Abstract
Purpose
Post-inflammatory hyperpigmentation (PIH) and solar lentigines are dark spots of skin from excessive melanin production due to injury or UV exposure. This 12-week single-center study assessed the efficacy and tolerability of a novel targeted pigment-correcting spot treatment gel suspension cream (Dark Spot Treatment) for improving mild-to-moderate PIH or solar lentigines.
Patients and Methods
Female participants (N = 41) aged 25–65 with mild-to-moderate facial dark spots applied Dark Spot Treatment daily for 12 weeks. Investigators assessed overall hyperpigmentation, skin tone evenness, and dark spot intensity, contrast, and size at Weeks 2, 4, 8, and 12. Participant self-assessments occurred at Weeks 1, 2, 4, 8, and 12. Tolerability was assessed by clinical grading and participant reporting.
Results
Dark Spot Treatment improved overall hyperpigmentation, skin tone evenness, and dark spot intensity and contrast at Weeks 2 through 12, and dark spot size at Weeks 4 through 12 (all p < 0.001 compared to baseline). Participant self-assessments showed high overall satisfaction. Dark Spot Treatment was well tolerated.
Conclusion
The novel pigment-correcting Dark Spot Treatment significantly improved the appearance of PIH and solar lentigines, had high participant satisfaction, and was well tolerated.
Keywords: post-inflammatory hyperpigmentation, age spots, solar lentigines, Dark Spot Treatment
Introduction
Hyperpigmentation of the skin is a dermatological condition where the color of the skin becomes darker due to excess melanin. This can occur in response to a variety of internal factors, such as hormonal changes, inflammation, or dermatoses (eg, acne, eczema, atopic dermatitis), or external factors, such as injury, specific medications/procedures, or UV exposure.1,2 The etiology of hyperpigmentation is complex and not entirely understood. Hyperpigmentation is the result of multiple factors leading to an increase in melanocyte activation, melanosome development, melanin production, and melanin transfer to surrounding keratinocytes that subsequently form dark spots.1,3,4 Post-inflammatory hyperpigmentation (PIH) and solar lentigines (“age spots”) are two forms of skin hyperpigmentation. PIH results from an overproduction of melanin in response to injury or inflammation, and presents as irregular shaped, darkly pigmented spots at the site of the injury/inflammation.2 Solar lentigines result from a similar overproduction of melanin in response to acute or chronic UV exposure. It is typically associated with older age and is located on body areas with high sun exposure, such as the face and hands.5
PIH is most common in darker skin tones (Fitzpatrick skin types III–VI), where it occurs with greater frequency and severity and is less likely to resolve quickly.3,6 Prevalence of PIH has been estimated in White/Caucasian, Hispanic, and Black/African American individuals at 25%, 48%, and 65%, respectively.7 Solar lentigines due to sun exposure are more likely to occur, and at a younger age, in individuals with lighter skin tones (Fitzpatrick skin types I–II).8 This is due to the lower amount of melanin in lighter skin tones, which increases susceptibility to UV-induced damage.8,9 Solar lentigines are estimated to occur in ~90% of individuals over 70 years of age.10
Both PIH and solar lentigines have no oncogenic potential and may resolve on their own over time. However, both dermatological conditions can significantly impact quality of life (QoL), affecting psychological and emotional well-being.1,3,11,12 Individuals with facial blemishes, including PIH and solar lentigines, may have feelings of isolation and loneliness, and report a negative impact on professional and social interactions. Strong correlations have been found between individuals with facial blemishes and a fear of negative perceptions and lower health-related QoL (HRQoL). A perception of better QoL without a facial blemish was a strong predictor of current overall HRQoL, suggesting that the presence of facial blemishes is the main driver of reduced HRQoL. Due to the negative impact of these conditions on QoL, individuals may choose to treat PIH or solar lentigines to reduce the hyperpigmentation of the dark spots.13
There are currently multiple treatment strategies for PIH and solar lentigines. The initial approach is proactive management by avoiding sun exposure and regularly applying sun protection. Current treatments may include topicals (eg, hydroquinone, retinoids, steroids), cryotherapy, chemical peels (eg, glycolic acid, salicylic acid), or laser therapy.5,12 However, many of these treatments are associated with negative side effects. Hydroquinone has been found to be effective for PIH and solar lentigines but is relatively slow to work and can cause hypersensitivity and acneiform eruptions.2,14,15 Retinoid treatment may reduce hyperpigmentation, but current evidence is limited on optimal combinations with other treatments.2,15 Chemical peels and cryotherapy may result in significant lightening, but long-term improvement rates are unclear, and recurrence is common. Cryotherapy has also been associated with pain and a risk of hyperpigmentation.2,15 Laser therapy has demonstrated efficacy for hyperpigmentation, but repeated procedures are more likely to cause PIH, especially in darker skin types.2,16,17 Current recommended regimens for both PIH and solar lentigines include combinations of these treatments to address the underlying hyperpigmentation.2
There is a current need for tolerable treatments to reduce hyperpigmentation due to PIH and solar lentigines and address the negative impact these dermatological conditions can have on QoL. Therefore, we conducted a 12-week, single-center clinical study in a diverse cohort of female participants with mild-to-moderate facial hyperpigmentation to assess the efficacy and tolerability of a targeted pigment-correcting spot treatment gel suspension cream (Dark Spot Tx [Even & Correct Dark Spot Cream, SkinMedica®, Allergan Aesthetics, an AbbVie Company, Irvine, CA, USA]). Dark Spot Tx combines a proprietary blend of lotus sprout extract, tranexamic acid, niacinamide, retinol, glycolic acid, vitamin C (tetrahexyldecyl ascorbate), kojic acid (kojic dipalmitate), and other ingredients, and is designed to address the multiple pathways involved in melanogenesis and reduce hyperpigmentation (Table 1).1,18–20
Table 1.
Target Pathways in the Melanin Life Cycle and Key Ingredients in Dark Spot Treatment
| Melanin Life Cycle Pathway | Dark Spot Treatment Ingredients |
|---|---|
| Melanocyte activation | Tranexamic acid |
| Niacinamide | |
| Vitamin C (tetrahexyldecyl ascorbate) | |
| Melanosome development | Lotus sprout extract |
| Melanin production | Lotus sprout extract |
| Phenylethyl resorcinol | |
| Kojic acid (kojic dipalmitate) | |
| Vitamin C (tetrahexyldecyl ascorbate) | |
| Melanin distribution | Niacinamide |
| Melanosome degradation | Lotus sprout extract |
| Melanin removal | Retinol |
| Glycolic acid |
Materials and Methods
Study Design
This 12-week single-center clinical study enrolled female participants aged 25–65 years with mild-to-moderate facial dark spots (score of 3–6 on a modified Griffiths scale). Female participants with Fitzpatrick skin types III–VI with at least 1 PIH spot ≥3 mm on the face were enrolled into a PIH subgroup. This subgroup was required to enroll at least 75% Black/African American, Asian, or Hispanic Caucasian participants. The solar lentigines subgroup enrolled female participants with Fitzpatrick skin types I–III and at least 1 age spot/solar lentigines ≥3 mm on the face. This subgroup was required to enroll approximately 50% Asian or Hispanic Caucasian participants. All study participants were also required to have not used facial treatments in the previous 6 months.
Exclusion criteria included known allergies to facial skin care products; history of skin cancer in the previous 5 years; breastfeeding, pregnant, or planning on becoming pregnant; current or previous use of oral/topical prescription acne medications; Accutane® within 6 months; Avita®, Differin®, Renova®, Retin-A®, Retin-A-Micro®, Soriatane®, or Tazorac® within 3 months; prescription strength skin lightening products within 3 months; over the counter (OTC) retinol-containing or other topical or systemic products within 4 weeks; or other OTC or other topical or systemic products known to affect skin aging or dyschromia within 2 weeks.
Treatment
All participants applied a once-daily treatment of Dark Spot Tx in the evening to only the dark facial spots, avoiding normal skin, for 12 weeks. All participants were also provided a basic skincare regimen to apply to the entire face (Facial Cleanser, Ultra Sheer Moisturizer [morning/evening], and Essential Defense Mineral Shield SPF 35 [morning and reapply as needed throughout the day], SkinMedica, Allergan Aesthetics, an AbbVie Company, Irvine, CA, USA). Participants avoided application of any topical moisturizing products or other treatment products to the face for at least 3 days prior to visit 1.
Assessments
Investigators performed clinical grading of the full face at baseline and Weeks 2, 4, 8, and 12 using the modified Griffiths 10-point scale for overall hyperpigmentation, skin tone evenness, and dark spot intensity, contrast, and size. Lower scores on this scale indicate improvement (0 = none, 1–3 = mild, 4–6 = moderate, 7–9 = severe). Standardized digital photographs were taken of the participants’ full faces (left, front, right) using a VISIA-CR photo station with a Canon Mark II digital SLR camera (Canfield Scientific, Parsippany, NJ, USA). Quantitative analysis of target dark spot skin brightness (L*) was evaluated using VAESTRO Image Analysis Software (Canfield Scientific).
Investigators performed clinical grading for tolerability using a 4-point scale (0 = none, 1 = mild, 2 = moderate, 3 = severe) by assessing signs of erythema, edema, and dryness. Using the same scale, participants reported the degree of burning, stinging, itching, and peeling globally on their face.
Participant self-assessments were made at Weeks 1, 2, 4, 8, and 12. Participants completed a sponsor-provided self-assessment questionnaire on treatment satisfaction, the effect of immediate and continued use, skin texture, and application experience.
Statistical Analyses
The per-protocol population included all participants who received treatment and completed the study in general accordance with the protocol. Number of participants, mean, median, standard deviation, minimum, and maximum of all scores/values were provided at all applicable time points. Analyses were conducted separately for PIH and solar lentigines, as well as combined based on average scores. Mean change from baseline was estimated at applicable post-baseline time points. Clinical grading of efficacy parameters and tolerability evaluations were analyzed using a Wilcoxon signed-rank test. Image analysis of skin color measurements were analyzed using a paired t-test. Questionnaires were tabulated and the frequency and percentage of all response options were reported for each question and time point. A binomial sign test was performed to test if the proportion of the combined designated favorable responses was equal to the combined designated unfavorable response for each applicable question. All analyses are 2-sided at a significance-level alpha = 0.05 unless otherwise stated.
Results
Participants
This study enrolled 41 female participants aged 25–64 years. The PIH group consisted of 19 female participants who were predominantly Black/African American (52.6%) and Hispanic (36.8%), with Fitzpatrick skin types IV–V. The solar lentigines group consisted of 22 females who were predominantly White/Caucasian (50.0%), Hispanic (22.7%), and Asian (18.2%), with Fitzpatrick skin types I–IV (Table 2).
Table 2.
Participant Demographics
| PIH (n = 19) | Solar Lentigines (n = 22) | All Participants (N = 41) | |
|---|---|---|---|
| Race and Ethnicity, n (%) | |||
| White/Caucasian | 1 (5.3) | 11 (50.0) | 12 (29.3) |
| Hispanic | 7 (36.8) | 5 (22.7) | 12 (29.3) |
| Black/African American | 10 (52.6) | 0 | 10 (24.4) |
| Asian | 1 (5.3) | 4 (18.2) | 5 (12.2) |
| Native American/Alaska Native | 0 | 1 (4.5) | 1 (2.4) |
| Asian/Caucasian | 0 | 1 (4.5) | 1 (2.4) |
| Fitzpatrick Skin Type, n (%) | |||
| I | 0 | 1 (4.5) | 1 (2.4) |
| II | 0 | 9 (40.9) | 9 (22.0) |
| III | 0 | 10 (45.5) | 10 (24.4) |
| IV | 10 (52.6) | 2 (9.1) | 12 (29.3) |
| V | 9 (47.4) | 0 | 9 (22.0) |
Abbreviation: PIH, post-inflammatory hyperpigmentation.
Efficacy Assessments
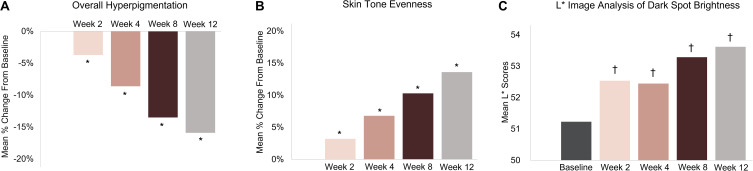
Dark Spot Tx resulted in a significant reduction in overall hyperpigmentation and a significant improvement in skin tone evenness in the overall group (combined PIH and solar lentigines) compared with baseline starting at Week 2 and continuing through Week 12 (p ≤ 0.018) (Figure 1A and B). Image analysis of target dark spot brightness (L*) showed significant improvement at all visits in the overall group (p ≤ 0.01) (Figure 1C).
Figure 1.
Mean percent change from baseline of overall hyperpigmentation (A) and skin tone evenness (B), and mean scores of the image analysis of dark spot brightness (L*) (C) in the overall group (PIH and solar lentigines combined). Skin tone evenness was graded on a scale from 0 to 9, where 0 indicated even, healthy skin and 9 indicated uneven, discolored appearance. A decrease in skin tone evenness score indicated an improvement. The results for skin tone evenness were inverted for clarity when interpreting the figure. Mean L* scores from images of the target dark spot. Increased L* indicates brighter skin. Overall group: n = 40 at Weeks 2 and 4; n = 41 at Weeks 8 and 12. *p ≤ 0.018 vs baseline (Wilcoxon signed-rank test). †p ≤ 0.01 vs baseline (paired t-test).
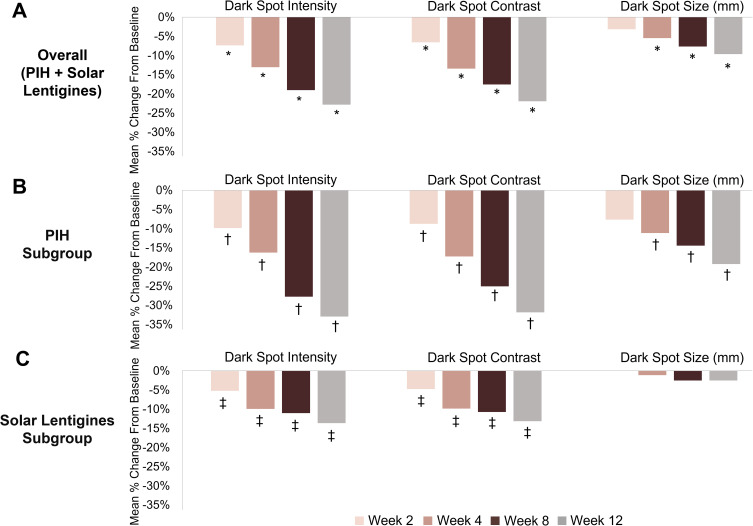
Dark spot intensity and dark spot contrast were significantly reduced with Dark Spot Tx at all follow-up visits, and dark spot size at Weeks 4, 8, and 12, compared to baseline in the overall group (p ≤ 0.008) (Figure 2A). Subgroup analysis showed similar results for the PIH subgroup with significant improvements in dark spot intensity and dark spot contrast starting at Week 2, and in dark spot size starting at Week 4 (p ≤ 0.031) (Figure 2B). In the solar lentigines subgroup, dark spot intensity and dark spot contrast were significantly reduced with Dark Spot Tx at all visits (p ≤ 0.016), and there was no significant change in dark spot size (Figure 2C).
Figure 2.
Mean percent change from baseline of dark spot intensity, contrast, and size in the overall group (A), PIH subgroup (B), and solar lentigines subgroup (C). Overall group: n = 40 at Weeks 2 and 4; n = 41 at Weeks 8 and 12. PIH subgroup: n = 22 at Weeks 2, 8, and 12; n = 21 at Week 4. Solar lentigines subgroup: n = 18 at Week 2; n = 19 at Weeks 4, 8, and 12. *p ≤ 0.008, †p ≤ 0.031, ‡p ≤ 0.016 vs baseline (Wilcoxon signed-rank test).
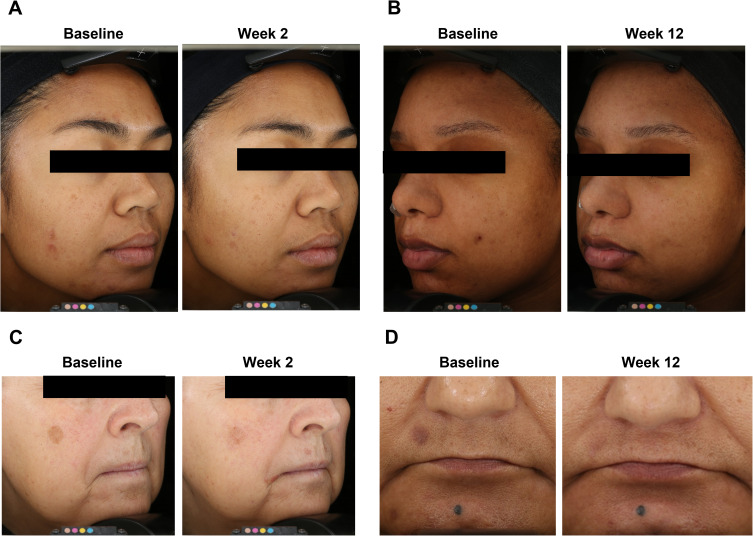
Representative images of 4 participants in Figure 3 show improvements in hyperpigmentation with the use of the Dark Spot Tx. In individuals with PIH (Figures 3A and B) or with solar lentigines (Figures 3C and D), improvements could be seen starting at Week 2 and continuing through to Week 12 in a range of ages and different skin types.
Figure 3.
Four representative participants at baseline and Week 2 and/or Week 12 after Dark Spot Tx. (A) A 37-year-old woman with Fitzpatrick skin type IV and PIH. (B) A 25-year-old woman with Fitzpatrick skin type V and PIH. (C) A 64-year-old woman with Fitzpatrick skin type II and solar lentigines. (D) A 51-year-old woman with Fitzpatrick skin type IV and solar lentigines.
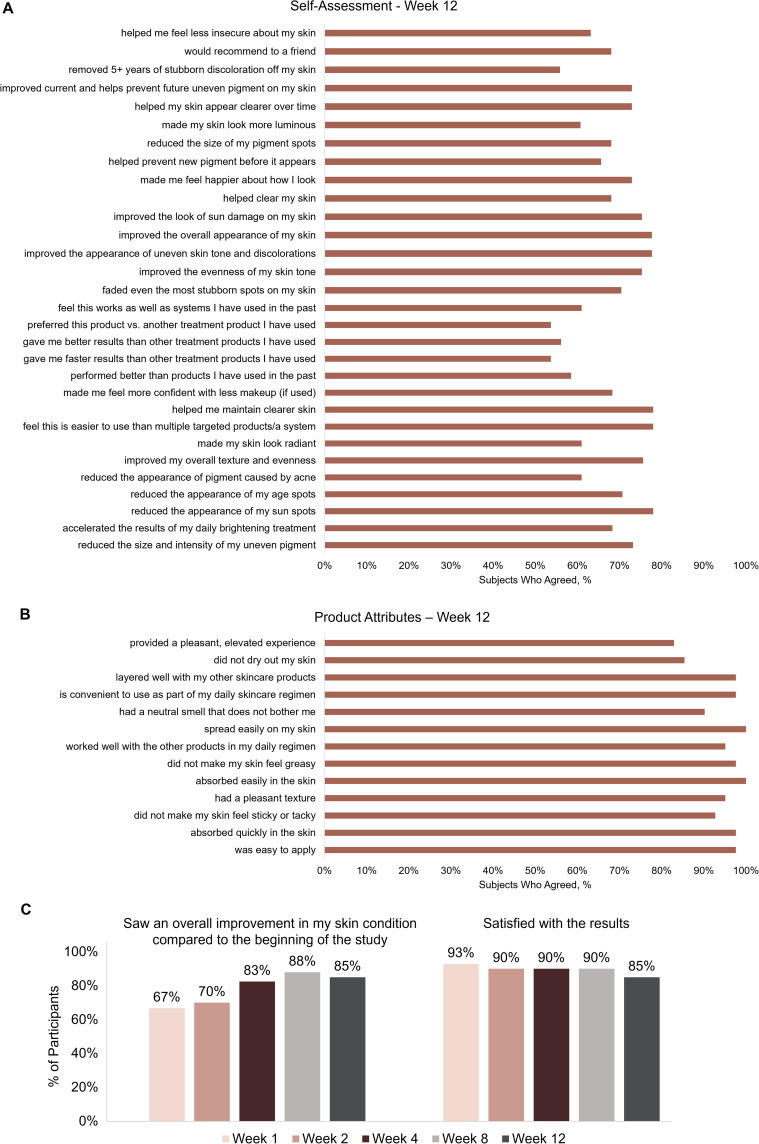
Participant self-assessment questionnaires showed consistent, high levels of self-perceived effectiveness across the questionnaire topics with the use of Dark Spot Tx during the 12-week study. Furthermore, 85% of the participants saw an overall improvement in their skin condition with Dark Spot Tx at Week 12, and ≥85% of the participants reported overall satisfaction with the results of the Dark Spot Tx at all time points (Figure 4).
Figure 4.
Participant self-assessment questionnaire at Week 12 (A), self-assessment product attributes questionnaire at Week 12 (B), and overall improvements and overall satisfaction at all weekly assessments (C). Participants completed self-assessment questionnaires regarding treatment satisfaction, effects of immediate and continued use, skin texture, and application experience.
Tolerability Assessments
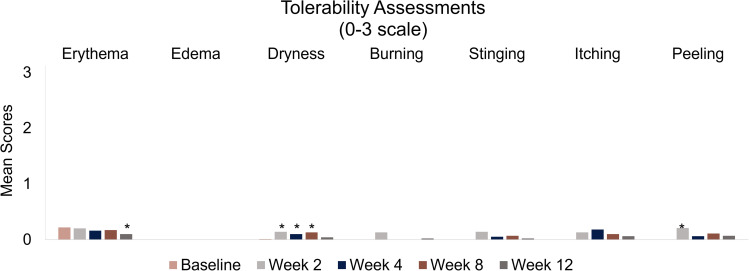
Investigator-assessed clinical grading and participant self-reporting of tolerability parameters (erythema, edema, dryness, burning, stinging, itching, peeling) showed that Dark Spot Tx was well tolerated during the 12-week study. The occurrence of erythema (Week 12), dryness (Weeks 2, 4, and 8), and peeling (Week 2) were significant compared with baseline; however, mean tolerability scores for all parameters were less than 0.25 (mild) at all study visits (Figure 5). No serious adverse events were reported.
Figure 5.
Tolerability parameters assessed by clinical grading and participant self-reporting at baseline and Weeks 2, 4, 8, and 12. Investigator-assessed clinical grading and participant self-reported tolerability was assessed on a 4-point scale (0 = none, 1 = mild, 2 = moderate, 3 = severe). All assessments were compared to baseline. *p ≤ 0.031 vs baseline (signed-rank test).
Discussion
This single-center 12-week study evaluated the efficacy and tolerability of a novel, targeted pigment-correcting dark spot treatment gel suspension cream for the treatment of PIH and solar lentigines. Significant improvements in overall hyperpigmentation, skin tone evenness, dark spot brightness, and dark spot intensity, contrast, and size were observed and supported by clinical investigator assessments, quantitative image analysis, and participant self-assessment questionnaires. Collectively, these results demonstrate the ability of Dark Spot Tx to target dark spots due to PIH and solar lentigines and improve multiple parameters of hyperpigmentation in a diverse cohort of females with varying ages, ethnicities, and Fitzpatrick skin types.
Erythema, edema, and dryness were assessed objectively by evaluating the clinical signs and symptoms of these parameters. Burning, stinging, itching, and peeling were assessed subjectively via participant self-reporting. All parameters had a mean score below 0.25 (mild) at all study visits demonstrating tolerability of the Dark Spot Tx, both objectively and subjectively. Glycolic acid (GA) is an established treatment for a range of dermatologic conditions, including PIH and solar lentigines; however, it may be associated with certain side effects (erythema, stinging, and burning) depending on the application strength and timing of the treatment.15,21 Similarly, topical retinols can be used to manage PIH and solar lentigines; however, they may be associated with redness, peeling, and irritation.3,14 Dark Spot Tx contains both GA and retinol, and demonstrated high tolerability across these parameters in this 12-week study.
Self-assessment questionnaires were completed by the participants and showed a high level of self-reported dark spot improvement and treatment satisfaction. Taken together with the previous results, this study shows the efficacy, tolerability, and participant satisfaction of the Dark Spot Tx. Medical treatment of dark spots from PIH and solar lentigines is not required, hence individuals with these conditions choose to initiate treatments to manage their hyperpigmentation and address QoL.1,3,11,13 Treatments that are not only effective in reducing the hyperpigmentation, but also tolerable and satisfy the individuals’ treatment goals, are of particular importance to the management of these conditions.
Current methods for managing PIH and solar lentigines include at-home topical treatments, such as hydroquinone, retinoids, or steroids and in-office treatments/procedures, such a cryotherapy, chemical peels, or laser therapy. These have varying degrees of effectiveness in treating hyperpigmentation and current practice include combinations of these treatments.2,5,12 Dark Spot Tx combines a unique blend of lotus seed extract, tranexamic acid, niacinamide, retinol, glycolic acid, vitamin C (tetrahexyldecyl ascorbate), kojic acid (kojic dipalmitate), and other ingredients to treat hyperpigmentation associated with PIH and solar lentigines. Using gel suspension technology, Dark Spot Tx was designed to stabilize and minimize degradation of the high load of powerful actives. This new treatment allows physicians and individuals with PIH and solar lentigines to customize their overall treatment regimen. Additional studies are needed to address optimal combinations of Dark Spot Tx with other treatments/procedures for targeted pigment correction.
One potential limitation of the study is the relatively small number of participants. However, results were consistent across all ethnicities and Fitzpatrick skin types for those participants included in the study. Future studies of additional diverse cohorts and a comparison in efficacy of Dark Spot Tx between the PIH and solar lentigines groups may further support the use of Dark Spot Tx for the treatment of PIH and solar lentigines.
Conclusion
The novel, targeted pigment-correcting spot treatment gel suspension cream is an effective and tolerable treatment for hyperpigmentation associated with PIH and solar lentigines. Improvements in facial dark spots were seen as early as Week 2, and continued through Week 12. These results were demonstrated in a broad range of ages, ethnicities, and skin types.
Ethics Approval
The study was conducted in compliance with all applicable guidelines for the protection of human subjects for research as outlined in the 1975 Declaration of Helsinki and 21 CFR 50. Institutional review board approval (Advarra IRB) was obtained prior to conduct of any study procedures. All subjects signed written informed consent forms and were willing to follow all study requirements and instructions.
Acknowledgments
Medical writing assistance was provided to the authors by Brian Neel, PhD, of Allergan Aesthetics, an AbbVie Company (Irvine, CA, USA), and was funded by Allergan Aesthetics, an AbbVie Company. Editorial assistance was provided to the authors by Angela Hadsell, of Allergan Aesthetics, an AbbVie Company, and funded by Allergan Aesthetics, an AbbVie Company.
The abstract of this manuscript was presented at the 2022 American Society for Dermatologic Surgery Annual Meeting and the 2022 Fall and 2023 Winter Clinical Dermatology Conferences as a poster presentation. The poster was published in the “March Issue & Poster Presentations from WCH23 Dermatology Conference®” in SKIN The Journal of Cutaneous Medicine, 7(2), s189. https://doi.org/10.25251/skin.7.supp.189
Funding Statement
Allergan Aesthetics, an AbbVie Company, funded this study and participated in study design, research, analysis, data collection, interpretation of data, review, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
Data Sharing Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/ then select “Home”.
Disclosure
Elizabeth T. Makino, Priscilla Huang, Tsing Cheng, and Rahul C. Mehta are employees of AbbVie and may hold AbbVie stock. Summer F. Acevedo was an employee of SGS Stephens Inc. at the time of the study and Cristiane de Oliveira is an employee of SGS Stephens Inc. Both served as principal investigators for the clinical study. The authors report no other conflicts of interest in this work.
References
- 1.Nautiyal A, Wairkar S. Management of hyperpigmentation: current treatments and emerging therapies. Pigm Cell Melanoma Res. 2021;34(6):1000–1014. doi: 10.1111/pcmr.12986 [DOI] [PubMed] [Google Scholar]
- 2.Plensdorf S, Livieratos M, Dada N. Pigmentation disorders: diagnosis and management. Am Fam Physician. 2017;96(12):797–804. [PubMed] [Google Scholar]
- 3.Davis EC, Callender VD. Postinflammatory hyperpigmentation a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3(7):20–31. [PMC free article] [PubMed] [Google Scholar]
- 4.Makino ET, Kadoya K, Sigler ML, Hino PD, Mehta RC. Development and clinical assessment of a comprehensive product for pigmentation control in multiple ethnic populations. J Drugs Dermatol. 2016;15(12):1562–1570. [PubMed] [Google Scholar]
- 5.Scarcella G, Gerber PA, Edge D, Nielsen MCE. Effective removal of solar lentigines by combination of pre– and post–fluorescent light energy treatment with picosecond laser treatment. Clin Case Rep. 2020;8(8):1429–1432. doi: 10.1002/ccr3.2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang BY, Ibrahim SA, Shokeen D, et al. Postinflammatory hyperpigmentation: protocol for development of a core outcome set for clinical trials. Arch Dermatol Res. 2022;314(4):357–361. doi: 10.1007/s00403-021-02239-6 [DOI] [PubMed] [Google Scholar]
- 7.Perkins AC, Cheng CE, Hillebrand GG, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol. 2011;25(9):1054–1060. doi: 10.1111/j.1468-3083.2010.03919.x [DOI] [PubMed] [Google Scholar]
- 8.Markiewicz E, Idowu OC. Melanogenic difference consideration in ethnic skin type: a balance approach between skin brightening applications and beneficial sun exposure. Clin Cosmet Invest Dermatol. 2020;13:215–232. doi: 10.2147/CCID.S245043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vashi NA, de Castro Maymone MB, Kundu RV. Aging differences in ethnic skin. J Clin Aesthet Dermatol. 2016;9(1):31–38. [PMC free article] [PubMed] [Google Scholar]
- 10.Ortonne JP. Pigmentary changes of the ageing skin. Brit J Dermatol. 1990;122(s35):21–28. doi: 10.1111/j.1365-2133.1990.tb16121.x [DOI] [PubMed] [Google Scholar]
- 11.Szymańczyk J, Trzeciakowski W, Ivonyak Y, et al. Blue laser (450 nm) treatment of solar lentigines. J Clin Med. 2021;10(21):4919. doi: 10.3390/jcm10214919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anvery N, Christensen RE, Dirr MA. Management of post‐inflammatory hyperpigmentation in skin of color: a short review. J Cosmet Dermatol. 2022;21(5):1837–1840. doi: 10.1111/jocd.14916 [DOI] [PubMed] [Google Scholar]
- 13.Balkrishnan R, McMichael AJ, Hu JY, et al. Correlates of health‐related quality of life in women with severe facial blemishes. Int J Dermatol. 2006;45(2):111–115. doi: 10.1111/j.1365-4632.2004.02371.x [DOI] [PubMed] [Google Scholar]
- 14.Plensdorf S, Martinez J. Common pigmentation disorders. Am Fam Physician. 2009;79(2):109–116. [PubMed] [Google Scholar]
- 15.Shenoy A, Madan R. Post-inflammatory hyperpigmentation: a review of treatment strategies. J Drugs Dermatol. 2020;19(8):763–768. doi: 10.36849/JDD.2020.4887 [DOI] [PubMed] [Google Scholar]
- 16.Hu S, Atmakuri M, Rosenberg J. Adverse events of nonablative lasers and energy-based therapies in subjects with Fitzpatrick skin phototypes IV to VI: a systematic review and meta-analysis. Aesthet Surg J. 2021;42(5):537–547. doi: 10.1093/asj/sjab398 [DOI] [PubMed] [Google Scholar]
- 17.Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3(1):11–20. doi: 10.1016/j.ijwd.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang M, Park SH, Park SJ, et al. p44/42 MAPK signaling is a prime target activated by phenylethyl resorcinol in its anti-melanogenic action. Phytomedicine. 2019;58:152877. doi: 10.1016/j.phymed.2019.152877 [DOI] [PubMed] [Google Scholar]
- 19.Konisky H, Balazic E, Jaller JA, Khanna U, Kobets K. Tranexamic acid in melasma: a focused review on drug administration routes. J Cosmet Dermatol. 2023;22(4):1197–1206. doi: 10.1111/jocd.15589 [DOI] [PubMed] [Google Scholar]
- 20.Searle T, Al‐Niaimi F, Ali FR. The top 10 cosmeceuticals for facial hyperpigmentation. Dermatol Ther. 2020;33(6):e14095. doi: 10.1111/dth.14095 [DOI] [PubMed] [Google Scholar]
- 21.Sharad J. Glycolic acid peel therapy – a current review. Clin Cosmet Invest Dermatol. 2013;6:281–288. doi: 10.2147/CCID.S34029 [DOI] [PMC free article] [PubMed] [Google Scholar]