Abstract
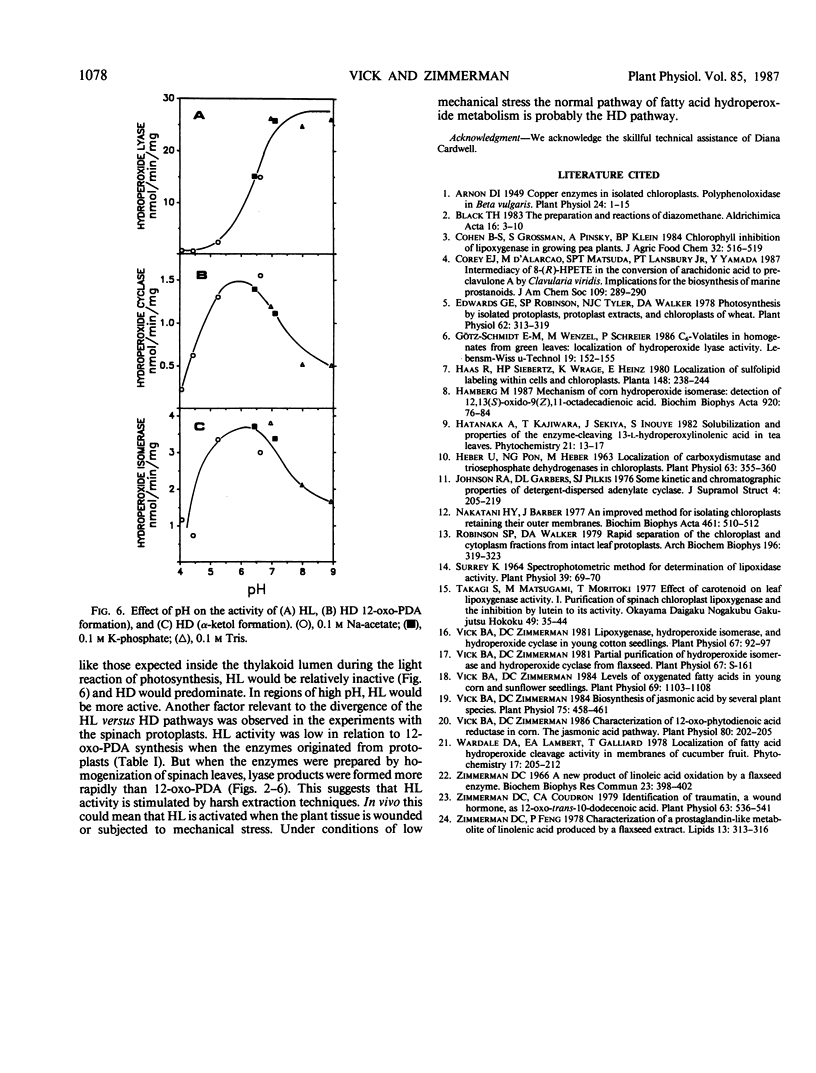
The metabolism of 13-hydroperoxylinolenic acid was examined in protoplasts and homogenates prepared from mature leaves of spinach (Spinacia oleracea L.). Chloroplast membranes were the principal site for metabolism of the compound by at least two highly hydrophobic enzyme systems, hydroperoxide lyase and hydroperoxide dehydrase, the new name for an enzyme system formerly known as hydroperoxide isomerase and hydroperoxide cyclase. Hydroperoxide lyase was most active above pH 7 and could be separated from hydroperoxide dehydrase by anion exchange chromatography. Hydroperoxide dehydrase, measured by the formation of both α-ketol product and 12-oxo-phytodienoic acid, had its optimum activity in the range of pH 5 to 7. Lyase was more active than dehydrase activity when the enzymes were extracted by homogenization. The reverse was true when the enzyme activities were measured in protoplasts, which are isolated by gentle extraction methods. The variation in enzyme activity ratios with extraction methods suggests that hydroperoxide lyase is activated by plant injury and thus may function in a wound response. In the absence of injury, the normal pathway of fatty acid hydroperoxide metabolism is probably by hydroperoxide dehydrase activity. The molecular weights of both the lyase and dehydrase were approximately 220,000, as estimated by gel filtration.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Robinson S. P., Tyler N. J., Walker D. A. Photosynthesis by isolated protoplasts, protoplast extracts, and chloroplasts of wheat: influence of orthophosphate, pyrophosphate, and adenylates. Plant Physiol. 1978 Aug;62(2):313–319. doi: 10.1104/pp.62.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Pon N. G., Heber M. Localization of Carboxydismutase & Triosephosphate Dehydrogenases in Chloroplasts. Plant Physiol. 1963 May;38(3):355–360. doi: 10.1104/pp.38.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Garbers D. L., Pilkis S. J. Some kinetic and chromatographic properties of detergent-dispersed adenylate cyclase. J Supramol Struct. 1976;4(2):205–219. doi: 10.1002/jss.400040208. [DOI] [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani H. Y., Barber J. An improved method for isolating chloroplasts retaining their outer membranes. Biochim Biophys Acta. 1977 Sep 14;461(3):500–512. [PubMed] [Google Scholar]
- Robinson S. P., Walker D. A. Rapid separation of the chloroplast and cytoplasmic fractions from intact leaf protoplasts. Arch Biochem Biophys. 1979 Sep;196(2):319–323. doi: 10.1016/0003-9861(79)90584-8. [DOI] [PubMed] [Google Scholar]
- Surrey K. Spectrophotometric Method for Determination of Lipoxidase Activity. Plant Physiol. 1964 Jan;39(1):65–70. doi: 10.1104/pp.39.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. Biosynthesis of jasmonic Acid by several plant species. Plant Physiol. 1984 Jun;75(2):458–461. doi: 10.1104/pp.75.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. Characterization of 12-oxo-phytodienoic Acid reductase in corn: the jasmonic Acid pathway. Plant Physiol. 1986 Jan;80(1):202–205. doi: 10.1104/pp.80.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. Levels of oxygenated Fatty acids in young corn and sunflower plants. Plant Physiol. 1982 May;69(5):1103–1108. doi: 10.1104/pp.69.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. Lipoxygenase, hydroperoxide isomerase, and hydroperoxide cyclase in young cotton seedlings. Plant Physiol. 1981 Jan;67(1):92–97. doi: 10.1104/pp.67.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenker H., Brandt H. P. Netzhautarterienverschluss und Wetter. Klin Monbl Augenheilkd. 1966;148(2):238–244. [PubMed] [Google Scholar]
- Zimmerman D. C. A new product of linoleic acid oxidation by a flaxseed enzyme. Biochem Biophys Res Commun. 1966 May 25;23(4):398–402. doi: 10.1016/0006-291x(66)90740-6. [DOI] [PubMed] [Google Scholar]
- Zimmerman D. C., Coudron C. A. Identification of Traumatin, a Wound Hormone, as 12-Oxo-trans-10-dodecenoic Acid. Plant Physiol. 1979 Mar;63(3):536–541. doi: 10.1104/pp.63.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]


