Abstract
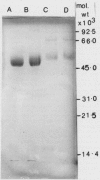
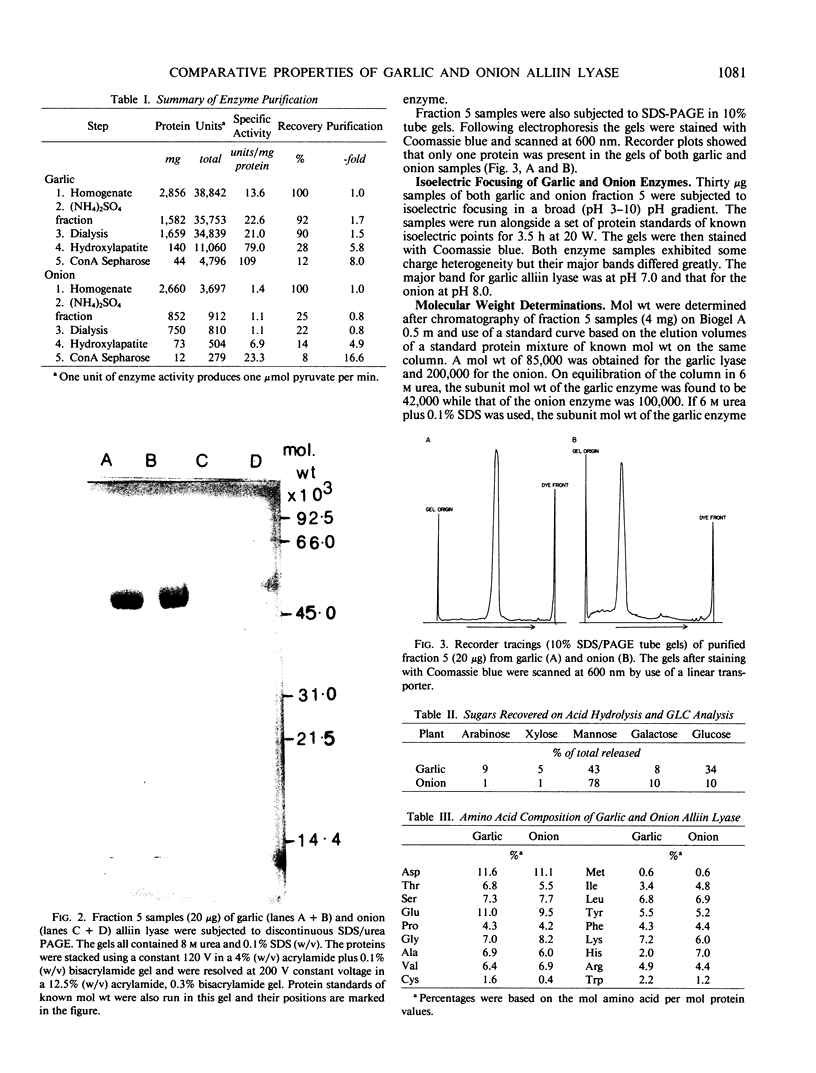
Garlic and onion alliin lyases, although from closely related species, have many differences. The two enzymes differ in their Km values, pH optima, and isoelectric points. There is a major difference in their molecular weight and subunit structure. The garlic holoenzyme has a molecular weight of 85,000 and consists of two subunits of molecular weight 42,000. The onion enzyme has a holoenzyme molecular weight of 200,000 composed of four subunits of molecular weight 50,000. The onion enzyme is much more difficult to dissociate into its subunits which suggests differences in subunit interaction between the two enzymes. The dimeric stucture of the garlic and the tetrameric structure of the onion enzyme is consistent with a coenzyme content (pyridoxal-5′-phosphate) equivalent to one mole per subunit. The two enzymes vary vastly in their spectra, the onion enzyme having a lower pyridoxal-5′-phosphate absorbance at 430 nanomoles and an inability to react with l-cysteine. Both enzymes are glycoproteins and bind to concanavalin A-Sepharose columns. The onion alliin lyase binds more tightly than the garlic enzyme. The amino acid content of both enzymes is similar as is the carbohydrate content. However, upon hydrolysis the onion lyase does yield more mannose units than the garlic enzyme which is consistent with the former's stronger affinity for concanavalin A.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter W. G., Etzler M. E. Isolation and characterization of cyanogen bromide fragments and a glycopeptide from the Dolichos biflorus lectin. Biochemistry. 1975 Nov 18;14(23):5118–5122. doi: 10.1021/bi00694a015. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- FOWDEN L. THE CHEMISTRY AND METABOLISM OF RECENTLY ISOLATED AMINO ACIDS. Annu Rev Biochem. 1964;33:173–204. doi: 10.1146/annurev.bi.33.070164.001133. [DOI] [PubMed] [Google Scholar]
- Giulian G. G., Moss R. L., Greaser M. Analytical isoelectric focusing using a high-voltage vertical slab polyacrylamide gel system. Anal Biochem. 1984 Nov 1;142(2):421–436. doi: 10.1016/0003-2697(84)90486-x. [DOI] [PubMed] [Google Scholar]
- Hamamoto A., Mazelis M. The C-S Lyases of Higher Plants : Isolation and Properties of Homogeneous Cystine Lyase from Broccoli (Brassica oleracea var botrytis) Buds. Plant Physiol. 1986 Mar;80(3):702–706. doi: 10.1104/pp.80.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Nevins D. J. Enzymic Dissociation of Zea Shoot Cell Wall Polysaccharides : II. Dissociation of (1 --> 3),(1 --> 4)-beta-d-Glucan by Purified (1 --> 3),(1 --> 4)-beta-d-Glucan 4-Glucanohydrolase from Bacillus subtilis. Plant Physiol. 1984 Jul;75(3):745–752. doi: 10.1104/pp.75.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazarian R. A., Goriachenkova E. V. Alliinase: ochistka i osnovnye fiziko-khimicheskie svoistva. Biokhimiia. 1978 Oct;43(10):1905–1913. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mazelis M., Creveling R. K. Purification and properties of S-alkyl-L-cysteine lyase from seedlings of Acacia farnesiana Willd. Biochem J. 1975 Jun;147(3):485–491. doi: 10.1042/bj1470485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelis M., Crews L. Purification of the alliin lyase of garlic, Allium sativum L. Biochem J. 1968 Aug;108(5):725–730. doi: 10.1042/bj1080725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Dunau M. L., Goldman D. A rapid sensitive silver stain for polypeptides in polyacrylamide gels. Anal Biochem. 1981 Jan 1;110(1):201–207. doi: 10.1016/0003-2697(81)90136-6. [DOI] [PubMed] [Google Scholar]
- Nock L. P., Mazelis M. The C-S lyases of higher plants: preparation and properties of homogeneous alliin lyase from garlic (Allium sativum). Arch Biochem Biophys. 1986 Aug 15;249(1):27–33. doi: 10.1016/0003-9861(86)90556-4. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Comparative biochemistry of plant glycoproteins. Biochem Soc Trans. 1979 Aug;7(4):783–799. doi: 10.1042/bst0070783. [DOI] [PubMed] [Google Scholar]
- Tobkin H. E., Jr, Mazelis M. Alliin lyase: preparation and characterization of the homogeneous enzyme from onion bulbs. Arch Biochem Biophys. 1979 Mar;193(1):150–157. doi: 10.1016/0003-9861(79)90018-3. [DOI] [PubMed] [Google Scholar]
- WADA H., SNELL E. E. The enzymatic oxidation of pyridoxine and pyridoxamine phosphates. J Biol Chem. 1961 Jul;236:2089–2095. [PubMed] [Google Scholar]