) wound integrity, and (3) space formation and preservation. Resorbable polymeric membranes are preferred because they can regenerate noncritical-size defects continuously, promote the healing process and economical price, and reduce surgical discomfort and consequences.
Membrane permeability impacts vasculature and bone progenitor cell growth over competitive soft tissue cells.215 Composite grafts of bone allografts or xenografts with a resorbable membrane provided clinical outcomes comparable to autologous bone transplants.216 These findings support that composite grafts might be a viable alternative to autogenous bone harvesting, overcoming key obstacles such as donor site complications. Differences in surgical procedures and operator skills should be addressed when estimating clinical outcomes of various combinations.
Maxillary Sinus Augmentation
Maxillary sinus augmentation can be accomplished using the lateral window method or the transrectal approach to create a gap between the sinus floor and the Schneiderian membrane to fill with biomaterials that encourage the creation of new osseous tissue.217 Several bone fillers have demonstrated effectiveness in increasing vertical bone height for future implant implantation. Table 6 depicts the implementation of polymers in sinus lifting.218–220 A comprehensive review discovered no statistically significant variations in implant longevity between bone auto grafts and bone replacement materials.221
Table 6.
Various Polymeric Scaffolds in Sinus Lifting
| Scaffolds | Outcome | Reference |
|---|---|---|
| Vancomycin/deferoxamine/dexamethasone (Van/DFO/Dex) liposome–alginate hydrogel composite |
|
[218] |
| Thermo-reversible poloxamers gel |
|
[219] |
| Injectable thermo-sensitive (CS/GP/GA) hydrogel loaded with erythropoietin (EPO) |
|
[220] |
Temporomandibular Joint Reconstruction
The TMJ’s two interacting structural constituents are the temporal bone and the mandibular condyle. TMJ deterioration can damage the disc, the surrounding fibrous tissue, the proliferative and hypertrophic fibrocartilage layers, or the condylar bone. Regenerative TMJ therapies have been offered to enhance clinical outcomes, long-term ossification, and adherence prevention. To reconstruct the TMJ, biphasic cartilage and bone engineering employing ex-vivo cell seeding and bioactive chemicals on an acellular scaffold is being employed. Among the several multipotent cell types studied for condylar cartilage regeneration, DPSCs have been extensively researched for their high reliability and multi-lineage development into chondrogenic cells, osteoblasts, and other important cell forms.222,223
Natural materials have been widely employed for TMJ disc regeneration. They have mechanical characteristics comparable to those of the natural disc, like cell adherence and penetration, cell growth, and proteoglycan accumulation.224
Synthetic polymers have been presented to produce synthetic ECM for TMJ fibrocartilage regeneration that outperforms natural materials in terms of mechanical strength and biodegradability.225 Poly-L-lactic-glycolic acid is one example the FDA has authorized for clinical use (PLGA). PLGA promotes mesenchymal stem cell colonization and proliferation while interacting with chondrocytes and other TMJ discal cells.226 PCL fibers are a form of pro-regenerative biomimetic nanofiber that the FDA has cleared for clinical use.227
Periodontal Tissue Regeneration, and Engineering
Periodontal regeneration attempts to heal the alveolar bone, periodontal ligament, and cementum that have been damaged by periodontitis. Scaling and root planning, curettage, and open flap cleaning are current therapy techniques with minimal success. Periodontal regeneration using GTR permits bone cells, fibroblasts, and PDL cells to be chosen to fill the periodontal defect. Melcher was the first to utilize a barrier membrane to control the natural wound-healing process in 1976. Bioactive substances and nanoparticles recently incorporated into polymeric scaffolds in order to create a smart scaffold that respond to internal signals.228 The use of regenerating biomaterials in GTR is widely established.229 A systematic review of GTR results with a collagen membrane revealed that pocket depth reduction is predictable with or without a bone replacement.230 Scaffolding materials, in addition to membranes, are commonly used in fault areas. On the other hand, polymeric scaffold materials are often osteoconductive, maintaining room for cells to migrate into the defect location; Figure 8 represents optimal characteristics for periodontal engineering scaffolds.
Figure 8.
Optimal characteristics for periodontal engineering scaffolds.
Different polymer matrices and scaffolds have been employed for periodontal regeneration of intraosseous abnormalities since the 1980s, as shown in Table 7.231–246 Likewise, 3D-printed scaffolds have recently been designed to improve on current supporting matrices. In contrast to traditional grafts’ brittleness, poorly processed porosities, and generic shapes, 3D scaffolds may be adapted to the unique demands of patients. To regenerate each periodontium tissue type ideally, compartmentalization, internal topographies, and pore diameters and angulations can be precisely engineered.13
Table 7.
Different Polymeric Biomaterials in Periodontal Tissue Regeneration and Engineering
| Scaffold | Target Tissue | Outcome | Reference |
|---|---|---|---|
| Guanidine appended polydiacetylene (G-PDA) | Cultivating human primary periodontal ligament (hPDL) cells was shown to be non-cytotoxic and cytocompatible. In vitro osteogenic differentiation |
[231] | |
| PCL fibers | Over 21 days, increased transcription of osteogenic marker (Runx2) overexpression of M1 signals | [232] | |
| Cellulose acetate (CA) and hydroxyapatite (HA) films | Improved cell attachment Increased cell survival |
[233] | |
| Dimethyloxalylglycine (DMOG) and nano silicate (nSi)/poly (lactic-co-glycolic acid) (PLGA) fibers | Increased osteogenesis and angiogenesis | [234] | |
| Bilayered (polycaprolactone (PCL)/ gelatin membrane | Improved osteogenesis and the regeneration of bone defects. | [235] | |
| Collagen | PDL | The most prevalent protein in the alveolar bone, PDL, and cementum ECMs. Biocompatible Mechanical characteristics are low. Safety issues include pathogen spread and immunological response. |
[236] |
| Gelatin | PDL; alveolar bone; cementum | Collagen hydrolysis byproduct There is no pathogen spread and no immunological response. Simple to modify for chemical and light crosslinking |
[237] |
| Chitosan | Alveolar bone; PDL; cementum | Biocompatible and antimicrobial characteristics resulting from chitin | [238] |
| Poly (lactic-co-glycolic acid) (PLGA | Alveolar bone; PDL | Biocompatible Controllable rate of deterioration There is no cell recognition pattern. |
[239] |
| Polycaprolactone (PCL) | Alveolar bone; PDL | Non - immunogenic The slow pace of deterioration |
[240] |
| PLGA + CaP | Alveolar bone | Made up of two layers (smooth outer layer and rough microporous inner layer) | [241] |
| Collagen + HA | Alveolar bone | Produced by freezing both collagen and HA or converting HA to collagen | [242] |
| Chitosan+β-TCP | Alveolar bone | Made by freezing and frying. HPDLC was sown into the scaffold to attract host cells and stimulate osteoblast development. |
[243] |
| PCL+ β-TCP + CaP coating | PDL; Alveolar bone | As the PDL layer, a PCL electro-spun scaffold was created. To increase the osteogenic potential of the PCL—TCP scaffold, a thin coating of CaP was placed on the surface. CaP coating increased bone development. |
[244] |
| Chitin + PLGA + BCG | Alveolar bone; cementum; PDL | PLGA was included to enhance structural performance and extend degradation time. BCG increased bone and cementum layer osteogenic ability. |
[245] |
| PCL + HA | Alveolar bone; PDL; cementum | Three layers of scaffolding were employed to simulate the architecture of the periodontium. There was no organized fiber insertion in the PDL or bone interface. | [246] |
A case study proved the effectiveness of using a 3D-printed PCL scaffold for periodontal regeneration. The graft failed because of the slow breakdown rate of PCL in comparison to surrounding tissue, resulting in graft exposure.34 Recent advancements in additive manufacturing technology enable the creation of nanoscale scaffolds with adjustable parameters such as fiber diameter, porosity, shape, and surface properties.
Growth factors, proteins, and medications can also be added to these polymeric matrices to regulate cellular responses and adjust the local inflammatory milieu to enhance periodontal tissue engineering, as seen in Figure 9.
Figure 9.
Requirements of tissue engineering.
Implant Coating
Dental implants are often used to replace missing teeth, restore function, and enhance patients’ life satisfaction.247,248 The long-term durability of dental implants may malfunction because of biological peri-implantitis (20%) caused by microbial infections and mechanical issues, including stress shielding, which may cause osteopenia and clenching-bruxism.249 Several investigators156,234,250–253 have assessed the effectiveness of these biopolymer-based hydrogels as coating materials for enhancing the characteristics of dental implants. In addition to surface changes, the coating of dental implants with various materials and biomolecules to accomplish particular advantages has been studied in the past few years.254,255
Ansari et al used the in-situ sol-gel approach to create polycaprolactone (PCL) and PCL/fluoride substituted HA (PCL-FHA) composites as a coating material for alkali-treated Ti6Al4V platform to increase protection against corrosion and in vitro biocompatibility.250 Joo et al251 synthesized and employed a hydrogel generated from hyaluronic acid and gelatin for the surface modification of polydimethylsiloxane (PDMS), frequently employed as an implant-supported covering material. In another study, Yang et al used carboxymethyl cellulose-dopamine (CMC-DA) hydrogel as a stimulator to cover the surface of magnesium alloys with HA.
The surface-modified alloys’ corrosion resistance and biocompatibility were then assessed. The outcomes showed that corrosion resistance of CMC-DA/HA coated AZ31 alloy (AZ31/CMC-DA/HA) enhanced 18.9-fold compared to naked AZ31 alloy. Furthermore, biocompatibility experiments revealed that AZ31/CMC-DA/HA induced a 21% boost in the multiplication of MC3T3-E1 cells following 5-day incubation compared to the raw AZ31 alloy, showing the coating’s superior cytocompatibility.153 Table 8 shows polymers as coating materials around implants210,250,251,256–260.
Table 8.
Polymeric Coating Materials Around Implants
| Coating | Type of Implant | Outcome | Reference |
|---|---|---|---|
| PCL/fluoride substituted HA (PCL-FHA) nanocomposite | Alkali-treated Ti6Al4V | Reduce the corrosion of the Ti6Al4V substrate. More viable human osteoblast-like MG63 cells Increased the proliferation and dissemination of these cells. |
[250] |
| Hyaluronic acid /gelatin/polydimethylsiloxane (PDMS) | Ti6Al4V | Bacterial adhesion inhibitor. Bacterial adhesion inhibitor (Pseudomonas aeruginosa; P. aeruginosa) Biocompatible hydrogel Improvement in coated implant biomedical applications |
[251] |
| Dopamine (DA)-loaded alginate-arginine-glycine-aspartic acid (RGD) (AlgR) hydrogel | Vaterite (CaCO3)-modified Ti implant (SLA/CaCO3 Ti) | Enhance bone regeneration and raise the Ti implants’ protection against bone destruction Following 14 days, there was a 2.3-fold elevation in ALP activity in human bone marrow stem cells (hBMSCs). Enhanced bioactivity and Osseo integration. |
[256] |
| Multifunctional hydrogel using sodium tetraborate (Na2B4O7), polyvinyl alcohol (PVA), silver nanoparticles (AgNPs), and tetraethyl orthosilicate (TEOS as a bone induction factor | Porous Ti (pTi) | Biocompatible three-dimensional (3D) composite devices boosted bone marrow mesenchymal stem cell proliferation and differentiation potential while inhibiting bacterial growth. | [210,257] |
| BMP2/PLGA/vancomycin/CS hydrogel | Rapid release of vancomycin for the first two days, followed by continuous release of rhBMP-2 for roughly 12 days Promotes Osseo integration as a coating around dental implants. |
[258] | |
| Keratin hydrogel | Dental Ti implants | Enhanced Osseo integration. | [259] |
| Photo-cross linkable gelatin methacryloyl (GelMA)/dopamine hydrochloride/antimicrobial peptide (AMP) / silicate nanoparticles (SNs) | Ti implants. | Significant decrease in bacterial CFU levels Increased expression of pathogenic markers. Enhanced Osseo integration capability |
[260] |
Oral Mucositis
Oral mucositis (OM) is a classic side event in oncological patients. It results from pathogenesis is a direct and indirect effect of tumor therapeutics that suppress physiologic cellular pathways and results in epithelial apoptosis.261 In vivo testing revealed that benzydamine hydrochloride-loaded lyophilized mucoadhesive displayed consistent structural, mechanical, and biological properties. It might be effective carrier for drug distribution for OM262 —carboxymethyl cellulose is functionalized with N-(2-aminoethyl) maleimide.
Through the interaction of the maleimide moiety and mucin, the polymer demonstrated outstanding mucoadhesive potential compared to carboxymethyl cellulose. The functionalized polymer may also regulate the release of benzydamine using Higuchi’s release model, proving it to be a reliable solution in bio-adhesive drug delivery.263
Liquid oral gel based on polyacrylate silver salt/polyvinylpyrrolidone Clinical OM and oral function were examined weekly after four weeks, until 5e10 days after radiation was completed. Standard Terminology Criteria for Adverse Events (CTCAE) v3.0 was used to evaluate the data of OM grade.264
Regenerative Endodontics
In regenerative endodontics, polymeric scaffolds have been utilized to produce an appropriate microenvironment for dentin-pulp system regeneration. Various scaffolds have shown clinical effectiveness in multiple applications, as shown in Table 9.265–269
Table 9.
Different Polymeric Scaffolds Utilized in Regenerative Endodontics
| Scaffold | Outcome | Reference |
|---|---|---|
| Granular 3-D chitosan scaffolds | It improved cell viability. Enhanced cellular differentiation. Hydrogel is highly conductive. Neural differentiation of dental pulp stem cells (DPSCs) |
[271] |
| Collagen/calcium phosphate bioceramic | Pulpal regeneration | [272] |
| Gelatin hydrogel loaded with fibroblast GF-2 | Regeneration of dentin-pulp system | [273] |
| PRF | Successful revitalization | [274] |
| PRF | Improved multiplication of pulpal cells. Increased levels of osteoprotegerin (OPG) expression. Enhanced alkaline phosphate (ALP) activity. |
[276] |
PRF scaffolds revealed apical closure, elimination of apical radiolucency, continued root extension, and dentinal wall thickening in permanent adolescent teeth with necrotic pulps.268
Bio-Gide collagen membranes (Geistlich, Wolhusen, Switzerland) have been found to enhance dentinal wall formation in the middle third of the root, strengthening the root and preventing cervical root fractures.270
Whole Tooth Regeneration
Whether treated or not, dental disorders such as tooth decay or periodontitis ultimately lead to tooth loss. Bioengineered edentulism management is a viable alternative to the advancement of tissue engineering.
Yang et al271 showed the reliability of incorporating fibrin glue with PRF as a matrix cultured with dental bud cells for dental regeneration purposes. PRF contains high amounts of tissue growth factor-β (TGF-β) and platelets-derived growth factor (PDGF), significantly affecting angiogenesis and complex tissue regeneration. Additionally, dental bud cells seeded on PRF/PRP frameworks were observed to regenerate an entire tooth structure.
Oral Drug Delivery Systems
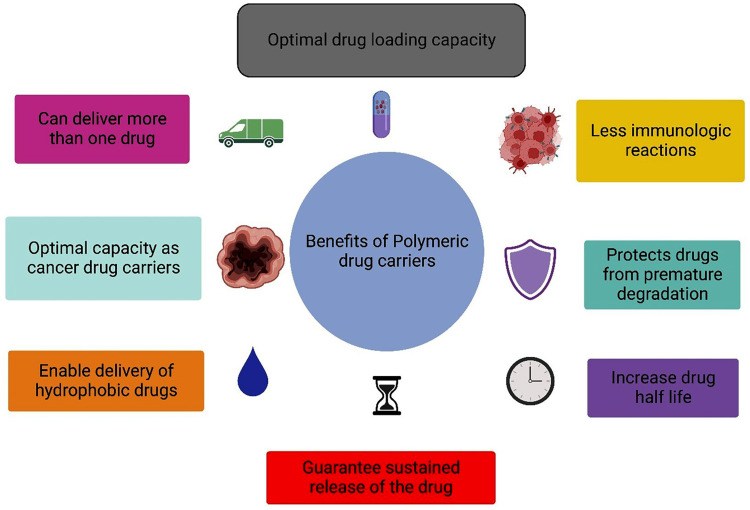
Administering medicinal substances into the oral cavity is an established way of treating local inflammation, discomfort, and illnesses of the mucosa and dentition. Polymeric drug carriers have astonishing characteristics that make them widely utilized as drug delivery carriers, as shown in Figure 10.
Figure 10.
Benefits of polymeric biomaterials as drug delivery systems.
An injectable chitosan sponge could be utilized for this objective because it promotes biomineralization in major-sized osseous deformities and is less expensive than growth factors such as BMPs.272,273 Bacterial cellulose is another substance that is very good for antibiotic dissemination.274 Nanoparticles or comparable technical solutions should be noted among unique formulations of different films, fibers, biodegradable matrices, local gels, or microspheres.276 Semi-solid gel preparations with antimicrobial, anti-inflammatory, and aseptic qualities commonly apply active compounds.
For the management of oral illnesses, several formulations have been developed, as represented in Table 10.275,277–281 In therapeutic strategies for oral cancer, they are frequently distinguished by bio-adhesive characteristics or response to stimuli.
Table 10.
Advantages of Polymeric Drug Carriers
| Bioactive | Characteristics | Function | Applications | References |
|---|---|---|---|---|
| Statins: Simvastatin (SMV): Atorvastatin (ATV) | Suppressors of 3-hydroxy-2-methyl-glutaryl coenzyme A (HMG-CoA) reductase, frequently utilized for arteriosclerosis and hyperlipidemia | Inhibition of osteoclastic actions. Up-regulation of BMP-2 levels |
Improved CAL levels Increased osteogenesis |
[275] |
| Metformin | Biguanide, an anti-hyperglycemic agent, is used to treat type II diabetes. | Enhances osteogenesis through LKB1/AMPK pathway | More Greater PD decline and CAL increase | [278] |
| Platelet-derived growth factor | Four isomers | Chemotaxis. Improved cellular multiplication. And development. Enhanced vascularity. |
Increased bone regeneration, the concentration of 0.3 mg/mL | [279] |
| Fibroblast growth factor | 22 subfamily proteins activate tyrosine kinase action. | Promotion of the healing process. Improved angiogenesis. |
Promotion of bone regeneration in periodontal defects. | [280] |
| Bone morphogenic protein | Belongs to the TGF-β superfamily | Stimulation of osteogenesis and chondrogenesis. Improved angiogenesis. |
BMP-2: Confirmation of bone regeneration capacity, but could result in root resorption and ankylosis BMP-6: Induction of bone, PDL, and cementum regeneration in periodontitis models in rats and dogs BMP-7: Enhances osteogenesis, and cementogenesis in Class III furcation defects in dogs | [281] |
| Amoxicillin | Broad spectrum antibiotic | Amoxicillin causes microorganism disintegration, while HAP NPs cause periodontal healing. | [282] |
They could also be employed with photodynamic treatment to administer photosensitizers.276 The ability to selectively destroy neoplastic cells while reducing cytotoxicity towards non-cancerous tissues and bactericidal action against bacteria building oral biofilm are further advantages of nanocarriers in oral cancer therapy.282
Bone Tissue Engineering
Despite the endogenous reparative capacity of bone, polymeric scaffolds have a critical role in enhancing the recovery process, shortening the healing period, and thus limiting postoperative complications and maximizing treatment success, as shown in Table 11.283–291 It is critical to achieving the goals of regenerative medicine by generating specific releases of biomolecules at the specific receptors. This was associated with the balance of biological signaling pathways in tissues, particularly bone.292
Table 11.
Numerous Polymeric Materials in Bone Tissue Engineering
| Framework | Strategy | Results | Purpose | Reference |
|---|---|---|---|---|
| Oxidized (Alg)/Gel including NCDs | Chemical cross-linked (NHS and EDC) and freeze-dried | Improved mechanical characteristics and proper pore size. | Osseous tissue engineering | [284] |
| CS hydrogel/3D-printed PCL/ encapsulated BMMSCs and BMP-2 | Injection and lyophilization, 3D printing | Greater osteogenesis, mineralization, and improved cellular maturation. | Bone tissue engineering | [283] |
| κ-Carrageenan/ PDGF-BB | Hydrogel beads | Improved loading and sustained release of the pharmaceutical agent. | Angiogenesis property for bone tissue engineering | [285] |
| Ch-LA /BMP-2 | Photocrosslinkable hydrogel | Greater compressive strength, elongation of crosslinking, better ALP function. | Bone tissue engineering | [286] |
| Polyacrylamide/ PVA/BG/HNT | Nanocomposite and freeze-thawing technique | Increased mineralization and better cellular attachment. | Bone regeneration | [287] |
| Desferrioxamine (DFO) –loaded SF-HAP | Nanocomposite and freeze-dried | Improved angiogenesis and osteogenesis. | Bone development | [288] |
| γ-Fe2O3/nano HAP/PVA | Nanocomposite and cyclic freeze-thawing technique | Better mechanical features and decline in porosity. | Bone tissue engineering | [289] |
| Type I Col- and CS–agarose hydrogel | 3D printing | Higher replicability, osteogenic activity, and angiogenesis. | Osteogenesis and adipogenesis | [290] |
| CMC/Gel/ nHAP | Cross-linked with tyrosinase | Greater mechanical strength enhancement of osteoblastic activity, differentiation, and proliferation | Osteoinduction | [291] |
Polyurethane foam/nano-hydroxyapatite composite was fabricated and potentially functioned as an extracellular environment in creating osteoblasts tissues.293 PLA membrane was adjusted by incorporating HAp NWs to exert barrier/-osteo induction dual roles in a rat mandibular deformity.294
Conclusions
It is necessary to acknowledge that this review can hardly avoid bias and errors to some extent, as there is no uniform evaluation of the scientific validity of the design, methodology, and results of the original literature such as a systematic review. However, it is still informative to learn about the application and research progress of hydrogels in maxillofacial and oral drug delivery. Compared with other materials such as nanoparticles, nanofibers, and thin films, many hydrogels have biocompatibility and unique stimulusresponsive properties that make them suitable as carriers or platforms for transporting drugs, cells, and others to target locations, which have unique advantages in local therapies and are therefore well suited for topical applications in the oral environment. This paper describes the application and research progress of hydrogels in maxillofacial and oral drug delivery. Different types of hydrogels have a wide range of applications in oral soft and hard tissue regeneration, antibacterial, and local drug targeting delivery due to their injectability, temperature sensitivity, pH sensitivity, biodegradability, and other properties. Many hydrogels do not have the function of repairing tissue defects themselves, but can be used as carriers for different drugs, growth factors, and even stem cells to achieve higher concentrations maintained over a long period of time. Some hydrogel systems are able to respond to stimuli such as temperature, pH, and light irradiation, which can modulate the drug release from the hydrogel on demand. In the future, how to obtain hydrogel systems with greater biocompatibility through smaller cost and simpler synthesis methods to achieve superior sustained local drug delivery efficacy are still important issues for researchers to focus on. There are many hydrogel drug delivery systems made with different materials and synthesis methods for complex oral and maxillofacial environments, but the high costs, complex synthesis steps, and toxic biodegradation by-products may be the key challenges affecting their further clinical applications.295,296 Meanwhile, in vivo studies and animal studies are relatively scarce, and the exploration of relevant hydrogels for clinical applications is even rarer. The stability of topical drug delivery and the biocompatibility of hydrogels need to be further investigated, and their use in the clinic requires more rigorous testing. It is expected that more clinical studies will be conducted in the future to verify the therapeutic effects of hydrogels as drug release platforms in different oral diseases, especially in the treatment of periodontal diseases, which currently have a relatively large number of clinical studies.
Acknowledgment
The authors thank the resources provided by the project 6PFE of the University of Life Sciences “King Mihai I” from Timisoara and the Research Institute for Biosecurity and Bioengineering from Timisoara.
Funding Statement
This paper is published from the project 6PFE of the University of Life Sciences “King Mihai I” from Timisoara and Research Institute for Biosecurity and Bioengineering from Timisoara.
Abbreviations
AgNPs, Silver nanoparticles; AMP, Antimicrobial peptide; ATV, Atorvastatin; BBR, Berberine; BC, Bacterial cellulose; BMP, Bone morphogenic protein; CA, Cellulose acetate; CAD/CAM, Computer-Aided Design And Manufacturing; CEC, N-carboxyethyl chitosan; CMC-DA, Carboxymethyl cellulose-dopamine; COAs, Clear overlay appliances; CoS, Chondroitin sulphate; CS, Chitosan; CTCAE, Common Terminology Criteria for Adverse Events; DFO, deferoxamine; DMOG, Dimethyloxalylglycine; DMSO, Dimethyl sulfoxide; DPSCs, Dental pulp stem cells; ECM, Extracellular matrix; EPO, Erythropoietin; FDA, Food and Drug Administration; GA, Gelatin; GBD, Global Burden of Diseases; GBR, Guided bone regeneration; GP, Glycerophosphate; G-PDA, Guanidine appended polydiacetylene; GTR, Guided tissue regeneration; HA, Hyaluronic acid; HA, hydroxyapatite; HBMSCs, human bone marrow stem cells; LIG, Lignin; MC3T3-E1 cells, Osteoblast precursor cell line derived from Mus musculus (mouse) calvaria; MNs, micro needles; nSi, nano silicate; OM, Oral mucositis; PA, peptide-amphiphile; PAA, Polyacrylic acid; PCL, Polycaprolactone; PCL-FHA, fluoride substituted HA; PDA, Polydopamine; PDEGMA, Poly di ethylene glycol methyl ether methacrylate; PDGF, Platelets derived growth factor; PDL, Periodontal ligament; PEEK, Polyether ether ketone; PEG, Poly ethylene glycol; PEI, Polyethylenimine; PEO, Polyethylene oxide; PES, Polyether sulfone; PET, Polyethylene terephthalate; PETG, Poly (ethylene terephthalate)-glycol; PHB, Poly-β-hydroxybutyrate; PLGA, Poly (lactic-co-glycolic acid); PLLA-CL, Poly l-lactic acid-co-caprolactone; PMMA, Poly methyl methacrylate; PPF, Poly propylene fumarate; PRF, Platelet rich fibrin; PRP, Platelet rich plasma; PS, Polystyrene; PU, Polyurethane; PVA, Poly vinyl alcohol; PVP, Poly vinyl pyrrolidone; RGD, Arginyl-glycyl-aspartic acid; rh TGF, Recombinant Human Transforming Growth Factor; SHED, Stem cells from human exfoliated deciduous teeth; SMV, Simvastatin; SNs, Silicate nanoparticles; TGF-β, tissue growth factor-β; TMJ, Temporomandibular Joint Reconstruction; Van, Vancomycin; β -TCP, β-tricalcium phosphate.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest.
References
- 1.James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latimer JM, Maekawa S, Yao Y, Wu DT, Chen M, Giannobile WV. Regenerative medicine technologies to treat dental, oral, and craniofacial defects. Front Bioeng Biotechnol. 2021;2021:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamdy TM. Polymers and ceramics biomaterials in orthopedics and dentistry: a review article. Egypt J Chem. 2018;61(4):723–730. [Google Scholar]
- 4.Hamouda IM. Current perspectives of nanoparticles in medical and dental biomaterials. J Biomed Res. 2012;26(3):143–151. doi: 10.7555/JBR.26.20120027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S, Yu JM, Gan YC, et al. Biomimetic natural biomaterials for tissue engineering and regenerative medicine: new biosynthesis methods, recent advances, and emerging applications. Milit MedRes. 2023;10(1):16. doi: 10.1186/s40779-023-00448-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahiwala A. Formulation approaches in enhancement of patient compliance to oral drug therapy. Expert Opin Drug Deliv. 2011;8(11):1521–1529. doi: 10.1517/17425247.2011.628311 [DOI] [PubMed] [Google Scholar]
- 7.X-F L, Zhao W-L, Zhu X-D, Zhang X-D. Fabrication methods of bioactive materials for bone regeneration. Bioact Mater Bone Regen. 2020;2020:3. [Google Scholar]
- 8.Sun Y, Chen L-G, Fan X-M, Pang JN. Ultrasound responsive smart implantable hydrogels for targeted delivery of drugs: reviewing current practices. Int J Nanomedicine. 2022;5001–5026. doi: 10.2147/IJN.S374247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin S-E, Jin H-E, Hong S. Targeted delivery system of nanobiomaterials in anticancer therapy: from cells to clinics. Biomed Res Int. 2014;2014:1–23. doi: 10.1155/2014/814208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu H, Fu H, Han Z, Sun Y. Biomaterials for bone tissue engineering scaffolds: a review. RSC Adv. 2019;9(45):26252–26262. doi: 10.1039/C9RA05214C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Chen X, Hong H, Hu R, Liu J, Liu C. Decellularized extracellular matrix scaffolds: recent trends and emerging strategies in tissue engineering. Bioact Mater. 2022;10:15–31. doi: 10.1016/j.bioactmat.2021.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao X, Han S, Zhang R, Liu G, Wu J. Progress in electrospun composite nanofibers: composition, performance and applications for tissue engineering. J Mater Chem B. 2019;7(45):7075–7089. doi: 10.1039/C9TB01730E [DOI] [PubMed] [Google Scholar]
- 13.Abdel Nasser Atia G, Shalaby HK, Zehravi M, et al. Locally applied repositioned hormones for oral bone and periodontal tissue engineering: a narrative review. Polymers. 2022;14(14):2964. doi: 10.3390/polym14142964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunatillake P, Mayadunne R, Adhikari R. Recent developments in biodegradable synthetic polymers. Biotechnol Annu Rev. 2006;12:301–347. [DOI] [PubMed] [Google Scholar]
- 15.Khorshidi S, Solouk A, Mirzadeh H, et al. A review of key challenges of electrospun scaffolds for tissue‐engineering applications. J Tissue Eng Regen Med. 2016;10(9):715–738. doi: 10.1002/term.1978 [DOI] [PubMed] [Google Scholar]
- 16.Negut I, Dorcioman G, Grumezescu V. Scaffolds for wound healing applications. Polymers. 2020;12(9):2010. doi: 10.3390/polym12092010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yi S, Ding F, Gong L, Gu X. Extracellular matrix scaffolds for tissue engineering and regenerative medicine. Curr Stem Cell Res Ther. 2017;12(3):233–246. doi: 10.2174/1574888X11666160905092513 [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Cheng J, Ao Q. Preparation of alginate-based biomaterials and their applications in biomedicine. Mar Drugs. 2021;19(5):264. doi: 10.3390/md19050264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seddiqi H, Oliaei E, Honarkar H, et al. Cellulose and its derivatives: towards biomedical applications. Cellulose. 2021;28(4):1893–1931. [Google Scholar]
- 20.El-Sherbiny IM, El-Baz NM. A review on bionanocomposites based on chitosan and its derivatives for biomedical applications. Eco Fndly Polym Nanocom. 2015;2015:173–208. [Google Scholar]
- 21.Sofi HS, Ashraf R, Beigh MA, Sheikh FA. Scaffolds Fabricated From Natural Polymers/Composites By Electrospinning For Bone Tissue Regeneration. In: Cutting-Edge Enabling Technologies for Regenerative Medicine. Springer; 2018:49–78. [DOI] [PubMed] [Google Scholar]
- 22.DeFrates KG, Moore R, Borgesi J, et al. Protein-based fiber materials in medicine: a review. Nanomaterials. 2018;8(7):457. doi: 10.3390/nano8070457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu M, Wu P, Xiao L, et al. Biomimetic mineralization of novel hydroxyethyl cellulose/soy protein isolate scaffolds promote bone regeneration in vitro and in vivo. Int J Biol Macromol. 2020;162:1627–1641. doi: 10.1016/j.ijbiomac.2020.08.029 [DOI] [PubMed] [Google Scholar]
- 24.Dutta PK. Chitin and Chitosan for Regenerative Medicine. Springer; 2016. [Google Scholar]
- 25.Russo L, Cipolla L. Glycomics: new challenges and opportunities in regenerative medicine. Chem Eur J. 2016;22(38):13380–13388. doi: 10.1002/chem.201602156 [DOI] [PubMed] [Google Scholar]
- 26.Tiwari S, Patil R, Bahadur P. Polysaccharide based scaffolds for soft tissue engineering applications. Polymers. 2018;11(1):1. doi: 10.3390/polym11010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sood A, Gupta A, Agrawal G. Recent advances in polysaccharides based biomaterials for drug delivery and tissue engineering applications. Carbohydr Polym Technol Appl. 2021;2:100067. [Google Scholar]
- 28.Islam S, Bhuiyan MA, Islam MN. Chitin and chitosan: structure, properties and applications in biomedical engineering. J Polym Environ. 2017;25(3):854–866. doi: 10.1007/s10924-016-0865-5 [DOI] [Google Scholar]
- 29.Llamas-Arriba MG, Hernández-Alcántara AM, Mohedano ML, et al. Lactic acid bacteria isolated from fermented doughs in Spain produce dextrans and riboflavin. Foods. 2021;10(9):2004. doi: 10.3390/foods10092004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maia J, Evangelista MB, Gil H, Ferreira L. Dextran-based materials for biomedical applications. Res Signpost. 2014;37661:31–53. [Google Scholar]
- 31.Ramasundaram S, Saravanakumar G, Sobha S, Oh TH. Dextran sulfate nanocarriers: design, strategies and biomedical applications. Int J Mol Sci. 2022;24(1):355. doi: 10.3390/ijms24010355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selyanin MA, Boykov PY, Khabarov VN, Polyak F. The history of hyaluronic acid discovery, foundational research and initial use. HA. 2015;1–8. [Google Scholar]
- 33.Litwiniuk M, Krejner A, Speyrer MS, Gauto AR, Grzela T. Hyaluronic acid in inflammation and tissue regeneration. Wounds. 2016;28(3):78–88. [PubMed] [Google Scholar]
- 34.Longinotti C. The use of hyaluronic acid based dressings to treat burns: a review. Burns Trauma. 2014;2(4):2321–3868. doi: 10.4103/2321-3868.142398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouvard C, Galy-Fauroux I, Grelac F, et al. Low-molecular-weight fucoidan induces endothelial cell migration via the PI3K/AKT pathway and modulates the transcription of genes involved in angiogenesis. Mar Drugs. 2015;13(12):7446–7462. doi: 10.3390/md13127075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Debele TA, Mekuria SL, Tsai H-C. Polysaccharide based nanogels in the drug delivery system: application as the carrier of pharmaceutical agents. Mater Sci Eng. 2016;68:964–981. doi: 10.1016/j.msec.2016.05.121 [DOI] [PubMed] [Google Scholar]
- 37.Larrañeta E, Henry M, Irwin NJ, Trotter J, Perminova AA, Donnelly RF. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr Polym. 2018;181:1194–1205. doi: 10.1016/j.carbpol.2017.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta KC, Haider A, Choi Y, Kang IK. Nanofibrous scaffolds in biomedical applications. Biomater Res. 2014;18(1):1–11. doi: 10.1186/2055-7124-18-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhatia S. Systems for Drug Delivery. Springer; 2016. [Google Scholar]
- 40.Wardrop D, Keeling D. The story of the discovery of heparin and warfarin. Br J Haematol. 2008;141(6):757–763. doi: 10.1111/j.1365-2141.2008.07119.x [DOI] [PubMed] [Google Scholar]
- 41.Laremore TN, Zhang F, Dordick JS, Liu J, Linhardt RJ. Recent progress and applications in glycosaminoglycan and heparin research. Curr Opin Chem Biol. 2009;13(5–6):633–640. doi: 10.1016/j.cbpa.2009.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joung YK, Bae JW, Park KD. Controlled release of heparin-binding growth factors using heparin-containing particulate systems for tissue regeneration. Expert Opin Drug Deliv. 2008;5(11):1173–1184. doi: 10.1517/17425240802431811 [DOI] [PubMed] [Google Scholar]
- 43.Rezaie AR, Giri H. Anticoagulant and signaling functions of antithrombin. J Thromb Haemost. 2020;18(12):3142–3153. doi: 10.1111/jth.15052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suhail M, Rosenholm JM, Minhas MU, et al. Nanogels as drug-delivery systems: a comprehensive overview. Ther Deliv. 2019;10(11):697–717. doi: 10.4155/tde-2019-0010 [DOI] [PubMed] [Google Scholar]
- 45.Pereira L, Cotas J. Introductory chapter: alginates-A general overview. Alginates Recent Uses Nat Polymer. 2020;2020:1. [Google Scholar]
- 46.Sabra W. The Promise and Challenge of Microbial Alginate Production: a Product with Novel Applications. In: Polysaccharides of Microbial Origin: Biomedical Applications. Springer; 2022:79–98. [Google Scholar]
- 47.Abdelraouf RM, Bayoumi RE, Hamdy TM. Effect of powder/water ratio variation on viscosity, tear strength and detail reproduction of dental alginate impression material (In vitro and clinical study). Polymers. 2021;13(17):2923. doi: 10.3390/polym13172923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ambrosetti R. Pullulan as a release modifier for drug delivery systems: effect of rheological behaviour of its solutions on the processability by spray drying; 2019.
- 49.Pacheco MS, Barbieri D, da Silva CF, de Moraes MA. A review on orally disintegrating films (ODFs) made from natural polymers such as pullulan, maltodextrin, starch, and others. Int J Biol Macromol. 2021;178:504–513. doi: 10.1016/j.ijbiomac.2021.02.180 [DOI] [PubMed] [Google Scholar]
- 50.Agrawal S, Budhwani D, Gurjar P, Telange D, Lambole V. Pullulan based derivatives: synthesis, enhanced physicochemical properties, and applications. Drug Deliv. 2022;29(1):3328–3339. doi: 10.1080/10717544.2022.2144544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mihaila SM, Popa EG, Reis RL, Marques AP, Gomes ME. Fabrication of endothelial cell-laden carrageenan microfibers for microvascularized bone tissue engineering applications. Biomacromolecules. 2014;15(8):2849–2860. doi: 10.1021/bm500036a [DOI] [PubMed] [Google Scholar]
- 52.Ocampo JIG, de Paula MMM, Bassous NJ, Lobo AO, Orozco CPO, Webster TJ. Osteoblast responses to injectable bone substitutes of kappa-carrageenan and nano hydroxyapatite. Acta biomater. 2019;83:425–434. doi: 10.1016/j.actbio.2018.10.023 [DOI] [PubMed] [Google Scholar]
- 53.Pacheco-Quito E-M, Ruiz-Caro R, Veiga M-D. Carrageenan: drug delivery systems and other biomedical applications. Mar Drugs. 2020;18(11):583. doi: 10.3390/md18110583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garnjanagoonchorn W, Wongekalak L, Engkagul A. Determination of chondroitin sulfate from different sources of cartilage. Chem Eng Process. 2007;46(5):465–471. doi: 10.1016/j.cep.2006.05.019 [DOI] [Google Scholar]
- 55.Manivong S, Cullier A, Audigie F, et al. New trends for osteoarthritis: biomaterials, models and modeling. Drug Discov Today. 2023;28:103488. doi: 10.1016/j.drudis.2023.103488 [DOI] [PubMed] [Google Scholar]
- 56.Pal D, Saha S. Chondroitin: a natural biomarker with immense biomedical applications. RSC Adv. 2019;9(48):28061–28077. doi: 10.1039/C9RA05546K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li T, Chen C, Brozena AH, et al. Developing fibrillated cellulose as a sustainable technological material. Nature. 2021;590(7844):47–56. doi: 10.1038/s41586-020-03167-7 [DOI] [PubMed] [Google Scholar]
- 58.Akram M, Taha I, Ghobashy MM. Low temperature pyrolysis of carboxymethylcellulose. Cellulose. 2016;23:1713–1724. doi: 10.1007/s10570-016-0950-x [DOI] [Google Scholar]
- 59.Zaki DY, Safwat EM, Nagi SM, et al. A novel dental re-mineralizing blend of hydroxyethyl-cellulose and cellulose nanofibers oral film loaded with nepheline apatite glass: preparation, characterization and in vitro evaluation of re-mineralizing effect. Carbohydr Polym Technol Appl. 2021;2:100035. doi: 10.1016/j.carpta.2021.100035 [DOI] [Google Scholar]
- 60.Upadhyay RK. Role of biological scaffolds, hydro gels and stem cells in tissue regeneration therapy. Adv Tissue Eng Regen Med Open Access. 2017;2(1):121. [Google Scholar]
- 61.Suamte L, Tirkey A, Babu PJ. Design of 3D smart scaffolds using natural, synthetic and hybrid derived polymers for skin regenerative applications. Smart Mater Med. 2022;2022:1. [Google Scholar]
- 62.Bhatt P, Kumar V, Goel R, et al. Structural modifications and strategies for native starch for applications in advanced drug delivery. Biomed Res Int. 2022;2022:1–14. doi: 10.1155/2022/2188940 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Zhang B, Liu N, Hao M, Zhou J, Xie Y, He Z. Plant-derived polysaccharides regulated immune status, gut health and microbiota of broilers: a review. Front Veter Sci. 2022;8:791371. doi: 10.3389/fvets.2021.791371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taghavizadeh Yazdi ME, Nazarnezhad S, Mousavi SH, et al. Gum tragacanth (GT): a versatile biocompatible material beyond borders. Molecules. 2021;26(6):1510. doi: 10.3390/molecules26061510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silva TH, Moreira-Silva J, Marques ALP, Domingues A, Bayon Y, Reis RL. Marine origin collagens and its potential applications. Mar Drugs. 2014;12(12):5881–5901. doi: 10.3390/md12125881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sapna G, Gokul S, Bagri‐Manjrekar K. Matrix metalloproteinases and periodontal diseases. Oral Dis. 2014;20(6):538–550. doi: 10.1111/odi.12159 [DOI] [PubMed] [Google Scholar]
- 67.Gursoy UK, Könönen E, Huumonen S, et al. Salivary type I collagen degradation end‐products and related matrix metalloproteinases in periodontitis. J Clin Periodontol. 2013;40(1):18–25. doi: 10.1111/jcpe.12020 [DOI] [PubMed] [Google Scholar]
- 68.McGuire JD, Walker MP, Dusevich V, Wang Y, Gorski JP. Enamel organic matrix: potential structural role in enamel and relationship to residual basement membrane constituents at the dentin enamel junction. Connect Tissue Res. 2014;55(sup1):33–37. doi: 10.3109/03008207.2014.923883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tjäderhane L, Carrilho MR, Breschi L, Tay FR, Pashley DH. Dentin basic structure and composition—an overview. Endod Topics. 2009;20(1):3–29. doi: 10.1111/j.1601-1546.2012.00269.x [DOI] [Google Scholar]
- 70.Gonçalves PF, Sallum EA, Sallum AW, Casati MZ, De toledo S, Junior FHN. Dental Cementum Reviewed: Development, Structure, Composition, Regeneration and Potential Functions. Piracicaba Dental School-UNICAMP; 2005. [Google Scholar]
- 71.Naomi R, Bahari H, Ridzuan PM, Othman F. Natural-based biomaterial for skin wound healing (Gelatin vs. collagen): expert review. Polymers. 2021;13(14):2319. doi: 10.3390/polym13142319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thombare N, Jha U, Mishra S, Siddiqui MZ. Guar gum as a promising starting material for diverse applications: a review. Int J Biol Macromol. 2016;88:361–372. doi: 10.1016/j.ijbiomac.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 73.Stoffels JMJ, Zhao C, Baron W. Fibronectin in tissue regeneration: timely disassembly of the scaffold is necessary to complete the build. Cell Mol Life Sci. 2013;70(22):4243–4253. doi: 10.1007/s00018-013-1350-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chantre CO, Campbell PH, Golecki HM, et al. Production-scale fibronectin nanofibers promote wound closure and tissue repair in a dermal mouse model. Biomaterials. 2018;166:96–108. doi: 10.1016/j.biomaterials.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Su K, Wang C. Recent advances in the use of gelatin in biomedical research. Biotechnol Lett. 2015;37(11):2139–2145. doi: 10.1007/s10529-015-1907-0 [DOI] [PubMed] [Google Scholar]
- 76.Chen S, Zhang Q, Nakamoto T, Kawazoe N, Chen G. Gelatin scaffolds with controlled pore structure and mechanical property for cartilage tissue engineering. Tissue Eng Part C Methods. 2016;22(3):189–198. doi: 10.1089/ten.tec.2015.0281 [DOI] [PubMed] [Google Scholar]
- 77.Ninan N, Muthiah M, Park I-K, Wong TW, Thomas S, Grohens Y. Natural polymer/inorganic material based hybrid scaffolds for skin wound healing. Polymer Rev. 2015;55(3):453–490. doi: 10.1080/15583724.2015.1019135 [DOI] [Google Scholar]
- 78.Cui X, Li J, Hartanto Y, et al. Advances in extrusion 3D bioprinting: a focus on multicomponent hydrogel‐based bioinks. Adv Healthcare Mater. 2020;9(15):1901648. doi: 10.1002/adhm.201901648 [DOI] [PubMed] [Google Scholar]
- 79.Philandrianos C, Andrac-Meyer L, Mordon S, et al. Comparison of five dermal substitutes in full-thickness skin wound healing in a porcine model. Burns. 2012;38(6):820–829. doi: 10.1016/j.burns.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 80.Parry DAD, Squire JM. Fibrous Proteins: Structures and Mechanisms. Springer; 2017. [Google Scholar]
- 81.Shanmugasundaram OL, Ahmed KSZ, Sujatha K, et al. Fabrication and characterization of chicken feather keratin/polysaccharides blended polymer coated nonwoven dressing materials for wound healing applications. Mater Sci Eng. 2018;92:26–33. doi: 10.1016/j.msec.2018.06.020 [DOI] [PubMed] [Google Scholar]
- 82.Yasuda H, Kuroda S, Shichinohe H, Kamei S, Kawamura R, Iwasaki Y. Effect of biodegradable fibrin scaffold on survival, migration, and differentiation of transplanted bone marrow stromal cells after cortical injury in rats. J Neurosurg. 2010;112(2):336–344. doi: 10.3171/2009.2.JNS08495 [DOI] [PubMed] [Google Scholar]
- 83.Noori A, Ashrafi SJ, Vaez-Ghaemi R, Hatamian-Zaremi A, Webster TJ. A review of fibrin and fibrin composites for bone tissue engineering. Int J Nanomedicine. 2017;12:4937. doi: 10.2147/IJN.S124671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khanna A, Oropeza BP, Huang NF. Engineering spatiotemporal control in vascularized tissues. Bioengineering. 2022;9(10):555. doi: 10.3390/bioengineering9100555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ziv-Polat O, Skaat H, Shahar A, Margel S. Novel magnetic fibrin hydrogel scaffolds containing thrombin and growth factors conjugated iron oxide nanoparticles for tissue engineering. Int J Nanomedicine. 2012;7:1259. doi: 10.2147/IJN.S26533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Egorikhina MN, Bronnikova II, Rubtsova YP, et al. Aspects of in vitro biodegradation of hybrid fibrin-collagen scaffolds. Polymers. 2021;13(20):3470. doi: 10.3390/polym13203470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang H-Y, Zhang Y-Q. Effect of regeneration of liquid silk fibroin on its structure and characterization. Soft Matter. 2013;9(1):138–145. doi: 10.1039/C2SM26945G [DOI] [Google Scholar]
- 88.Bhattacharjee P, Naskar D, Maiti TK, Bhattacharya D, Kundu SC. Investigating the potential of combined growth factors delivery, from non-mulberry silk fibroin grafted poly (ɛ-caprolactone)/hydroxyapatite nanofibrous scaffold, in bone tissue engineering. Appl Mater Today. 2016;5:52–67. doi: 10.1016/j.apmt.2016.09.007 [DOI] [Google Scholar]
- 89.Ko E, Lee JS, Kim H, et al. Electrospun silk fibroin nanofibrous scaffolds with two-stage hydroxyapatite functionalization for enhancing the osteogenic differentiation of human adipose-derived mesenchymal stem cells. ACS Appl Mater Interfaces. 2017;10(9):7614–7625. doi: 10.1021/acsami.7b03328 [DOI] [PubMed] [Google Scholar]
- 90.Wang S, Cao X, Shen M, Guo R, Bányai I, Shi X. Fabrication and morphology control of electrospun poly (γ-glutamic acid) nanofibers for biomedical applications. Colloids Surf B Biointerfaces. 2012;89:254–264. doi: 10.1016/j.colsurfb.2011.09.029 [DOI] [PubMed] [Google Scholar]
- 91.Sofi HS, Ashraf R, Khan AH, Beigh MA, Majeed S, Sheikh FA. Reconstructing nanofibers from natural polymers using surface functionalization approaches for applications in tissue engineering, drug delivery and biosensing devices. Mater Sci Eng. 2019;94:1102–1124. doi: 10.1016/j.msec.2018.10.069 [DOI] [PubMed] [Google Scholar]
- 92.Najar IN, Das S. Poly-glutamic acid (PGA)-Structure, synthesis, genomic organization and its application: a Review. Int J Pharm Sci Res. 2015;6(6):2258. [Google Scholar]
- 93.Wang G, Liu Q, Yang YM, et al. Applications and functions of γ-poly-glutamic acid and its derivatives in medicine. Curr Pharm Biotechnol. 2021;22(11):1404–1411. doi: 10.2174/1389201021999201118161155 [DOI] [PubMed] [Google Scholar]
- 94.d’Ischia M, Napolitano A, Ball V, Chen C-T, Buehler MJ. Polydopamine and eumelanin: from structure–property relationships to a unified tailoring strategy. Acc Chem Res. 2014;47(12):3541–3550. doi: 10.1021/ar500273y [DOI] [PubMed] [Google Scholar]
- 95.Manivasagan P, Kim J, Jang E-S. Recent progress in multifunctional conjugated polymer nanomaterial-based synergistic combination phototherapy for microbial infection theranostics. Coord Chem Rev. 2022;470:214701. doi: 10.1016/j.ccr.2022.214701 [DOI] [Google Scholar]
- 96.Ralph J, Lapierre C, Boerjan W. Lignin structure and its engineering. Curr Opin Biotechnol. 2019;56:240–249. doi: 10.1016/j.copbio.2019.02.019 [DOI] [PubMed] [Google Scholar]
- 97.Sugiarto S, Leow Y, Tan CL, Wang G, Kai D. How far is Lignin from being a biomedical material? Bioact Mater. 2022;8:71–94. doi: 10.1016/j.bioactmat.2021.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Domínguez-Robles J, Martin NK, Fong ML, et al. Antioxidant PLA composites containing lignin for 3D printing applications: a potential material for healthcare applications. Pharmaceutics. 2019;11(4):165. doi: 10.3390/pharmaceutics11040165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pillai CKS. Challenges for natural monomers and polymers: novel design strategies and engineering to develop advanced polymers. Des Monomers Polym. 2010;13(2):87–121. doi: 10.1163/138577210X12634696333190 [DOI] [Google Scholar]
- 100.Zhu J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31(17):4639–4656. doi: 10.1016/j.biomaterials.2010.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Geckil H, Xu F, Zhang X, Moon S, Demirci U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine. 2010;5(3):469–484. doi: 10.2217/nnm.10.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bacakova L, Zikmundova M, Pajorova J, et al. Nanofibrous scaffolds for skin tissue engineering and wound healing based on synthetic polymers. Appl Nanobiotechnol. 2019;2019:1. [Google Scholar]
- 103.Kluge JA, Mauck RL. Synthetic/biopolymer nanofibrous composites as dynamic tissue engineering scaffolds. Biomed Appl Polymer Nanofibers. 2011;2011:101–130. [Google Scholar]
- 104.Zhang Z, Ortiz O, Goyal R, Kohn J. Biodegradable polymers. In: Handbook of Polymer Applications in Medicine and Medical Devices. Elsevier; 2014;303–335. [Google Scholar]
- 105.D’souza AA, Shegokar R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv. 2016;13(9):1257–1275. doi: 10.1080/17425247.2016.1182485 [DOI] [PubMed] [Google Scholar]
- 106.Zustiak SP, Leach JB. Hydrolytically degradable poly (ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules. 2010;11(5):1348–1357. doi: 10.1021/bm100137q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ayres CE, Jha BS, Sell SA, Bowlin GL, Simpson DG. Nanotechnology in the design of soft tissue scaffolds: innovations in structure and function. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2(1):20–34. doi: 10.1002/wnan.55 [DOI] [PubMed] [Google Scholar]
- 108.Calvo P, Remunan‐Lopez C, Vila‐Jato JL, Alonso MJ. Novel hydrophilic chitosan‐polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci. 1997;63(1):125–132. doi: [DOI] [Google Scholar]
- 109.Howard MD, Jay M, Dziubla TD, Lu X. PEGylation of nanocarrier drug delivery systems: state of the art. J Biomed Nanotechnol. 2008;4(2):133–148. doi: 10.1166/jbn.2008.021 [DOI] [Google Scholar]
- 110.Wang P, Chu W, Zhuo X, et al. Modified PLGA–PEG–PLGA thermosensitive hydrogels with suitable thermosensitivity and properties for use in a drug delivery system. J Mater Chem B. 2017;5(8):1551–1565. doi: 10.1039/C6TB02158A [DOI] [PubMed] [Google Scholar]
- 111.Hodge J, Quint C. The improvement of cell infiltration in an electrospun scaffold with multiple synthetic biodegradable polymers using sacrificial PEO microparticles. J Biomed Mater Res A. 2019;107(9):1954–1964. doi: 10.1002/jbm.a.36706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Trakunjae C, Boondaeng A, Apiwatanapiwat W, et al. Enhanced polyhydroxybutyrate (PHB) production by newly isolated rare actinomycetes Rhodococcus sp. strain BSRT1-1 using response surface methodology. Sci Rep. 2021;11(1):1896. doi: 10.1038/s41598-021-81386-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kasper FK, Tanahashi K, Fisher JP, Mikos AG. Synthesis of poly (propylene fumarate). Nat Protoc. 2009;4(4):518–525. doi: 10.1038/nprot.2009.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang S, Yaszemski MJ, Gruetzmacher JA, Lu L. Photo-crosslinked poly(ε-caprolactone fumarate) networks: roles of crystallinity and crosslinking density in determining mechanical properties. Polymer. 2008;49(26):5692–5699. doi: 10.1016/j.polymer.2008.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jayabalan M, Shalumon KT, Mitha MK, Ganesan K, Epple M. Effect of hydroxyapatite on the biodegradation and biomechanical stability of polyester nanocomposites for orthopaedic applications. Acta Biomater. 2010;6(3):763–775. doi: 10.1016/j.actbio.2009.09.015 [DOI] [PubMed] [Google Scholar]
- 116.Mirmohammadi SA, Imani M, Uyama H, Atai M. In situ photocrosslinkable nanohybrids based on poly (ε-caprolactone fumarate)/polyhedral oligomeric silsesquioxane: synthesis and characterization. J Polym Res. 2013;20(12):1–13. doi: 10.1007/s10965-013-0297-z [DOI] [Google Scholar]
- 117.Abdulkareem A, Kasak P, Nassr MG, et al. Surface modification of poly (lactic acid) film via cold plasma assisted grafting of fumaric and ascorbic acid. Polymers. 2021;13(21):3717. doi: 10.3390/polym13213717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim K, Fisher JP. Nanoparticle technology in bone tissue engineering. J Drug Target. 2007;15(4):241–252. doi: 10.1080/10611860701289818 [DOI] [PubMed] [Google Scholar]
- 119.Mohamed RM, Yusoh K. A review on the recent research of polycaprolactone (PCL). Adv Mat Res. 2016;1134:249–255. doi: 10.4028/www.scientific.net/AMR.1134.249 [DOI] [Google Scholar]
- 120.Yadav BK, Patel G. Mechanical properties and biodegradability of electrospun methylcellulose-polyvinylalcohol single-layered nanofibers compared to polycaprolactone/methylcellulose-polyvinylalcohol/polycaprolactone multilayered nanofibers. Int J Polym Mater Polym Biomater. 2021;2021:1–8. [Google Scholar]
- 121.W-J L, Tuli R, Huang X, Laquerriere P, Tuan RS. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials. 2005;26(25):5158–5166. doi: 10.1016/j.biomaterials.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 122.Malikmammadov E, Tanir TE, Kiziltay A, Hasirci V, Hasirci N. PCL and PCL-based materials in biomedical applications. J Biomater Sci Polym Ed. 2018;29(7–9):863–893. doi: 10.1080/09205063.2017.1394711 [DOI] [PubMed] [Google Scholar]
- 123.Rasal RM, Janorkar AV, Hirt DE. Poly (lactic acid) modifications. Prog Polym Sci. 2010;35(3):338–356. doi: 10.1016/j.progpolymsci.2009.12.003 [DOI] [Google Scholar]
- 124.Choudhury S, Das A. Advances in generation of three-dimensional skin equivalents: pre-clinical studies to clinical therapies. Cytotherapy. 2021;23(1):1–9. doi: 10.1016/j.jcyt.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gaaz TS, Sulong AB, Akhtar MN, Kadhum AAH, Mohamad AB, Al-Amiery AA. Properties and applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. Molecules. 2015;20(12):22833–22847. doi: 10.3390/molecules201219884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rahmani Del Bakhshayesh A, Annabi N, Khalilov R, et al. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif Cells, Nanomed Biotechnol. 2018;46(4):691–705. doi: 10.1080/21691401.2017.1349778 [DOI] [PubMed] [Google Scholar]
- 127.Sharma S, Parmar A, Kori S, Sandhir R. PLGA-based nanoparticles: a new paradigm in biomedical applications. TrAC Trends Anal Chem. 2016;80:30–40. doi: 10.1016/j.trac.2015.06.014 [DOI] [Google Scholar]
- 128.Alsaab HO, Alharbi FD, Alhibs AS, et al. PLGA-based nanomedicine: history of advancement and development in clinical applications of multiple diseases. Pharmaceutics. 2022;14(12):2728. doi: 10.3390/pharmaceutics14122728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zou Y, Li D, Shen M, Shi X. Polyethylenimine‐based nanogels for biomedical applications. Macromol Biosci. 2019;19(11):1900272. doi: 10.1002/mabi.201900272 [DOI] [PubMed] [Google Scholar]
- 130.Husain MSB, Gupta A, Alashwal BY, Sharma S. Synthesis of PVA/PVP based hydrogel for biomedical applications: a review. Energ Sources Part A. 2018;40(20):2388–2393. doi: 10.1080/15567036.2018.1495786 [DOI] [Google Scholar]
- 131.Zayed M, Baker M, Ghazal H. Journal of textiles, coloration and polymer science; 2023.
- 132.Gugerell A, Kober J, Laube T, et al. Electrospun poly (ester-urethane)-and poly (ester-urethane-urea) fleeces as promising tissue engineering scaffolds for adipose-derived stem cells. PLoS One. 2014;9(3):e90676. doi: 10.1371/journal.pone.0090676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lizundia E, Sipponen MH, Greca LG, et al. Multifunctional lignin-based nanocomposites and nanohybrids. Green Chem. 2021;23(18):6698–6760. doi: 10.1039/d1gc01684a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kaur G, Narayanan G, Garg D, Sachdev A, Matai I. Biomaterials-based regenerative strategies for skin tissue wound healing. ACS Appl Bio Mater. 2022;5(5):2069–2106. doi: 10.1021/acsabm.2c00035 [DOI] [PubMed] [Google Scholar]
- 135.Mohammadalizadeh Z, Bahremandi-Toloue E, Karbasi S. Synthetic-based blended electrospun scaffolds in tissue engineering applications. J Mater Sci. 2022;34:1–60. doi: 10.1007/s10856-022-06709-9 [DOI] [Google Scholar]
- 136.Bottino FA, Cinquegrani AR, Di Pasquale G, Leonardi L, Pollicino A. Chemical modifications, mechanical properties and surface photo-oxidation of films of polystyrene (PS). Polym Test. 2004;23(4):405–411. doi: 10.1016/j.polymertesting.2003.10.001 [DOI] [Google Scholar]
- 137.Motealleh B, Zahedi P, Rezaeian I, Moghimi M, Abdolghaffari AH, Zarandi MA. Morphology, drug release, antibacterial, cell proliferation, and histology studies of chamomile‐loaded wound dressing mats based on electrospun nanofibrous poly (ɛ‐caprolactone)/polystyrene blends. J Biomed Mater Res B Appl Biomater. 2014;102(5):977–987. doi: 10.1002/jbm.b.33078 [DOI] [PubMed] [Google Scholar]
- 138.Chantre CO, Gonzalez GM, Ahn S, et al. Porous biomimetic hyaluronic acid and extracellular matrix protein nanofiber scaffolds for accelerated cutaneous tissue repair. ACS Appl Mater Interfaces. 2019;11(49):45498–45510. doi: 10.1021/acsami.9b17322 [DOI] [PubMed] [Google Scholar]
- 139.Ding X, Zhao H, Li Y, et al. Synthetic peptide hydrogels as 3D scaffolds for tissue engineering. Adv Drug Deliv Rev. 2020;160:78–104. doi: 10.1016/j.addr.2020.10.005 [DOI] [PubMed] [Google Scholar]
- 140.Sughanthy SAP, Ansari MNM, Atiqah A. Dynamic mechanical analysis of polyethylene terephthalate/hydroxyapatite biocomposites for tissue engineering applications. J Mater Res Technol. 2020;9(2):2350–2356. doi: 10.1016/j.jmrt.2019.12.066 [DOI] [Google Scholar]
- 141.Bahari N, Hashim N, Md Akim A, Maringgal B. Recent advances in honey-based nanoparticles for wound dressing: a review. Nanomaterials. 2022;12(15):2560. doi: 10.3390/nano12152560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rahimpour A. UV photo-grafting of hydrophilic monomers onto the surface of nano-porous PES membranes for improving surface properties. Desalination. 2011;265(1–3):93–101. doi: 10.1016/j.desal.2010.07.037 [DOI] [Google Scholar]
- 143.Babaeijandaghi F, Shabani I, Seyedjafari E, et al. Accelerated epidermal regeneration and improved dermal reconstruction achieved by polyethersulfone nanofibers. Tissue Eng Part A. 2010;16(11):3527–3536. doi: 10.1089/ten.tea.2009.0829 [DOI] [PubMed] [Google Scholar]
- 144.Li H, Williams GR, Wu J, Wang H, Sun X, Zhu L-M. Poly(N-isopropylacrylamide)/poly(l-lactic acid-co-ɛ-caprolactone) fibers loaded with ciprofloxacin as wound dressing materials. Mater Sci Eng. 2017;79:245–254. doi: 10.1016/j.msec.2017.04.058 [DOI] [PubMed] [Google Scholar]
- 145.Farahani M, Shafiee A. Wound healing: from passive to smart dressings. Adv Healthcare Mater. 2021;10(16):2100477. doi: 10.1002/adhm.202100477 [DOI] [PubMed] [Google Scholar]
- 146.Liu S, Ma J, Xu L, et al. An electrospun polyurethane scaffold-reinforced zwitterionic hydrogel as a biocompatible device. J Mater Chem B. 2020;8(12):2443–2453. doi: 10.1039/C9TB02870F [DOI] [PubMed] [Google Scholar]
- 147.Harijan M, Singh M. Zwitterionic polymers in drug delivery: a review. J Mol Recognit. 2022;35(1):e2944. doi: 10.1002/jmr.2944 [DOI] [PubMed] [Google Scholar]
- 148.Islam S. Microneedles in pain management of Cancer; 2021.
- 149.Cai SS, Li T, Akinade T, Zhu Y, Leong KW. Drug delivery carriers with therapeutic functions. Adv Drug Deliv Rev. 2021;176:113884. doi: 10.1016/j.addr.2021.113884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tuan-Mahmood T-M, McCrudden MTC, Torrisi BM, et al. Microneedles for intradermal and transdermal drug delivery. Eur J Pharm Sci. 2013;50(5):623–637. doi: 10.1016/j.ejps.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bhatnagar S, Gadeela PR, Thathireddy P, Venuganti VVK. Microneedle-based drug delivery: materials of construction. J Chem Sci. 2019;131(9):1–28. doi: 10.1007/s12039-019-1666-x [DOI] [Google Scholar]
- 152.Avcil M, Çelik A. Microneedles in drug delivery: progress and challenges. Micromachines. 2021;12(11):1321. doi: 10.3390/mi12111321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang Y, Wu Y, Wei Y, Zeng T, Cao B, Liang J. Preparation and characterization of hydroxyapatite coating on AZ31 magnesium alloy induced by carboxymethyl cellulose-dopamine. Materials. 2021;14(8):1849. doi: 10.3390/ma14081849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bilal M, Mehmood S, Raza A, Hayat U, Rasheed T, Iqbal HMN. Microneedles in smart drug delivery. Adv Wound Care. 2021;10(4):204–219. doi: 10.1089/wound.2019.1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Donnelly RF, Larrañeta E. Microarray patches: potentially useful delivery systems for long-acting nanosuspensions. Drug Discov Today. 2018;23(5):1026–1033. doi: 10.1016/j.drudis.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 156.Sheng T, Luo B, Zhang W, et al. Microneedle-mediated vaccination: innovation and translation. Adv Drug Deliv Rev. 2021;179:113919. doi: 10.1016/j.addr.2021.113919 [DOI] [PubMed] [Google Scholar]
- 157.Ali A, Ahmed S. Recent advances in edible polymer based hydrogels as a sustainable alternative to conventional polymers. J Agric Food Chem. 2018;66(27):6940–6967. doi: 10.1021/acs.jafc.8b01052 [DOI] [PubMed] [Google Scholar]
- 158.Kakde I, Bansode D. Microrobotics a new approach in ocular drug delivery system: a review; 2022.
- 159.Ceylan H, Giltinan J, Kozielski K, Sitti M. Mobile microrobots for bioengineering applications. Lab Chip. 2017;17(10):1705–1724. doi: 10.1039/C7LC00064B [DOI] [PubMed] [Google Scholar]
- 160.Dabbagh SR, Sarabi MR, Birtek MT, Seyfi S, Sitti M, Tasoglu S. 3D-printed microrobots from design to translation. Nat Commun. 2022;13(1):1–24. doi: 10.1038/s41467-022-33409-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nelson BJ, Kaliakatsos IK, Abbott JJ. Microrobots for minimally invasive medicine. Annu Rev Biomed Eng. 2010;12:55–85. doi: 10.1146/annurev-bioeng-010510-103409 [DOI] [PubMed] [Google Scholar]
- 162.Bunea AI, Glückstad J. Strategies for optical trapping in biological samples: aiming at microrobotic surgeons. Laser Photon Rev. 2019;13(4):1800227. doi: 10.1002/lpor.201800227 [DOI] [Google Scholar]
- 163.Bhattarai DP, Aguilar LE, Park CH, Kim CS. A review on properties of natural and synthetic based electrospun fibrous materials for bone tissue engineering. Membranes. 2018;8(3):62. doi: 10.3390/membranes8030062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Samadian H, Farzamfar S, Vaez A, et al. A tailored polylactic acid/polycaprolactone biodegradable and bioactive 3D porous scaffold containing gelatin nanofibers and Taurine for bone regeneration. Sci Rep. 2020;10(1):1–12. doi: 10.1038/s41598-020-70155-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Costa RG, Oliveira J, Paula G, et al. Electrospinning of polymers in solution: part II: applications and perspectives. Polímeros. 2012;22:178–185. doi: 10.1590/S0104-14282012005000018 [DOI] [Google Scholar]
- 166.Lin M, Dai Y, Xia F, Zhang X. Advances in non-covalent crosslinked polymer micelles for biomedical applications. Mater Sci Eng. 2021;119:111626. doi: 10.1016/j.msec.2020.111626 [DOI] [PubMed] [Google Scholar]
- 167.Bleul R, Thiermann R, Maskos M. Techniques to control polymersome size. Macromolecules. 2015;48(20):7396–7409. doi: 10.1021/acs.macromol.5b01500 [DOI] [Google Scholar]
- 168.Ghorbanizamani F, Tok K, Moulahoum H, et al. Dye-loaded polymersome-based lateral flow assay: rational design of a COVID-19 testing platform by repurposing SARS-CoV-2 antibody cocktail and antigens obtained from positive human samples. ACS Sens. 2021;6(8):2988–2997. doi: 10.1021/acssensors.1c00854 [DOI] [PubMed] [Google Scholar]
- 169.Hosta-Rigau L, Jensen BEB, Fjeldsø KS, et al. Surface‐adhered composite poly(vinyl alcohol) physical hydrogels: polymersome‐aided delivery of therapeutic small molecules; 2012:1. [DOI] [PubMed]
- 170.Wayakanon K, Thornhill MH, Douglas CWI, et al. Polymersome‐mediated intracellular delivery of antibiotics to treat Porphyromonas gingivalis‐infected oral epithelial cells. FASEB J. 2013;27(11):4455–4465. doi: 10.1096/fj.12-225219 [DOI] [PubMed] [Google Scholar]
- 171.Mittal P, Saharan A, Verma R, et al. Dendrimers: a new race of pharmaceutical nanocarriers. Biomed Res Int. 2021;2021:8844030. doi: 10.1155/2021/8844030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gillies ER, Frechet JM. Dendrimers and dendritic polymers in drug delivery. Drug Discov Today. 2005;10(1):35–43. doi: 10.1016/S1359-6446(04)03276-3 [DOI] [PubMed] [Google Scholar]
- 173.Dwivedi N, Shah J, Mishra V, Tambuwala M, Kesharwani P. Nanoneuromedicine for management of neurodegenerative disorder. J Drug Deliv Sci Technol. 2019;49:477–490. doi: 10.1016/j.jddst.2018.12.021 [DOI] [Google Scholar]
- 174.Bapat RA, Chaubal TV, Joshi CP, et al. An overview of application of silver nanoparticles for biomaterials in dentistry. Mater Sci Eng. 2018;91:881–898. doi: 10.1016/j.msec.2018.05.069 [DOI] [PubMed] [Google Scholar]
- 175.Kesharwani P, Jain K, Jain PS. Dendrimer as nanocarrier for drug delivery. Prog Polym Sci. 2014;39(2):268–307. doi: 10.1016/j.progpolymsci.2013.07.005 [DOI] [Google Scholar]
- 176.Madani M, Hosny S, Alshangiti DM, et al. Green synthesis of nanoparticles for varied applications: green renewable resources and energy-efficient synthetic routes. Nanotechnol Rev. 2022;11(1):731–759. doi: 10.1515/ntrev-2022-0034 [DOI] [Google Scholar]
- 177.Tao Y, Endo M, Kaneko K. A review of synthesis and nanopore structures of organic polymer aerogels and carbon aerogels. Recent Pat Chem Eng. 2008;1(3):192–200. doi: 10.2174/2211334710801030192 [DOI] [Google Scholar]
- 178.Liu Z, Ran Y, Xi J, Wang J. Polymeric hybrid aerogels and their biomedical applications. Soft Matter. 2020;16(40):9160–9175. doi: 10.1039/D0SM01261K [DOI] [PubMed] [Google Scholar]
- 179.Neamtu I, Rusu AG, Diaconu A, Nita LE, Chiriac AP. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017;24(1):539–557. doi: 10.1080/10717544.2016.1276232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li X, Tsibouklis J, Weng T, et al. Nano carriers for drug transport across the blood–brain barrier. J Drug Target. 2017;25(1):17–28. doi: 10.1080/1061186X.2016.1184272 [DOI] [PubMed] [Google Scholar]
- 181.Kwon GS, Kataoka KJ. Block copolymer micelles as long-circulating drug vehicles. Adv Drug Deliv Rev. 2012;64:237–245. doi: 10.1016/j.addr.2012.09.016 [DOI] [Google Scholar]
- 182.Hamdy TM. Polymerization shrinkage in contemporary resin-based dental composites: a Review Article. Egypt J Chem. 2021;64(6):3087–3092. [Google Scholar]
- 183.Haugen HJ, Basu P, Sukul M, Mano JF, Reseland JE. Injectable Biomaterials for Dental Tissue Regeneration. Int J Mol Sci. 2020;21(10):3442. doi: 10.3390/ijms21103442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ifkovits JL, Burdick JA. Photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007;13(10):2369–2385. doi: 10.1089/ten.2007.0093 [DOI] [PubMed] [Google Scholar]
- 185.Sosnik A, Seremeta KP. Polymeric hydrogels as technology platform for drug delivery applications. Gels. 2017;3(3):25. doi: 10.3390/gels3030025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ghobashy MM, El-Sawy NM, Kodous AS. Nanocomposite of cosubstituted carbonated hydroxyapatite fabricated inside Poly (sodium hyaluronate-acrylamide) hydrogel template prepared by gamma radiation for osteoblast cell regeneration. Radiat Phys Chem. 2021;183:109408. doi: 10.1016/j.radphyschem.2021.109408 [DOI] [Google Scholar]
- 187.Khojasteh A, Kheiri L, Motamedian SR, Khoshkam V. Guided bone regeneration for the reconstruction of alveolar bone defects. Ann Maxillofac Surg. 2017;7(2):263. doi: 10.4103/ams.ams_76_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Neto AMD, Sartoretto SC, Duarte IM, et al. In vivo comparative evaluation of biocompatibility and biodegradation of bovine and porcine collagen membranes. Membranes. 2020;10(12):423. doi: 10.3390/membranes10120423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Colobatiu L, Gavan A, Potarniche A-V, et al. Evaluation of bioactive compounds-loaded chitosan films as a novel and potential diabetic wound dressing material. React Funct Polym. 2019;145:104369. doi: 10.1016/j.reactfunctpolym.2019.104369 [DOI] [Google Scholar]
- 190.Oryan A, Kamali A, Moshiri A, Baharvand H, Daemi H. Chemical crosslinking of biopolymeric scaffolds: current knowledge and future directions of crosslinked engineered bone scaffolds. Int J Biol Macromol. 2018;107:678–688. doi: 10.1016/j.ijbiomac.2017.08.184 [DOI] [PubMed] [Google Scholar]
- 191.Wathoni N, Motoyama K, Higashi T, Okajima M, Kaneko T, Arima H. Physically crosslinked-sacran hydrogel films for wound dressing application. Int J Biol Macromol. 2016;89:465–470. doi: 10.1016/j.ijbiomac.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 192.Ahi ZB, Renkler NZ, Gul Seker M, Tuzlakoglu K. Biodegradable Polymer Films with a Natural Antibacterial Extract as Novel Periodontal Barrier Membranes. Int J Biomater. 2019;2019:7932470. doi: 10.1155/2019/7932470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chi M, Qi M, L A, et al. Novel bioactive and therapeutic dental polymeric materials to inhibit periodontal pathogens and biofilms. Int J Mol Sci. 2019;20(2). doi: 10.3390/ijms20020278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Atia GA, Shalaby HK, Zehravi M, et al. Drug-loaded chitosan scaffolds for periodontal tissue regeneration. Polymers. 2022;14(15):3192. doi: 10.3390/polym14153192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Procopio A, Lagreca E, Jamaledin R, et al. Recent fabrication methods to produce polymer-based drug delivery matrices (Experimental and In Silico Approaches). Pharmaceutics. 2022;14(4):872. doi: 10.3390/pharmaceutics14040872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sherstneva AA, Demina TS, Monteiro APF, Akopova TA, Grandfils C, Ilangala AB. Biodegradable microparticles for regenerative medicine: a state of the art and trends to clinical application. Polymers. 2022;14(7):1314. doi: 10.3390/polym14071314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lu J, Ren B, Wang L, Li M, Liu Y. Preparation and evaluation of IL-1ra-loaded dextran/PLGA microspheres for inhibiting periodontal inflammation in vitro. Inflammation. 2020;43:168–178. doi: 10.1007/s10753-019-01107-w [DOI] [PubMed] [Google Scholar]
- 198.Perez-Puyana V, Jiménez-Rosado M, Romero A, Guerrero A. Polymer-based scaffolds for soft-tissue engineering. Polymers. 2020;12(7):1566. doi: 10.3390/polym12071566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Stratton S, Shelke NB, Hoshino K, Rudraiah S, Kumbar SG. Bioactive polymeric scaffolds for tissue engineering. Bioact Mater. 2016;1(2):93–108. doi: 10.1016/j.bioactmat.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang L, Morsi Y, Wang Y, Li Y, Ramakrishna S. Review scaffold design and stem cells for tooth regeneration. Jpn Dent Sci Rev. 2013;49(1):14–26. doi: 10.1016/j.jdsr.2012.09.001 [DOI] [Google Scholar]
- 201.Albuquerque MTP, Valera MC, Nakashima M, Nör JE, Bottino MC. Tissue-engineering-based strategies for regenerative endodontics. J Dent Res. 2014;93(12):1222–1231. doi: 10.1177/0022034514549809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bottino MC, Kamocki K, Yassen GH, et al. Bioactive nanofibrous scaffolds for regenerative endodontics. J Dent Res. 2013;92(11):963–969. doi: 10.1177/0022034513505770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Baylan N, Bhat S, Ditto M, Lawrence JG, Lecka-Czernik B, Yildirim-Ayan E. Polycaprolactone nanofiber interspersed collagen type-I scaffold for bone regeneration: a unique injectable osteogenic scaffold. Biomed Mater. 2013;8(4):045011. doi: 10.1088/1748-6041/8/4/045011 [DOI] [PubMed] [Google Scholar]
- 204.Tai H, Mather ML, Howard D, et al. Control of pore size and structure of tissue engineering scaffolds produced by supercritical fluid processing. Eur Cell Mater. 2007;14:64–77. doi: 10.22203/eCM.v014a07 [DOI] [PubMed] [Google Scholar]
- 205.Palit MC, Hedge KS, Bhat SS, Sargod SS, Mantha S, Chattopadhyay S. Tissue engineering in endodontics: root canal revascularization. J Clin Pediatr Dent. 2014;38(4):291–297. doi: 10.17796/jcpd.38.4.j5285857278615r1 [DOI] [PubMed] [Google Scholar]
- 206.Barron JA, Krizman DB, Ringeisen BR. Laser printing of single cells: statistical analysis, cell viability, and stress. Ann Biomed Eng. 2005;33:121–130. doi: 10.1007/s10439-005-8971-x [DOI] [PubMed] [Google Scholar]
- 207.Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G, Forgacs G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication. 2010;2(2):022001. doi: 10.1088/1758-5082/2/2/022001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Galler KM, Cavender A, Yuwono V, et al. Self-assembling peptide amphiphile nanofibers as a scaffold for dental stem cells. Tissue Eng Part A. 2008;14(12):2051–2058. doi: 10.1089/ten.tea.2007.0413 [DOI] [PubMed] [Google Scholar]
- 209.Atia GA, Shalaby HK, Ali NG, et al. New challenges and prospective applications of three-dimensional bioactive polymeric hydrogels in oral and craniofacial tissue engineering: a narrative review. Pharmaceuticals. 2023;16(5):702. doi: 10.3390/ph16050702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Pan Y, Zhao Y, Kuang R, et al. Injectable hydrogel-loaded nano-hydroxyapatite that improves bone regeneration and alveolar ridge promotion. Mater Sci Eng. 2020;116:111158. doi: 10.1016/j.msec.2020.111158 [DOI] [PubMed] [Google Scholar]
- 211.Chang H-C, Yang C, Feng F, Lin F-H, Wang C-H, Chang P-C. Bone morphogenetic protein-2 loaded poly (D, L-lactide-co-glycolide) microspheres enhance osteogenic potential of gelatin/hydroxyapatite/β-tricalcium phosphate cryogel composite for alveolar ridge augmentation. J Formos Med Assoc. 2017;116(12):973–981. doi: 10.1016/j.jfma.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 212.Zeng J, Mamitimin M, Song Y, Sun W, Wu Z, Qi X. Chairside administrated antibacterial hydrogels containing berberine as dental temporary stopping for alveolar ridge preservation. Eur Polym J. 2021;160:110808. doi: 10.1016/j.eurpolymj.2021.110808 [DOI] [Google Scholar]
- 213.J-t G, Jiao K, Li J, et al. Polyphosphate-crosslinked collagen scaffolds for hemostasis and alveolar bone regeneration after tooth extraction. Bioact Mater. 2022;15:68–81. doi: 10.1016/j.bioactmat.2021.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Serino G, Biancu S, Iezzi G, Piattelli A. Ridge preservation following tooth extraction using a polylactide and polyglycolide sponge as space filler: a clinical and histological study in humans. Clin Oral Implants Res. 2003;14(5):651–658. doi: 10.1034/j.1600-0501.2003.00970.x [DOI] [PubMed] [Google Scholar]
- 215.Mancini L, Romandini M, Fratini A, Americo LM, Panda S, Marchetti E. Biomaterials for periodontal and peri-implant regeneration. Materials. 2021;14(12):3319. doi: 10.3390/ma14123319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wickramasinghe ML, Dias GJ, Premadasa K. A novel classification of bone graft materials. J Biomed Mater Res B Appl Biomater. 2022;110(7):1724–1749. doi: 10.1002/jbm.b.35029 [DOI] [PubMed] [Google Scholar]
- 217.Nguyen TT, Wu DT, Ramamoorthi M, Syrbu J, Tran SD. Scaffolds for maxillary sinus augmentation. In: Handbook of Tissue Engineering Scaffolds: Volume One. Elsevier; 2019:369–386. [Google Scholar]
- 218.Xun X, Qiu J, Zhang J, et al. Triple-functional injectable liposome–hydrogel composite enhances bacteriostasis and osteo/angio-genesis for advanced maxillary sinus floor augmentation. Colloids Surf B Biointerfaces. 2022;217:112706. doi: 10.1016/j.colsurfb.2022.112706 [DOI] [PubMed] [Google Scholar]
- 219.Cossellu G, Farronato G, Farronato D, Ceschel G, Angiero F. Space-maintaining management in maxillary sinus lifting: a novel technique using a resorbable polymeric thermo-reversible gel. Int J Oral Maxillofac Surg. 2017;46(5):648–654. doi: 10.1016/j.ijom.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 220.Li D, Zhao L, Cong M, et al. Injectable thermosensitive chitosan/gelatin-based hydrogel carried erythropoietin to effectively enhance maxillary sinus floor augmentation in vivo. Dent Mater. 2020;36(7):e229–e240. doi: 10.1016/j.dental.2020.04.016 [DOI] [PubMed] [Google Scholar]
- 221.Al-Nawas B, Schiegnitz E. Augmentation procedures using bone substitute materials or autogenous bone-a systematic review and meta-analysis. Eur J Oral Implantol. 2014;7(Suppl 2):S219–S234. [PubMed] [Google Scholar]
- 222.Bousnaki M, Bakopoulou A, Papadogianni D, et al. Fibro/chondrogenic differentiation of dental stem cells into chitosan/alginate scaffolds towards temporomandibular joint disc regeneration. J Mater Sci Mater Med. 2018;29(7):1–17. [DOI] [PubMed] [Google Scholar]
- 223.Van bellinghen X, Idoux-Gillet Y, Pugliano M, et al. Temporomandibular joint regenerative medicine. Int J Mol Sci. 2018;19(2):446. doi: 10.3390/ijms19020446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Acri TM, Shin K, Seol D, et al. Tissue Engineering for the Temporomandibular Joint. Adv Healthc Mater. 2019;8(2):e1801236. doi: 10.1002/adhm.201801236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Pina S, Ribeiro VP, Marques CF, et al. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials. 2019;12(11):1824. doi: 10.3390/ma12111824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Uematsu K, Hattori K, Ishimoto Y, et al. Cartilage regeneration using mesenchymal stem cells and a three-dimensional poly-lactic-glycolic acid (PLGA) scaffold. Biomaterials. 2005;26(20):4273–4279. doi: 10.1016/j.biomaterials.2004.10.037 [DOI] [PubMed] [Google Scholar]
- 227.Eap S, Morand D, Clauss F, et al. Nanostructured thick 3D nanofibrous scaffold can induce bone. Biomed Mater Eng. 2015;25(s1):79–85. doi: 10.3233/BME-141248 [DOI] [PubMed] [Google Scholar]
- 228.Hamdy TM. Dental biomaterial scaffolds in tooth tissue engineering: a review. Curr Oral Health Rep. 2023;10(1):14–21. doi: 10.1007/s40496-023-00329-0 [DOI] [Google Scholar]
- 229.Ramseier CA, Rasperini G, Batia S, Giannobile WV. Advanced reconstructive technologies for periodontal tissue repair. Periodontol 2000. 2012;59(1):185–202. doi: 10.1111/j.1600-0757.2011.00432.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Stoecklin-Wasmer C, Rutjes AWS, Da Costa BR, Salvi GE, Jüni P, Sculean A. Absorbable collagen membranes for periodontal regeneration: a systematic review. J Dent Res. 2013;92(9):773–781. doi: 10.1177/0022034513496428 [DOI] [PubMed] [Google Scholar]
- 231.Das EC, Babu J, Dhawan S, et al. Synthetic osteogenic matrix using polymeric dendritic peptides for treating human periodontal defects–design and in vitro evaluation. Mater Today. 2019;15:199–216. [Google Scholar]
- 232.Daghrery A, Ferreira JA, Xu J, et al. Tissue-specific melt electrowritten polymeric scaffolds for coordinated regeneration of soft and hard periodontal tissues. Bioact Mater. 2023;19:268–281. doi: 10.1016/j.bioactmat.2022.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Dascălu C-A, Maidaniuc A, Pandele AM, et al. Synthesis and characterization of biocompatible polymer-ceramic film structures as favorable interface in guided bone regeneration. Appl Surf Sci. 2019;494:335–352. doi: 10.1016/j.apsusc.2019.07.098 [DOI] [Google Scholar]
- 234.Shang L, Liu Z, Ma B, et al. Dimethyloxallyl glycine/nanosilicates-loaded osteogenic/angiogenic difunctional fibrous structure for functional periodontal tissue regeneration. Bioact Mater. 2021;6(4):1175–1188. doi: 10.1016/j.bioactmat.2020.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jamalpour MR, Yadegari A, Vahdatinia F, et al. 3D-printed bi-layered polymer/hydrogel construct for interfacial tissue regeneration in a canine model. Dent Mater. 2022;38(8):1316–1329. doi: 10.1016/j.dental.2022.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Momose T, Miyaji H, Kato A, et al. Collagen hydrogel scaffold and fibroblast growth factor-2 accelerate periodontal healing of class II furcation defects in dog. Open Dent J. 2016;10:347. doi: 10.2174/1874210601610010347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Nakamura S, Kubo T, Ijima H. Heparin-conjugated gelatin as a growth factor immobilization scaffold. J Biosci Bioeng. 2013;115(5):562–567. doi: 10.1016/j.jbiosc.2012.11.011 [DOI] [PubMed] [Google Scholar]
- 238.Varoni EM, Vijayakumar S, Canciani E, et al. Chitosan-based trilayer scaffold for multitissue periodontal regeneration. J Dent Res. 2018;97(3):303–311. doi: 10.1177/0022034517736255 [DOI] [PubMed] [Google Scholar]
- 239.Shang S, Yang F, Cheng X, Walboomers XF, Jansen JA. The effect of electrospun fibre alignment on the behaviour of rat periodontal ligament cells; 2010. [DOI] [PubMed]
- 240.Batool F, Morand D-N, Thomas L, et al. Synthesis of a novel electrospun polycaprolactone scaffold functionalized with ibuprofen for periodontal regeneration: an in vitro and in vivo study. Materials. 2018;11(4):580. doi: 10.3390/ma11040580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Reis ECC, Borges APB, Araújo MVF, Mendes VC, Guan L, Davies JE. Periodontal regeneration using a bilayered PLGA/calcium phosphate construct. Biomaterials. 2011;32(35):9244–9253. doi: 10.1016/j.biomaterials.2011.08.040 [DOI] [PubMed] [Google Scholar]
- 242.Liu Z, Yin X, Ye Q, et al. Periodontal regeneration with stem cells-seeded collagen-hydroxyapatite scaffold. J Biomater Appl. 2016;31(1):121–131. doi: 10.1177/0885328216637978 [DOI] [PubMed] [Google Scholar]
- 243.Liao F, Chen Y, Li Z, et al. A novel bioactive three-dimensional β-tricalcium phosphate/chitosan scaffold for periodontal tissue engineering. J Mater Sci Mater Med. 2010;21:489–496. doi: 10.1007/s10856-009-3931-x [DOI] [PubMed] [Google Scholar]
- 244.Costa PF, Vaquette C, Zhang Q, Reis RL, Ivanovski S, Hutmacher DW. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J Clin Periodontol. 2014;41(3):283–294. doi: 10.1111/jcpe.12214 [DOI] [PubMed] [Google Scholar]
- 245.Sowmya S, Mony U, Jayachandran P, et al. Tri‐layered nanocomposite hydrogel scaffold for the concurrent regeneration of cementum, periodontal ligament, and alveolar bone. Adv Healthcare Mater. 2017;6(7):1601251. doi: 10.1002/adhm.201601251 [DOI] [PubMed] [Google Scholar]
- 246.Lee CH, Hajibandeh J, Suzuki T, Fan A, Shang P, Mao JJ. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng Part A. 2014;20(7–8):1342–1351. doi: 10.1089/ten.tea.2013.0386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zafar MS, Fareed MA, Riaz S, Latif M, Habib SR, Khurshid Z. Customized therapeutic surface coatings for dental implants. Coatings. 2020;10(6):568. doi: 10.3390/coatings10060568 [DOI] [Google Scholar]
- 248.Gaviria L, Salcido JP, Guda T, Ong JL. Current trends in dental implants. J Korean Assoc Oral Maxillofac Surg. 2014;40(2):50. doi: 10.5125/jkaoms.2014.40.2.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Accioni F, Vázquez J, Merinero M, Begines B, Alcudia A. Latest trends in surface modification for dental implantology: innovative developments and analytical applications. Pharmaceutics. 2022;14(2):455. doi: 10.3390/pharmaceutics14020455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ansari Z, Kalantar M, Kharaziha M, Ambrosio L, Raucci MG. Polycaprolactone/fluoride substituted-hydroxyapatite (PCL/FHA) nanocomposite coatings prepared by in-situ sol-gel process for dental implant applications. Prog Org Coat. 2020;147:105873. doi: 10.1016/j.porgcoat.2020.105873 [DOI] [Google Scholar]
- 251.Joo H, Park J, Sutthiwanjampa C, et al. Surface coating with hyaluronic acid-gelatin-crosslinked hydrogel on gelatin-conjugated poly (dimethylsiloxane) for implantable medical device-induced fibrosis. Pharmaceutics. 2021;13(2):269. doi: 10.3390/pharmaceutics13020269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Catauro M, Bollino F, Giovanardi R, Veronesi P. Modification of Ti6Al4V implant surfaces by biocompatible TiO2/PCL hybrid layers prepared via sol-gel dip coating: structural characterization, mechanical and corrosion behavior. Mater Sci Eng. 2017;74:501–507. doi: 10.1016/j.msec.2016.12.046 [DOI] [PubMed] [Google Scholar]
- 253.Clifford A, D’Elia A, Deering J, Lee BEJ, Grandfield K, Zhitomirsky I. Electrochemical fabrication and characterization of pectin hydrogel composite materials for bone tissue repair. ACS Appl Polymer Mater. 2020;2(8):3390–3396. doi: 10.1021/acsapm.0c00480 [DOI] [Google Scholar]
- 254.Barkarmo S, Wennerberg A, Hoffman M, et al. Nano‐hydroxyapatite‐coated PEEK implants: a pilot study in rabbit bone. J Biomed Mater Res A. 2013;101(2):465–471. doi: 10.1002/jbm.a.34358 [DOI] [PubMed] [Google Scholar]
- 255.Kulkarni Aranya A, Pushalkar S, Zhao M, LeGeros RZ, Zhang Y, Saxena D. Antibacterial and bioactive coatings on titanium implant surfaces. J Biomed Mater Res A. 2017;105(8):2218–2227. doi: 10.1002/jbm.a.36081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wang M, Wang C, Zhang Y, Lin Y. Controlled release of dopamine coatings on titanium bidirectionally regulate osteoclastic and osteogenic response behaviors. Mater Sci Eng. 2021;129:112376. doi: 10.1016/j.msec.2021.112376 [DOI] [PubMed] [Google Scholar]
- 257.Zheng S, Zhong H, Cheng H, et al. Engineering multifunctional hydrogel with osteogenic capacity for critical-size segmental bone defect repair. Front Bioeng Biotechnol. 2022;10:899457. doi: 10.3389/fbioe.2022.899457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Song W, Xiao Y. Sequential drug delivery of vancomycin and rhBMP-2 via pore-closed PLGA microparticles embedded photo-crosslinked chitosan hydrogel for enhanced osteointegration. Int J Biol Macromol. 2021;182:612–625. doi: 10.1016/j.ijbiomac.2021.03.181 [DOI] [PubMed] [Google Scholar]
- 259.Campbell DI, Duncan WJ. The effect of a keratin hydrogel coating on osseointegration: an histological comparison of coated and non-coated dental titanium implants in an ovine model. J Maxillofac Oral Surg. 2014;13:159–164. doi: 10.1007/s12663-013-0482-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Cheng H, Yue K, Kazemzadeh-Narbat M, et al. Mussel-inspired multifunctional hydrogel coating for prevention of infections and enhanced osteogenesis. ACS Appl Mater Interfaces. 2017;9(13):11428–11439. doi: 10.1021/acsami.6b16779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Naidu MUR, Ramana GV, Rani PU, Suman A, Roy P, Roy P. Chemotherapy-induced and/or radiation therapy-induced oral mucositis-complicating the treatment of cancer. Neoplasia. 2004;6(5):423–431. doi: 10.1593/neo.04169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mehravaran M, Haeri A, Rabbani S, Mortazavi SA, Torshabi M. Preparation and characterization of benzydamine hydrochloride-loaded lyophilized mucoadhesive wafers for the treatment of oral mucositis. J Drug Deliv Sci Technol. 2022;78:103944. doi: 10.1016/j.jddst.2022.103944 [DOI] [Google Scholar]
- 263.Pornpitchanarong C, Rojanarata T, Opanasopit P, Ngawhirunpat T, Bradley M, Patrojanasophon P. Maleimide-functionalized carboxymethyl cellulose: a novel mucoadhesive polymer for transmucosal drug delivery. Carbohydr Polym. 2022;288:119368. doi: 10.1016/j.carbpol.2022.119368 [DOI] [PubMed] [Google Scholar]
- 264.Chin H-L, Chung K-P, Liu H-C, Chen R-S, Chang -H-H, Chen M-H. Efficacy of polyacrylate silver salt/polyvinylpyrrolidone-based liquid oral gel in management of concurrence chemoradiotherapy-induced oral mucositis. J Formos Med Assoc. 2023;122:723–730. doi: 10.1016/j.jfma.2022.12.007 [DOI] [PubMed] [Google Scholar]
- 265.Feng X, Lu X, Huang D, et al. 3D porous chitosan scaffolds suit survival and neural differentiation of dental pulp stem cells. Cell Mol Neurobiol. 2014;34:859–870. doi: 10.1007/s10571-014-0063-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Moussa DG, Aparicio C. Present and future of tissue engineering scaffolds for dentin‐pulp complex regeneration. J Tissue Eng Regen Med. 2019;13(1):58–75. doi: 10.1002/term.2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ishimatsu H, Kitamura C, Morotomi T, et al. Formation of dentinal bridge on surface of regenerated dental pulp in dentin defects by controlled release of fibroblast growth factor–2 from gelatin hydrogels. J Endod. 2009;35(6):858–865. doi: 10.1016/j.joen.2009.03.049 [DOI] [PubMed] [Google Scholar]
- 268.Shivashankar VY, Johns DA, Vidyanath S, Kumar MR. Platelet rich fibrin in the revitalization of tooth with necrotic pulp and open apex. J Conserv Dent. 2012;15(4):395. doi: 10.4103/0972-0707.101926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Huang FM, Yang SF, Zhao JH, Chang YC. Platelet-rich fibrin increases proliferation and differentiation of human dental pulp cells. J Endod. 2010;36(10):1628–1632. doi: 10.1016/j.joen.2010.07.004 [DOI] [PubMed] [Google Scholar]
- 270.Jiang X, Liu H, Peng C. Clinical and radiographic assessment of the efficacy of a collagen membrane in regenerative endodontics: a randomized, controlled clinical trial. J Endod. 2017;43(9):1465–1471. doi: 10.1016/j.joen.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 271.Yang KC, Wang CH, Chang HH, Chan WP, Chi CH, Kuo TF. Fibrin glue mixed with platelet‐rich fibrin as a scaffold seeded with dental bud cells for tooth regeneration. J Tissue Eng Regen Med. 2012;6(10):777–785. doi: 10.1002/term.483 [DOI] [PubMed] [Google Scholar]
- 272.Wieckiewicz M, W Boening K, Grychowska N, Paradowska-Stolarz A. Clinical application of chitosan in dental specialities. Mini Rev Med Chem. 2017;17(5):401–409. doi: 10.2174/1389557516666160418123054 [DOI] [PubMed] [Google Scholar]
- 273.Nayef L, Mekhail M, Benameur L, Rendon JS, Hamdy R, Tabrizian M. A combinatorial approach towards achieving an injectable, self-contained, phosphate-releasing scaffold for promoting biomineralization in critical size bone defects. Acta biomater. 2016;29:389–397. doi: 10.1016/j.actbio.2015.10.020 [DOI] [PubMed] [Google Scholar]
- 274.Junka A, Bartoszewicz M, Dziadas M, et al. Application of bacterial cellulose experimental dressings saturated with gentamycin for management of bone biofilm in vitro and ex vivo. J Biomed Mater Res B Appl Biomater. 2020;108(1):30–37. doi: 10.1002/jbm.b.34362 [DOI] [PubMed] [Google Scholar]
- 275.Pankaj D, Sahu I, Kurian IG, Pradeep AR. Comparative evaluation of subgingivally delivered 1.2% rosuvastatin and 1% metformin gel in treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol. 2018;89(11):1318–1325. doi: 10.1002/jper.17-0434 [DOI] [PubMed] [Google Scholar]
- 276.Abrahamse H, Hamblin MR. New photosensitizers for photodynamic therapy. Biochem J. 2016;473(4):347–364. doi: 10.1042/bj20150942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Elavarasu S, Suthanthiran TK, Naveen D. Statins: a new era in local drug delivery. J Pharm Bioallied Sci. 2012;4(Suppl 2):S248–51. doi: 10.4103/0975-7406.100225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Javed F, Al-Askar M, Al-Rasheed A, Al-Hezaimi K. Significance of the platelet-derived growth factor in periodontal tissue regeneration. Arch Oral Biol. 2011;56(12):1476–1484. doi: 10.1016/j.archoralbio.2011.06.020 [DOI] [PubMed] [Google Scholar]
- 279.Murakami S. Periodontal tissue regeneration by signaling molecule(s): what role does basic fibroblast growth factor (FGF-2) have in periodontal therapy? Periodontol 2000. 2011;56(1):188–208. doi: 10.1111/j.1600-0757.2010.00365.x [DOI] [PubMed] [Google Scholar]
- 280.Anusuya GS, Kandasamy M, Jacob Raja SA, Sabarinathan S, Ravishankar P, Kandhasamy B. Bone morphogenetic proteins: signaling periodontal bone regeneration and repair. J Pharm Bioallied Sci. 2016;8(Suppl 1):S39–s41. doi: 10.4103/0975-7406.191964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Abourehab MAS, Rajendran RR, Singh A, et al. Alginate as a promising biopolymer in drug delivery and wound healing: a review of the state-of-the-art. Int J Mol Sci. 2022;23(16):9035. doi: 10.3390/ijms23169035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Venkatesan J, Lee J-Y, Kang DS, et al. Antimicrobial and anticancer activities of porous chitosan-alginate biosynthesized silver nanoparticles. Int J Biol Macromol. 2017;98:515–525. doi: 10.1016/j.ijbiomac.2017.01.120 [DOI] [PubMed] [Google Scholar]
- 283.Dong L, Wang SJ, Zhao XR, Zhu YF, Yu JK. 3D- printed poly(ε-caprolactone) scaffold integrated with cell-laden chitosan hydrogels for bone tissue engineering. Sci Rep. 2017;7(1):13412. doi: 10.1038/s41598-017-13838-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Ghanbari M, Salavati-Niasari M, Mohandes F. Thermosensitive alginate–gelatin–nitrogen-doped carbon dots scaffolds as potential injectable hydrogels for cartilage tissue engineering applications. RSC Adv. 2021;11(30):18423–18431. doi: 10.1039/D1RA01496J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Santo VE, Frias AM, Carida M, et al. Carrageenan-based hydrogels for the controlled delivery of PDGF-BB in bone tissue engineering applications. Biomacromolecules. 2009;10(6):1392–1401. doi: 10.1021/bm8014973 [DOI] [PubMed] [Google Scholar]
- 286.Kim S, Kang Y, Mercado-Pagán ÁE, Maloney WJ, Yang Y. In vitro evaluation of photo-crosslinkable chitosan-lactide hydrogels for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2014;102(7):1393–1406. doi: 10.1002/jbm.b.33118 [DOI] [PubMed] [Google Scholar]
- 287.Kumar A, Han SS. Enhanced mechanical, biomineralization, and cellular response of nanocomposite hydrogels by bioactive glass and halloysite nanotubes for bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2021;128:112236. doi: 10.1016/j.msec.2021.112236 [DOI] [PubMed] [Google Scholar]
- 288.Wang K, Cheng W, Ding Z, et al. Injectable silk/hydroxyapatite nanocomposite hydrogels with vascularization capacity for bone regeneration. J Mater Sci Technol. 2020;2020:1. [Google Scholar]
- 289.Hou R, Zhang G, Du G, et al. Magnetic nanohydroxyapatite/PVA composite hydrogels for promoted osteoblast adhesion and proliferation. Colloids Surf B Biointerfaces. 2013;103:318–325. doi: 10.1016/j.colsurfb.2012.10.067 [DOI] [PubMed] [Google Scholar]
- 290.Duarte Campos DF, Blaeser A, Korsten A, et al. The stiffness and structure of three-dimensional printed hydrogels direct the differentiation of mesenchymal stromal cells toward adipogenic and osteogenic lineages. Tissue Eng Part A. 2015;21(3–4):740–756. doi: 10.1089/ten.TEA.2014.0231 [DOI] [PubMed] [Google Scholar]
- 291.Mishra D, Bhunia BK, Banerjee I, et al. Enzymatically crosslinked carboxymethyl–chitosan/gelatin/nano-hydroxyapatite injectable gels for in situ bone tissue engineering application. Mater Sci Eng. 2011;31:1295–1304. doi: 10.1016/j.msec.2011.04.007 [DOI] [Google Scholar]
- 292.Qasim M, Chae DS, NYJIjon L. Advancements and frontiers in nano-based 3D and 4D scaffolds for bone and cartilage tissue engineering. Int J Nanomedicine. 2019;14:4333. doi: 10.2147/IJN.S209431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Christy PN, Basha SK, Kumari VS. Nano zinc oxide and nano bioactive glass reinforced chitosan/poly(vinyl alcohol) scaffolds for bone tissue engineering application. Mater Today Commun. 2022;31:103429. doi: 10.1016/j.mtcomm.2022.103429 [DOI] [Google Scholar]
- 294.Han J, Ma B, Liu H, et al. Hydroxyapatite nanowires modified polylactic acid membrane plays barrier/osteoinduction dual roles and promotes bone regeneration in a rat mandible defect model. J Biomed Mater Res A. 2018;106(12):3099–3110. doi: 10.1002/jbm.a.36502 [DOI] [PubMed] [Google Scholar]
- 295.Alkhursani SA, Ghobashy MM, Alshangiti DM, et al. Plastic waste management and safety disinfection processes for reduced the COVID-19 Hazards. Int J Sustain Eng. 2023;16(1):1–14. doi: 10.1080/19397038.2023.2188396 [DOI] [Google Scholar]
- 296.Alkhursani SA, Ghobashy MM, Al-Gahtany SA, et al. Application of nano-inspired scaffolds-based biopolymer hydrogel for bone and periodontal tissue regeneration. Polymers. 2022;14(18):3791. doi: 10.3390/polym14183791 [DOI] [PMC free article] [PubMed] [Google Scholar]