Abstract
Biomolecular condensates are essential for cellular functionality, yet the complex interplay among the diverse molecular interactions that mediate their formation remains poorly understood. Here, using coarse-grained molecular dynamics simulations, we address the contribution of cation−π interactions to the stability of condensates formed via liquid–liquid phase separation. We found greater stabilization of up to 80% via cation−π interactions in condensates formed from peptides with higher aromatic residue content or less charge clustering. The contribution of cation−π interactions to droplet stability increases with increasing ionic strength, suggesting a trade-off between cation−π and electrostatic interactions. Cation−π interactions, therefore, can compensate for reduced electrostatic interactions, such as occurs at higher salt concentrations and in sequences with less charged residue content or clustering. Designing condensates with desired biophysical characteristics therefore requires quantification not only of the individual interactions but also cross-talks involving charge–charge, π–π, and cation−π interactions.
Biological mesoscale condensates have been found to regulate the functionality of some proteins and nucleic acids.1−4 Furthermore, anomalous protein condensate behavior has been shown to underlie several diseases and to arise from mutations affecting the specific amino acid sequences of the participating proteins and their interchain interactions.5−9 These findings have sparked interest in designing condensates to function as membraneless organelles to control cellular activities10,11 and to serve as therapeutic delivery agents12 and as biomaterials with tunable stability and dynamics.10,12,13 Liquid–liquid phase separation14−18 has emerged in the past decade as the primary mechanism by which biological systems attain such high-density membrane-less compartmentalization, producing condensates whose constituent proteins convert readily between their different conformations, engage in interactions through multivalent sticker motifs,19,20 and exhibit highly preserved mobility in liquid condensates.17,21−23
The genesis of biomolecular condensates, which are often formed by intrinsically disordered proteins (IDPs) that can be viewed as multivalent polymers,24 may be driven simply by long-range electrostatic interactions between charged residues in polyelectrolytic IDPs with high net charges (in the presence of oppositely charged polymers) or in polyampholytic IDPs.25,26 Typically, natural IDP sequences deviate from ideal polyelectrolyte or polyampholyte sequences, as they often comprise polar, aromatic, and hydrophobic residues. Indeed, not only charge–charge interactions,25,27,28 but also hydrogen bonding,29 π–π stacking,30 and cation−π18,31,32 interactions play crucial roles in the formation of biological condensates and in shaping their properties.
Several computational33−37 and experimental38−43 studies have endeavored to decipher the molecular grammar of the set of interactions that dominate condensate stability and dynamics. The interactions that drive the condensate phase may be classified as acting over short- or long-range intracellular distances. Earlier studies broadly defined a balance between the short- and long-range interactions that may stabilize the condensates, yet with diverging properties emerging depending on the sequence enrichment of these interactions.44−46 Although all nonelectrostatic interactions may be classified as short-range interactions as they act on a length scale of ∼1 nm, cation−π interactions18,31 are of special interest as they can be viewed as bridging between electrostatic and aromatic interactions.47,48 Recent experiments suggest the existence of multivalent cation−π interactions that play a dominant role in synaptophysin and synapsin coacervation49 and in Tau phase separation.50,51 Biomolecular condensates have been shown to retain their stability in extremely harsh conditions such as at very high salt concentrations.52,53 This observation indicates that nonionic interactions, namely, cation−π and π–π interactions, play a crucial role in these condensates, but their exact quantification is yet elusive. It has been postulated that IDP enrichment with Arg and Tyr often facilitates IDP recruitment in a preformed dense phase through cation−π interactions in addition to electrostatic interactions.54,55 FUS proteins undergo extensive phase separation due to the formation of cation−π interactions between the Arg and Tyr residues in their tails.56 We have shown that replacing charged residues with aromatic residues (and thereby replacing electrostatic interactions with π–π interactions while maintaining a net charge of zero) reduces condensate stability.44 In principle, mutating polyampholytes to aromatic residues may allow the formation of cation−π interactions in addition to π–π interactions. Although both cation−π and π–π interactions have a short-range nature, they may differ in their effect on condensate characteristics.
The current study aims to quantify the effects on condensate stability and dynamics that arise from cation−π interactions in the context of their trade-offs with other intracondensate interactions. Variation in the contribution and strength of the cation−π interactions may have different origins, including changes in the relative content of charged and aromatic residues, the organization of the charged residues, or the salt concentration of the solution. These changes in the IDP sequence or in the ionic strength of the solution may alter the content and strength of charged–charged and aromatic interactions, thus tuning potential cross-talks between them and affecting the formation of cation−π interactions (Figure 1A).
Figure 1.

Interplay between charge–charge, π–π, and cation−π interactions within intrinsically disordered proteins that form biomolecular condensates. (A) A schematic illustration of frustration between purely electrostatic or aromatic interactions and cation−π interactions in an intrinsically disordered protein (IDP). The formation of a cation−π interaction may depend on breaking charge–charge and aromatic interactions, thereby making positively charged and aromatic residues available to interact with each other. This scenario may depend on the sequence characteristics and conformations of the IDPs, as well as on solvent salt concentrations, which modulate the overall strength of electrostatic interactions. Accordingly, cation−π, charge–charge, and π–π interactions in IDPs may not be additive, but rather exhibit compensatory behavior. (B) The five 40-amino acid sequences (designated S1–S5) designed for the current research to explore the interplay between cation−π, charge–charge, and π–π interactions. The peptides comprise three types of residues: positively charged (blue), negatively charged (red), and aromatic (green). Each sequence has a net charge of zero. The sequences differ in terms of their aromatic residue content (ϕ) and charged residue content (fraction of charged residues, FCR). Some of the sequences also have different charged residue clustering coefficients, κ, which was achieved by shuffling the residues within the sequence. Sequences S2–S4 have identical numbers of charged and aromatic residues but differ with respect to their organization, as reflected by their different κ values. Sequences S2 and S5 were obtained from the S1 sequence by gradually replacing its charged residues with aromatic residues (which reduces FCR and, consequently, the value of κ).
The formation of cation−π interactions can be indirectly regulated by changing solvent salt concentration, which tunes the prevalence of electrostatic interactions in the sequences. For a more direct approach, it is known that manipulating the content and organization of charged residues can influence the balance between their short- and long-range interactions as well as their stability,44 which consequently may directly affect their propensity to form cation−π interactions. Therefore, to gain understanding into the complex blending of long-range and short-range interactions between IDPs in biomolecular condensates, we designed five IDP sequences (designated S1–S5; Figure 1B) that differed with respect to their aromatic residue content (ϕ), their charged residue content (as indicated by the fraction of charged residues, FCR), and/or their charged residue clustering coefficients (κ)57 and/or sequence charge decoration in the sequences (SCD).58 IDPs S1–S5 comprise 40 residues of which 4 (S1) to 24 (S5) residues are aromatic, such that aromatic residue content ranges from 0.1 (S1) to 0.6 (S5) and FCR is correspondingly 0.9 (S1) to 0.4 (S5). All five designed sequences have a net charge of zero and are composed of only three types of residues: positively charged, negatively charged, and aromatic residues. The number of positively and negatively charged residues in the sequences ranges from 18 (S1) to eight (S5), with S2 and S4 all having 14 positively and negatively charged residues but differing in terms of how their charged residues are organized along the sequence. Differences in charged residue organization manifest in different charged residue clustering coefficients, κ, which have an overall range of κ = 0.47–0.21 for S1–S5 and of κ = 0.36–0.03 for S2–S4 (see Figure 1B).
A coarse-grained molecular dynamics model was employed to simulate multiple copies of each of the designed sequences to study their self-condensation in an aqueous solution. Each residue in each of the five 40-residue sequences was modeled as a bead that could be positively charged, negatively charged, or have aromatic characteristics (see Figure 1).
The potential energy function consists of several terms: bonded and angular terms, long-range electrostatic interactions among all charged beads, both intra- and intermolecularly, and short-range dispersion interactions. Short-range dispersion interactions are applied to model interactions between the aromatic residues (i.e., π–π interactions) and between aromatic beads and positively charged beads (i.e., cation−π interactions). In addition, an excluded volume potential is applied to all bead pairs. The bonded and angular interactions are modeled with a harmonic potential. As IDPs have high conformational flexibility, which is important in the formation of biological network-based condensates, the studied sequences are modeled as completely flexible without any dihedral angle constraint.
The electrostatic interactions between
the charged beads are defined
using the Debye–Hückel potential44,59,60Eelectrostatic = 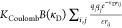 where qi and qj denote
the charge of the ith and jth bead, rij denotes the
interbead distance, ε is the solvent dielectric constant, and KCoulomb = 4πε0 = 332 kcal/mol. B(κD) is
a function of solvent salt concentration and the radius (a) of ions produced by the dissociation of the salt, and if can be
expressed as
where qi and qj denote
the charge of the ith and jth bead, rij denotes the
interbead distance, ε is the solvent dielectric constant, and KCoulomb = 4πε0 = 332 kcal/mol. B(κD) is
a function of solvent salt concentration and the radius (a) of ions produced by the dissociation of the salt, and if can be
expressed as 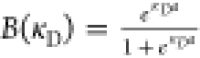 . The Debye–Hückel electrostatic
interactions of an ion pair act over a length scale of the order of
. The Debye–Hückel electrostatic
interactions of an ion pair act over a length scale of the order of  , which is called the Debye screening length.
The Poisson–Boltzmann equation leads to the following relation
of κD to ionic strength:
, which is called the Debye screening length.
The Poisson–Boltzmann equation leads to the following relation
of κD to ionic strength:  where NA is
the Avogadro number, e is the charge of an electron, ρA is the solvent density, I denotes solvent ionic strength, kB is
the Boltzmann constant, and T is the temperature.
The short-range dispersion interactions between a pair of aromatic
beads (i.e., π–π) or between an
aromatic bead and a positively charged bead (i.e.,
cation−π) are modeled using the Lennard-Jones potential: Eπ–π/cation−π =
where NA is
the Avogadro number, e is the charge of an electron, ρA is the solvent density, I denotes solvent ionic strength, kB is
the Boltzmann constant, and T is the temperature.
The short-range dispersion interactions between a pair of aromatic
beads (i.e., π–π) or between an
aromatic bead and a positively charged bead (i.e.,
cation−π) are modeled using the Lennard-Jones potential: Eπ–π/cation−π =  where σij denotes the optimal distance between beads i and j that are in contact with each other
and σij = 7 Å.
The parameter ε denotes the strength of the short-range dispersion
interaction. We used ε = 0.2 kcal/mol, as this value was shown
to reproduce the experimentally determined radius of gyration (Rg)
of several IDPs as estimated from their simulations using a coarse-grained
model that included only electrostatic and hydrophobic interactions.44 Given that cation−π interactions
in proteins are of similar strength to π–π interactions,61,62 we modeled both types of interactions at the same epsilon value
(i.e., επ–π =
εcation−π = 0.2 kcal/mol). Our model
assumes that the cation−π interaction strength is independent
of the salt concentration. We have observed that the same cation−π
interaction strength reproduces their experimentally determined Rg
values for two different salt concentrations (i.e., 20 and 40 mM).
In addition, we heuristically calibrated the cation−π
interaction strength to reproduce experimental Rg of these IDPs. We
observed that cation−π interaction strength of 0.2 kcal/mol
along with aromatic strength of 0.2 kcal/mol serves best to reproduce
highest correlation between experimental and simulated Rg’s
(Figures S1–S4). We note that while
ions may play an important role in determining the thermodynamic stability
of the condensate phase,26,63 our model assumes that
once the condensates are formed nearly all counterions are released
to the bulk. Accordingly, the probability of competition between the
residue–residue cation−π and ion–residue
cation−π within a condensate phase seems to be low unless
there is a significant trapping of ions in condensates.
where σij denotes the optimal distance between beads i and j that are in contact with each other
and σij = 7 Å.
The parameter ε denotes the strength of the short-range dispersion
interaction. We used ε = 0.2 kcal/mol, as this value was shown
to reproduce the experimentally determined radius of gyration (Rg)
of several IDPs as estimated from their simulations using a coarse-grained
model that included only electrostatic and hydrophobic interactions.44 Given that cation−π interactions
in proteins are of similar strength to π–π interactions,61,62 we modeled both types of interactions at the same epsilon value
(i.e., επ–π =
εcation−π = 0.2 kcal/mol). Our model
assumes that the cation−π interaction strength is independent
of the salt concentration. We have observed that the same cation−π
interaction strength reproduces their experimentally determined Rg
values for two different salt concentrations (i.e., 20 and 40 mM).
In addition, we heuristically calibrated the cation−π
interaction strength to reproduce experimental Rg of these IDPs. We
observed that cation−π interaction strength of 0.2 kcal/mol
along with aromatic strength of 0.2 kcal/mol serves best to reproduce
highest correlation between experimental and simulated Rg’s
(Figures S1–S4). We note that while
ions may play an important role in determining the thermodynamic stability
of the condensate phase,26,63 our model assumes that
once the condensates are formed nearly all counterions are released
to the bulk. Accordingly, the probability of competition between the
residue–residue cation−π and ion–residue
cation−π within a condensate phase seems to be low unless
there is a significant trapping of ions in condensates.
To examine
cross-talks between cation−π aromatic stacking
interactions, we also simulated a selected subset of systems at a
higher cation−π strength (εcation−π = 0.3 kcal/mol while επ–π =
0.2 kcal/mol). In order to avoid overlap between beads, each bead
interacts with all other beads through a steep repulsion interaction
defined by Erepulsion = 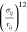 with σij = 4 Å. The initial configuration of the studied
IDPs was designed by randomly placing 100 copies of each studied ID
in a box with dimensions of 300 × 300 × 300 Å. Multiple
copies for each of the simulations were performed by solving the Langevin
equation to achieve proper averaging at several temperatures and salt
concentrations. A clustering algorithm was used to distinguish the
individual condensates and their component polymers at each time step.
with σij = 4 Å. The initial configuration of the studied
IDPs was designed by randomly placing 100 copies of each studied ID
in a box with dimensions of 300 × 300 × 300 Å. Multiple
copies for each of the simulations were performed by solving the Langevin
equation to achieve proper averaging at several temperatures and salt
concentrations. A clustering algorithm was used to distinguish the
individual condensates and their component polymers at each time step.
To study the interplay among cation−π, charge–charge, and aromatic π–π interactions between IDPs in biomolecular condensates, we independently studied 100 copies of each of the five sequences S1–S5. Each system was studied by using two related coarse-grained molecular dynamics models. Both models allowed the peptides to take part in electrostatic (charge–charge) and aromatic (π–π) interactions, but they differed regarding whether they included or excluded the formation of cation−π interactions. Each system was studied at different solution salt concentrations to manipulate the strength of the electrostatics interactions and thereby allow for examination of their cross-talks between all three types of interactions potentially occurring within the condensate. At each salt concentration, each of these systems was simulated over a large temperature range that covers its transition from weakly interacting free chains to a condensed assembly of highly interacting chains. These simulations were used to construct phase diagrams for liquid–liquid phase separation in each system, with the concentration of the dilute and dense phases plotted for different temperatures.
In Figure 2, the left and middle panels show phase diagrams (temperature T versus solvent density ρ) for selected peptide systems, highlighting the critical temperature, TC, beyond which separation does not occur and therefore refers to the stability of the condensate. Each system was studied under both the cation−π-inclusive (filled symbols) and cation−π-exclusive models. The arrows in the phase diagrams indicate TC and are positioned to show the difference in TC (ΔTC) between these two models. Figure 2 (right) summarizes the dependence of ΔTC on some key molecular features of the systems, as discussed below.
Figure 2.
Effects of cation−π
interactions on the stability
of peptide condensates having different properties. The effect of
cation−π interactions is explored as a function of salt
concentration of the solution (A); the aromatic residue content, ϕ,
of the sequence (B); and the charge clustering coefficient, κ,
of the sequence (C). Representative phase diagrams for the liquid–liquid
phase separation of selected peptides are plotted (left and middle
panels). Each peptide was simulated using coarse-grained models that
include (filled symbols) and exclude (empty symbols) cation−π
interactions at NaCl concentrations of 0.02 M (circles) and 0.06 M
(triangles). For each temperature in a phase diagram, an error bar
has been shown as the standard deviation obtained from three independent
simulation trajectories. Critical temperature has been obtained by
fitting density–temperature data with the ρdense phase – ρdilute phase = 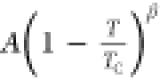 , where ρdense phase and ρdilute phase denote
the polymer bead density in the dense phase and in the dilute phase,
respectively, and β is a critical exponent that, following other
studies, was set to 0.325. The estimation of critical temperature
has also been validated by a change in slope of condensate phase density
along temperature. The effect of cation−π interactions
on the stability of each peptide condensate is indicated by the change
in the critical temperatures (TC) obtained
from simulations performed using the cation−π-inclusive
and cation−π-exclusive models (i.e.,
ΔTC = TC(incl_cation−π) – TC(excl_cation−π)). ΔTC is schematically illustrated
in each phase diagram by the blue arrows. The right panels summarize
the relationship between normalized ΔTC (in %) and salt concentration, κ, and ϕ. The
dependence of ΔTC on salt concentration
is examined for two cation−π interaction strengths ((εcation−π = 0.2 or 0.3 kcal/mol). The linear fit
is shown for each of these correlations (dashed or solid lines). The
peptides discussed in each panel are indicated on it. At moderate
and high aromatic content, we have observed a re-entrant phase behavior
with condensate phase density being lowered as temperature is decreased.
This observation stems from the fact that short-range interactions
are dominant in this regime, and as temperature is lowered, kinetic
arresting may play an important role once electrostatic interactions
are diminished in conjunction with lowering temperature.
, where ρdense phase and ρdilute phase denote
the polymer bead density in the dense phase and in the dilute phase,
respectively, and β is a critical exponent that, following other
studies, was set to 0.325. The estimation of critical temperature
has also been validated by a change in slope of condensate phase density
along temperature. The effect of cation−π interactions
on the stability of each peptide condensate is indicated by the change
in the critical temperatures (TC) obtained
from simulations performed using the cation−π-inclusive
and cation−π-exclusive models (i.e.,
ΔTC = TC(incl_cation−π) – TC(excl_cation−π)). ΔTC is schematically illustrated
in each phase diagram by the blue arrows. The right panels summarize
the relationship between normalized ΔTC (in %) and salt concentration, κ, and ϕ. The
dependence of ΔTC on salt concentration
is examined for two cation−π interaction strengths ((εcation−π = 0.2 or 0.3 kcal/mol). The linear fit
is shown for each of these correlations (dashed or solid lines). The
peptides discussed in each panel are indicated on it. At moderate
and high aromatic content, we have observed a re-entrant phase behavior
with condensate phase density being lowered as temperature is decreased.
This observation stems from the fact that short-range interactions
are dominant in this regime, and as temperature is lowered, kinetic
arresting may play an important role once electrostatic interactions
are diminished in conjunction with lowering temperature.
To study the interplay between cation−π interactions and salt concentration, we studied the S2 peptide at three salt concentrations (0.02 0.04, and 0.06 M). We selected the S2 peptide for this purpose as it has a medium aromatic residue content (ϕ = 0.3; i.e., 30% of its residues are aromatic), which indicates that positively charged and aromatic residues are present in almost equal proportions and so maximizes the potential for cation−π crosstalk, and a medium charge clustering coefficient (κ = 0.36) (see Figure 1A). Figure 2A shows that as the salt concentration increases from the left to middle panel, condensate stability, as indicated by the value of TC, decreases when using the cation−π-exclusive model (empty symbols), as expected from salt-related weakening of electrostatic interactions. The stability of the condensates simulated using the cation−π-inclusive model (filled symbols) also decreases as salt concentration increases, but only slightly. The finding that the stability of the condensates is much less sensitive to salt concentration under the cation−π-inclusive model indicates that the formation of cation−π interactions compensates for the loss of stability due to loss of electrostatic interactions. A 3-fold increase in salt concentration enhances condensate TC by 15–60% (Figure 3A, right panel) because of the formation of cation−π interactions. The increase in ΔTC at higher salt concentrations reflects a trade-off between electrostatic and cation−π interactions.
Figure 3.
The energetic gain due to cation−π formation in biomolecular condensates. The energetic gain, ΔEDroplet, is estimated as the energy gained by each polymer within the condensate using the cation−π-inclusive model. (A) The energetic gain due to cation−π interactions is shown for peptide S2 simulated at different salt concentrations and for two cation−π interaction strengths (εcation−π = 0.2 or 0.3 kcal/mol, fitted to solid and dashed lines, respectively). (B) The energetic gain due to cation−π interactions for peptides S2–S4, which differ with respect to charge clustering (κ). Changing κ can reduce cation−π stabilization; however, this effect strongly depends on salt concentration. At a salt concentration of 0.02 M, the energetic gain arising from the formation of cation−π interactions varies by 6–13 kcal/mol depending on the κ value. At a higher salt concentration of 0.06 M, characterized by weak electrostatic interactions, the energetic gain from cation−π interactions is independent of variation in κ and each polymer gains nearly 10 kcal/mol energetic stabilization in the condensate phase under the cation−π-inclusive model compared with the cation−π-exclusive model. (C) Illustrative snapshots of two S2 polymers interacting within a condensate droplet formed at a salt concentration of 0.06 M and T/Tc = 0.4 and modeled using the cation−π-inclusive (left) and cation−π-exclusive models. In these snapshots, positively and negatively charged residues are colored blue and red, respectively, and the aromatic residues are shown in green.
The trade-off is illustrated by the droplet gaining energy at higher salt concentrations despite the breakage of electrostatic interactions. The formation of cation−π interactions in the dense phase results in an energetic gain of ∼6–10 kcal/mol per peptide at salt concentrations of 0.02–0.06 M (Figure 3A). This increase in stability arising from the formation of cation−π interactions was obtained using a model that assumes identical strengths for cation−π and π–π interactions (i.e., επ–π = εcation−π = 0.2). To examine the effect of even stronger cation−π interactions on ΔTC, we simulated the S2 peptide for εcation−π = 0.3 (Figure 2A, upper values, dashed line), which enhanced the stability of its condensate to ∼50% and ∼80% at low and high salt concentrations, respectively (Figure 2A). For the stronger cation−π interactions, the energetic stabilization per polymer in the condensate increases to be between 10 and 14 kcal/mol depending on the salt concentration (Figure 3A, dashed line).
Next, we examined the effect of aromatic residue content, ϕ, on the formation of cation−π interactions in the condensate and thus on stability, Tc. We note that a change in ϕ is naturally coupled with a change in the charged residue content and therefore also changes the κ value. The interplay between ϕ and Tc occurring during the formation of cation−π interactions is examined by studying the phase separation of sequences S1, S2, and S5, which have ϕ values of 0.1–0.6 (i.e., FCR values of 0.9–0.4; see Figure 1). Figure 2B (right panel) shows that, using the cation−π inclusive model and at salt concentration of 0.02 M (filled round symbols), the gain in stability of the condensate phase is higher for ϕ = 0.6 (sequence S5; ΔTC = 30%) than for ϕ = 0.1 (sequence S1; ΔTC = 10%). The value of ΔTC for condensates of these sequences including and excluding cation−π interactions is 20–45%, respectively, when simulated at a higher salt concentration of 0.06 M. The condensate phase is significantly less dense when cation−π interactions are excluded rather than included, and the difference in critical temperature between systems simulated with and without cation−π interactions is enhanced for higher aromaticity content. The enhancement of the stability of the droplet phase that is contributed by cation−π interactions for higher ϕ values is greater at higher salt concentrations than at lower salt concentrations (Figure 2B). When aromatic content is lower (ϕ = 0.1), electrostatics play the dominant role, whereas when aromatic content is higher (ϕ = 0.6) the aromatic interactions dominate. However, at an intermediate aromatic content (ϕ = 0.3) we observe maximal energetic stabilization (ΔTC ≈ 70% compared with ΔTC values of 20% and 45% for ϕ = 0.1 or 0.6, respectively), which is achieved via cation−π crosstalk with the charge–charge and π–π interactions (Figure 2B and Figure S7).
The observed interplay between salt concentration and ϕ concerning the extent of formation of cation−π interactions (with consequent effects on ΔTC), suggests that variations in the charged residue clustering patterns (represented by κ) may also modulate cation−π interactions, as κ affects electrostatic interactions. To estimate the existence of trade-offs between cation−π and κ, we examined the properties of the condensates formed by sequences S2 and S4, which have identical charged and aromatic residue contents (ϕ = 0.3, which also ensures a potentially high number of cation−π interactions) and with κ of 0.03–0.36 (see Figure 1B). Figure 2C (right panel) shows the correlation between ΔTC and κ for two different salt concentrations of 0.02 (circles) and 0.06 M (triangles). As κ increases, the cation−π interactions have a reduced effect on the stability of the condensate, as indicated by the strong negative correlation between ΔTC and κ at a salt concentration of 0.02 M. Figure 2C shows a ΔTC of ∼50% for the S4 peptide with κ = 0.03 compared with ΔTC of ∼15% for the S2 peptide with κ = 0.36. As charge clustering in the sequences decreases (i.e., at lower κ values), more electrostatic interactions are disrupted, which enables more positively charged residues to engage in cation−π interactions. Consequently, cation−π interactions have less of an effect for sequences with higher κ values. This trend depends on salt concentration. As electrostatic interactions weaken as salt concentration increases, the effect of charge clustering (i.e., of the κ value) on the electrostatic energy also reduces. Consequently, at a salt concentration of 0.06 M, ΔTC exhibits weaker dependence on κ than that observed at a salt concentration of 0.02 M. At salt concentrations of both 0.02 and 0.06 M (Figure 2B; right panel) ΔTC is positive, yet ΔTC is greater at the higher salt concentration, consistent with Figure 2A (right panel).
The relation between salt concentration, κ value, and the ability to form cation−π interactions is supported by the energetic analysis (Figure 3B). It is evident that, at a low salt concentration of 0.02 M (Figure 3B, circles), the energy of each polymer decreases by ∼13 kcal/mol as a result of cation−π interactions with neighboring sequences in the droplet for sequences with low κ values (i.e., S4) compared with a condensate having no cation−π interactions, whereas the additional stabilization per polymer is only ∼6 kcal/mol for sequences with higher κ values (i.e., S2) (Figure 3B). At a higher salt concentration of 0.06 M, the energetic gain per polymer in the droplet phase is much less sensitive to κ and remains nearly uniform at ∼10 kcal/mol. Figure 3C shows illustrative snapshots of S2 droplets formed with and without cation−π interactions, respectively, as indicated by interactions between blue and green residues, which occur only in one case.
The incorporation of
cation−π interactions in the
condensate, which in all the cases studied here increased condensate
stability, is expected to reduce internal diffusion by individual
polymers within the droplet. However, given the sensitivity of the
energetic contribution of the cation−π interactions to
the molecular properties of the constituent peptides, the effect of
cation−π interactions on the liquidlike nature of the
droplet may also depend on these molecular properties. To quantify
the effect of cation−π interactions on peptide diffusivity
in the condensate phase, we calculated the difference between the
extent of internal diffusion under the cation−π-inclusive
and cation−π-exclusive models. In order to focus on the
effect of cation−π interactions on translational diffusion
in the dense phase, we normalized the translational diffusion coefficient
of each peptide in the droplet by its diffusion coefficient in the
bulk (Ddroplet/Dbulk). Accordingly, the effect of cation−π interactions
on translational diffusion is probed by the difference between the
two Ddroplet/Dbulk ratios for systems in which cation−π interactions are
included or excluded (Figure 4). The diffusion constants were obtained from the slope of
the linear fit to the mean squared displacement (MSD) of the polymer
in the dense phase or in the bulk. MSD in three dimensions is related
to the diffusion constant as follows, D = 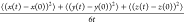 . When the diffusion coefficient in the
dense phase was calculated, we ensured that the peptides were part
of the condensate for the entire time window of interest.
. When the diffusion coefficient in the
dense phase was calculated, we ensured that the peptides were part
of the condensate for the entire time window of interest.
Figure 4.
Cation−π interactions influence the shape and liquid-like nature of peptide condensates. The change in diffusivity (A and C) and shape anisotropy (B and D) for condensates simulated using the cation−π-inclusive and cation−π-exclusive models as a function of charge clustering (κ) (for peptide sequences S2–S4; panels A and B) and aromatic residue content (ϕ) (for peptide sequences S1, S2, and S5; panels C and D) at salt concentrations of 0.02 M (circles, fitted with solid line) and 0.06 M (triangles, fitted with dashed line). The change in diffusivity is calculated as the difference of the ratio DDroplet/DBulk obtained using the cation−π-inclusive and cation−π-exclusive models.
For peptides S2 and S4 that have identical aromatic residue contents of ϕ = 0.3, the diffusivity of the peptides in the condensate phase is observed to reduce when cation−π interactions are included as κ reduces from 0.36 to 0.03 at a low salt concentration of 0.02 M (Figure 4A; circles). For example, a ∼60% lower normalized diffusion coefficient is measured in condensates with κ = 0.03, compared with a reduction of ∼30% for peptides with κ = 0.36. This reduced diffusivity due to cation−π interactions in condensates formed by peptides of low κ is not observed at the higher salt concentration of 0.06 M (Figure 4A; triangles).
The different manifestation of the cation−π interactions on the liquid nature of peptides S2 and S4 at low and high salt concentrations is consistent with the difference in energetic gain of these systems at these salt concentrations (Figure 3B). The electrostatic interactions are weaker under conditions of higher compared with lower salt concentration, which makes sufficient positively charged residues available to participate in cation−π interactions. The participation of more positively charged residues in cation−π interactions at the high salt concentration of 0.06 M enhances condensate stability through an energetic gain of approximately −10 kcal/mol per monomer (Figure 3B) and reduces peptide diffusivity by ∼50% (Figure 4A, left panel), for various κ values.
The gradual replacement of electrostatic residues by aromatic residues, leading to increased aromatic residue content ϕ (as observed in the series of peptides S1, S2, and S5) increases the diffusivity of the corresponding condensate phase of these peptides when cation−π interactions are included. The maximal loss of diffusivity of the condensate phase upon inclusion of cation−π interactions (compared with their exclusion) occurs at intermediate ϕ values (associated with ∼60% reduced diffusivity), thus strongly supporting the earlier finding of greater energetic stabilization (ΔTC) of each polymer in the condensate at ϕ = 0.3 (Figure 2B, right panel; Figure S10). At higher ϕ, aromatic interactions limit condensate diffusivity to such an extent that the inclusion of cation−π interactions has no effect. The linkage between the effect of cation−π interactions on the energetics of the condensate and peptide diffusion within it can also be understood as an effect on the density of the condensate.
Similarly to the condensate density, the overall shape of the condensate
phase may also be affected by the formation of cation−π
interactions. The spherical shape of the condensate can be estimated
by the shape anisotropy parameter defined as shape anistropy =  . A shape anisotropy parameter value of
3 indicates that the x, y, and z axes of the condensate are of similar length and describes
a spherical shape. The effect of cation−π interactions
on the sphericity of the condensate is probed by the change in the
shape anisotropy parameter upon turning on cation−π interactions.
. A shape anisotropy parameter value of
3 indicates that the x, y, and z axes of the condensate are of similar length and describes
a spherical shape. The effect of cation−π interactions
on the sphericity of the condensate is probed by the change in the
shape anisotropy parameter upon turning on cation−π interactions.
Figure 4 suggests that a larger deviation from a spherically shaped condensate occurs at lower values for charge clustering (Figure 4A, right panel) and aromatic residue content (Figure 4B, right panel) as a consequence of the enhanced formation of cation−π interactions under these conditions. Interestingly, at a higher salt concentration, the condensate phase more closely resembles a spherical shape than it does at a low salt concentration, which can be attributed to cation−π interactions becoming more likely as electrostatic interactions weaken (Figure 3A). At a lower salt concentration, the effect of cation−π formation on condensate shape is more prominent because lowering κ significantly weakens electrostatic interactions.
To support our claim that cation−π interactions are indeed essential in stabilizing biomolecular condensates, we performed a sequence analysis of intrinsically disordered regions (IDRs) that are involved in liquid–liquid phase separation (LLPS) by examining the interplay between their charged and aromatic residues. The IDRs that are prone to phase separate via LLPS were obtained from the LLPSDB database64 and were compared to IDRs extracted from the human genomes. In this analysis, 12100 IDR regions from the human genome (having IUPred 2a score >0.5) and 900 IDRs from the LLPS databases have been analyzed. We have also ensured that the IDRs are sufficiently long and comprise at least 50 residues. This sequence analysis shows that enrichment of aromatic residues in the human IDRs leads toward a depletion of positive and negatively charged residues as reflected by the reduction in the mean fraction of positive or negative residues (f+ and f–, respectively) as the fraction of aromatic content, ϕ, increases (Figure 5A). This reduction of f+ and f– as ϕ increases results in similar net charge for all IDRs which tend to be slightly negative because f– is often greater than f+.
Figure 5.
Interplay between charged and aromatic residues in IDRs. The relationship between the content of charged and aromatic residues are shown for IDRs in the human proteome (A) and for IDRs in that are prone to LLPS (obtained from the LLPSDB). (B) The fraction of positive (f+, blue circles) and negative charges (f–, red circles) are plotted against the aromatic content (ϕ) in each IDR. The IDRs are binned based on their aromatic content. For the IDRs in proteomes, the mean fraction of positive and negative charges decay linearly as the IDRs are enriched with aromatic residues. The linear fits obtained are as follows, f+ = 0.13–0.28ϕ and f– = 0.145–0.13ϕ. The depletion of positive and negatively charged residues are nearly similar as aromatic residues enrich sequences leading to nearly polyampholyte behavior (i.e., NCR is close to zero). For IDRs that are prone to LLPS, the linear fits obtained are as follows: f+ = 0.13–0.39ϕ and f– = 0.17–1.05ϕ. The greater depletion of the negatively charged residues than of the positively charged residues in these sequences lead toward an increase in the net positive charge of the IDR regions.
The interplay between the fraction of charged residues and the fraction of aromatic residues is different for IDRs that form LLPS. In such IDRs forming condensates, the depletion of negatively charged residues is much greater than the depletion of the positively charged residues as the IDRs become more enriched with aromatic residues (Figure 5B). This suggests a conservation of positively charged residues with aromatic residues over negatively charged residues, leading to a positive net charge of LLPS IDRs (namely, their net charge is coupled to their aromatic content). Such conserved positive charges can interact with aromatic ones under suitable conditions leading toward additional stabilization of the condensate and compensatory mechanism for the reduced stability due to electrostatics weakening.
In the current study, we built on our earlier work44,45 to show that the replacement of charged residues with aromatic residues in disordered peptides leads to trade-offs between short- and long-range interactions during their formation of biomolecular condensates via liquid–liquid phase separation. In particular, our simulations indicate that the formation of cation−π interactions may depend on the composition and organization of charged and aromatic residues in disordered peptide sequences and on environmental conditions (salt concentration). Bioinformatic analysis of IDR regions in LLPS prone proteins suggests a conservation of positively charged residues once aromatic residues enrich the sequences, leading toward a possibility of extensive cation−π interactions. Experimental studies of LLPS have shown that nonionic interactions are fairly important to infer stability to condensates at very high salt concentrations,52,53 highlighting the possibility of compensating interactions due to the loss of charge–charge interactions at high salt concentration. The strong effect salt may have on the interplay between the various molecular forces in the condensate may suggest that detailed computational studies with explicit ions and water molecules are needed to further quantify interactions between IDPs in condensates.
The formation of cation−π interactions depends on breaking charge–charge and π–π interactions. The stability of charge–charge interactions may depend on various factors, including the fraction of charged residues in the sequence (which, in the studied peptides S1–S5, was in inverse proportion to their aromatic residue content), their organizational pattern (represented by the κ value), and solvent salt concentration. We found that decreasing the fraction of charged residues in a disordered peptide, reducing its κ, or increasing the solvent salt concentration led to a loss of electrostatic interactions that reduce condensate stability. However, our simulations showed that such condensate destabilization is offset by 20–80% by the consequent increase in the formation of cation−π interactions. The gain in stability arising from increased cation−π interactions is ∼10–14 kcal/mol per peptide compared with a peptide in a condensate in which cation−π interactions do not occur. The increased stability bestowed by cation−π interactions is linked with a reduction in diffusivity of 10–60% as a function of either sequence charge clustering or aromatic content.
The existence of trade-offs65,66 between charge–charge, π–π, and cation−π interactions suggests that these interactions are not additive and that the loss of some can be compensated for by a gain in others. Such a mechanism suggests weak sensitivity to the loss of interactions, as they can be recovered via the formation of other interactions, as was shown here by the formation of cation−π interactions. Moreover, the trade-off mechanism may have implications for the design of synthetic condensates by leveraging the complex molecular grammar of condensate stability and dynamics to create peptides with the desired properties. Our findings suggest that the stability and dynamics of protein condensates can be tuned by manipulating the fraction of charged residues in the sequence and their clustering pattern to optimize trade-offs among charge–charge, π–π, and cation−π interactions.
Acknowledgments
This work was funded by the Israeli Science Foundation (ISF center of excellence grant no. 2253/18 and 2072/22) for funding. Y.L. holds The Morton and Gladys Pickman professional chair in Structural Biology.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpclett.3c01642.
IDR sequence information for calibration of structure-based coarse-grained model, comparison of experimentally obtained and simulated Rgs for IDR sequences (S1–S4) along variation of cation−π and aromatic strength parameters, phase diagrams for studied sequences with different amount of aromatic content along salt concentration variation, effect of sequence charge clustering on the stability of condensate phase at different salt concentrations, interchain enthalpy variation in condensates along aromatic content variation in studied sequences at different salt concentrations, interchain enthalpy variation in condensates as sequence charge clustering is perturbed in studied sequences at different salt concentrations, relative diffusivity of polymers in condensates with respect to bulk as a function of temperature for variants with differential aromatic content in sequences at different salt, relative diffusivity of polymers in condensates with respect to bulk as a function of temperature for variants with differential charge-clustering in sequences at different salt, and shape-anisotropy variation along aromatic content at different salt concentrations (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Case L. B.; Zhang X.; Ditlev J. A.; Rosen M. K. Stoichiometry controls activity of phase-separated clusters of actin signaling proteins. Science 2019, 363 (6431), 1093–1097. 10.1126/science.aau6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzmann T. M.; Jahnel M.; Pozniakovsky A.; Mahamid J.; Holehouse A. S.; Nüske E.; Richter D.; Baumeister W.; Grill S. W.; Pappu R. V. Phase separation of a yeast prion protein promotes cellular fitness. Science 2018, 359 (6371), eaao5654. 10.1126/science.aao5654. [DOI] [PubMed] [Google Scholar]
- Sabari B. R.Biomolecular Condensates and Gene Activation in Development and Disease. In Developmental Cell; Cell Press: 2020; Vol. 55, pp 84–96. [DOI] [PubMed] [Google Scholar]
- Alberti S.; Gladfelter A.; Mittag T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 2019, 176 (3), 419–434. 10.1016/j.cell.2018.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo M.; Fuxreiter M. Protein condensation diseases: therapeutic opportunities. Nat. Commun. 2022, 13 (1), 5550. 10.1038/s41467-022-32940-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami M.; Taylor J. P.; Parker R. Altered ribostasis: RNA-protein granules in degenerative disorders. Cell 2013, 154 (4), 727–736. 10.1016/j.cell.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J.; Kim N. C.; Wang Y. D.; Scarborough E. A.; Moore J.; Diaz Z.; MacLea K. S.; Freibaum B.; Li S.; Molliex A.; et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 2013, 495 (7442), 467–473. 10.1038/nature11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensah M. A.; Niskanen H.; Magalhaes A. P.; Basu S.; Kircher M.; Sczakiel H. L.; Reiter A. M. V.; Elsner J.; Meinecke P.; Biskup S.; et al. Aberrant phase separation and nucleolar dysfunction in rare genetic diseases. Nature 2023, 614 (7948), 564–571. 10.1038/s41586-022-05682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.; Protter D. S.; Rosen M. K.; Parker R. Formation and Maturation of Phase-Separated Liquid Droplets by RNA-Binding Proteins. Mol. Cell 2015, 60 (2), 208–219. 10.1016/j.molcel.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y.; Farag M.; Lee D.; Zeng X.; Kim K.; Son H. i.; Guo X.; Su J.; Peterson N.; Mohammed J.; et al. Programmable synthetic biomolecular condensates for cellular control. Nat. Chem. Biol. 2023, 19, 518. 10.1038/s41589-022-01252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian M. V.; Wang W.; Dabdoub J. B.; Tong M.; Caldwell R. M.; Benman W.; Schuster B. S.; Deiters A.; Good M. C. Designer membraneless organelles sequester native factors for control of cell behavior. Nat. Chem. Biol. 2021, 17 (9), 998–1007. 10.1038/s41589-021-00840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshareedah I.; Moosa M. M.; Banerjee P. R. Programmable Viscoelasticity in Protein-RNA Condensates with Disordered Sticker-Spacer Polypeptides. bioRxiv 2021, 1. 10.1101/2021.01.24.427968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do S.; Lee C.; Lee T.; Kim D.-N.; Shin Y. Engineering DNA-based synthetic condensates with programmable material properties, compositions, and functionalities. Sci. Adv. 2022, 8 (41), eabj1771. 10.1126/sciadv.abj1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne C. P.; Eckmann C. R.; Courson D. S.; Rybarska A.; Hoege C.; Gharakhani J.; Julicher F.; Hyman A. A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 2009, 324 (5935), 1729–1732. 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- Elbaum-Garfinkle S.; Kim Y.; Szczepaniak K.; Chen C. C.; Eckmann C. R.; Myong S.; Brangwynne C. P. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl. Acad. Sci. U.S.A. 2015, 112 (23), 7189–7194. 10.1073/pnas.1504822112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. F.; Brangwynne C. P.; Gharakhani J.; Hyman A. A.; Julicher F. Spatial organization of the cell cytoplasm by position-dependent phase separation. Phys. Rev. Lett. 2013, 111 (8), 088101 10.1103/PhysRevLett.111.088101. [DOI] [PubMed] [Google Scholar]
- Brangwynne C. P.; Mitchison T. J.; Hyman A. A. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. P Natl. Acad. Sci. USA 2011, 108 (11), 4334–4339. 10.1073/pnas.1017150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Choi J. M.; Holehouse A. S.; Lee H. O.; Zhang X.; Jahnel M.; Maharana S.; Lemaitre R.; Pozniakovsky A.; Drechsel D.; et al. A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 2018, 174 (3), 688–699. 10.1016/j.cell.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. M.; Dar F.; Pappu R. V. LASSI: A lattice model for simulating phase transitions of multivalent proteins. Plos Comput. Biol. 2019, 15 (10), e1007028–e1007028. 10.1371/journal.pcbi.1007028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.-M.; Holehouse A. S.; Pappu R. V. Physical Principles Underlying the Complex Biology of Intracellular Phase Transitions. Ann. Rev. Biophys. 2020, 49, 107. 10.1146/annurev-biophys-121219-081629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamagata K.; Kanbayashi S.; Honda M.; Itoh Y.; Takahashi H.; Kameda T.; Nagatsugi F.; Takahashi S. Liquid-like droplet formation by tumor suppressor p53 induced by multivalent electrostatic interactions between two disordered domains. Sci. Rep-Uk 2020, 10 (1), 580. 10.1038/s41598-020-57521-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamagata K.; Iwaki N.; Hazra M. K.; Kanbayashi S.; Takahashi H.; Kimura M.; Oikawa H.; Takahashi S.; et al. Molecular principles of recruitment and dynamics of guest proteins in liquid droplets. Sci. Rep-Uk 2021, 11, 19323. 10.1038/s41598-021-98955-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichheld S. E.; Muiznieks L. D.; Keeley F. W.; Sharpe S. Direct observation of structure and dynamics during phase separation of an elastomeric protein. Proc. Natl. Acad. Sci. U.S.A. 2017, 114 (22), E4408–E4415. 10.1073/pnas.1701877114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peran I.; Mittag T. Molecular structure in biomolecular condensates. Current Opinion in Structural Biology 2019, 60, 17. 10.1016/j.sbi.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra M. K.; Levy Y. Charge pattern affects the structure and dynamics of polyampholyte condensates. Phys. Chem. Chem. Phys. 2020, 22, 19368. 10.1039/D0CP02764B. [DOI] [PubMed] [Google Scholar]
- Hazra M. K.; Levy Y. Affinity of disordered protein complexes is modulated by entropy-energy reinforcement. PNAS 2023, 119, e2120456119. 10.1073/pnas.2120456119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.; Eisen A.; Lin Y. H.; Chan H. S. A Lattice Model of Charge-Pattern-Dependent Polyampholyte Phase Separation. J. Phys. Chem. B 2018, 122 (21), 5418–5431. 10.1021/acs.jpcb.7b11723. [DOI] [PubMed] [Google Scholar]
- Lin Y.; Currie S. L.; Rosen M. K. Intrinsically disordered sequences enable modulation of protein phase separation through distributed tyrosine motifs. J. Biol. Chem. 2017, 292, 19110. 10.1074/jbc.M117.800466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy A. C.; Dignon G. L.; Kan Y.; Zerze G. H.; Parekh S. H.; Mittal J.; Fawzi N. L. Molecular interactions underlying liquid-liquid phase separation of the FUS low-complexity domain. Nature Structural & Molecular Biology 2019, 26 (7), 637. 10.1038/s41594-019-0250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon R. M. C.; Chong P. A.; Tsang B.; Kim T. H.; Bah A.; Farber P.; Lin H.; Forman-Kay J. D. Pi-Pi contacts are an overlooked protein feature relevant to phase separation. Elife 2018, 7, 1–48. 10.7554/eLife.31486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer A.; Farag M.; Borcherds W. M.; Peran I.; Martin E. W.; Pappu R. V.; Mittag T. Deciphering how naturally occurring sequence features impact the phase behaviours of disordered prion-like domains. Nat. Chem. 2022, 14 (2), 196–207. 10.1038/s41557-021-00840-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.; Ng S. C.; Tompa P.; Lee K. A.; Chan H. S. Polycation-pi interactions are a driving force for molecular recognition by an intrinsically disordered oncoprotein family. PLoS Comput. Biol. 2013, 9 (9), e1003239 10.1371/journal.pcbi.1003239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekhi S.; Sundaravadivelu Devarajan D.; Howard M. P.; Kim Y. C.; Nikoubashman A.; Mittal J. Role of Strong Localized vs Weak Distributed Interactions in Disordered Protein Phase Separation. J. Phys. Chem. B 2023, 127 (17), 3829–3838. 10.1021/acs.jpcb.3c00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignon G. L.; Zheng W.; Kim Y. C.; Best R. B.; Mittal J. Sequence determinants of protein phase behavior from a coarse-grained model. PLoS Comput. Biol. 2018, 14 (1), e1005941 10.1371/journal.pcbi.1005941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignon G. L.; Best R. B.; Mittal J. Biomolecular Phase Separation: From Molecular Driving Forces to Macroscopic Properties. Annu. Rev. Phys. Chem. 2020, 71, 53–75. 10.1146/annurev-physchem-071819-113553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W.; Dignon G. L.; Jovic N.; Xu X.; Regy R. M.; Fawzi N. L.; Kim Y. C.; Best R. B.; Mittal J. Molecular Details of Protein Condensates Probed by Microsecond Long Atomistic Simulations. J. Phys. Chem. B 2020, 124 (51), 11671–11679. 10.1021/acs.jpcb.0c10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J. A.; Reinhardt A.; Aguirre A.; Chew P. Y.; Russell K. O.; Espinosa J. R.; Garaizar A.; Collepardo-Guevara R. Physics-driven coarse-grained model for biomolecular phase separation with near-quantitative accuracy. Nat. Comput. Sci. 2021, 1 (11), 732–743. 10.1038/s43588-021-00155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy A. C.; Dignon G. L.; Kan Y.; Zerze G. H.; Parekh S. H.; Mittal J.; Fawzi N. L. Molecular interactions underlying liquid–liquid phase separation of the FUS low-complexity domain. Nature Structural and Molecular Biology 2019, 26 (7), 637–648. 10.1038/s41594-019-0250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan V. H.; Dignon G. L.; Zerze G. H.; Chabata C. V.; Silva R.; Conicella A. E.; Amaya J.; Burke K. A.; Mittal J.; Fawzi N. L. Mechanistic View of hnRNPA2 Low-Complexity Domain Structure, Interactions, and Phase Separation Altered by Mutation and Arginine Methylation. Mol. Cell 2018, 69 (3), 465–479. 10.1016/j.molcel.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E. W.; Holehouse A. S.; Peran I.; Farag M.; Incicco J. J.; Bremer A.; Grace C. R.; Soranno A.; Pappu R. V.; Mittag T. Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science 2020, 367 (6478), 694. 10.1126/science.aaw8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E. W.; Holehouse A. S.; Grace C. R.; Hughes A.; Pappu R. V.; Mittag T. Sequence Determinants of the Conformational Properties of an Intrinsically Disordered Protein Prior to and upon Multisite Phosphorylation. J. Am. Chem. Soc. 2016, 138 (47), 15323–15335. 10.1021/jacs.6b10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. P.; Farber P. J.; Sekhar A.; Lin Y. H.; Huang R.; Bah A.; Nott T. J.; Chan H. S.; Baldwin A. J.; Forman-Kay J. D.; et al. Structural and hydrodynamic properties of an intrinsically disordered region of a germ cell-specific protein on phase separation. Proc. Natl. Acad. Sci. U.S.A. 2017, 10.1073/pnas.1706197114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy A. C.; Tang W. S.; Jovic N.; Janke A. M.; Seo D. H.; Perdikari T. M.; Mittal J.; Fawzi N. L. Molecular interactions contributing to FUS SYGQ LC-RGG phase separation and co-partitioning with RNA polymerase II heptads. Nat. Struct Mol. Biol. 2021, 28 (11), 923–935. 10.1038/s41594-021-00677-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra M. K.; Levy Y. Biophysics of Phase Separation of Disordered Proteins Is Governed by Balance between Short- And Long-Range Interactions. J. Phys. Chem. B 2021, 125 (9), 2202–2211. 10.1021/acs.jpcb.0c09975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessen J.; Das S.; Pal T.; Chan H. S. Analytical Formulation and Field-Theoretic Simulation of Sequence-Specific Phase Separation of Protein-Like Heteropolymers with Short- and Long-Spatial-Range Interactions. J. Phys. Chem. B 2022, 126 (45), 9222–9245. 10.1021/acs.jpcb.2c06181. [DOI] [PubMed] [Google Scholar]
- Alshareedah I.; Kaur T.; Ngo J.; Seppala H.; Kounatse L. A. D.; Wang W.; Moosa M. M.; Banerjee P. R. Interplay between Short-Range Attraction and Long-Range Repulsion Controls Reentrant Liquid Condensation of Ribonucleoprotein-RNA Complexes. J. Am. Chem. Soc. 2019, 141 (37), 14593–14602. 10.1021/jacs.9b03689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cabau C.; Salvatella X.. Regulation of biomolecular condensate dynamics by signaling. In Current Opinion in Cell Biology; Elsevier Ltd: 2021; Vol. 69, pp 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.; Lin Y. H.; Vernon R. M.; Forman-Kay J. D.; Chan H. S. Comparative roles of charge, pi, and hydrophobic interactions in sequence-dependent phase separation of intrinsically disordered proteins. P Natl. Acad. Sci. USA 2020, 117 (46), 28795–28805. 10.1073/pnas.2008122117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.; Lee S. E.; Jeong S.; Lee J.; Park D.; Chang S. Multivalent electrostatic pi–cation interaction between synaptophysin and synapsin is responsible for the coacervation. Molecular Brain 2021, 14 (1), 137. 10.1186/s13041-021-00846-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreon J. C.; Jain A.; Choi K. J.; Tsoi P. S.; Mackenzie K. R.; Jung S. Y.; Ferreon A. C. Acetylation disfavors tau phase separation. Int. J. Mol. Sci. 2018, 19 (5), 1360. 10.3390/ijms19051360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M.; Hess D.; Eglinger J.; Fritsch A. W.; Kreysing M.; Weinert B. T.; Choudhary C.; Matthias P. Acetylation of intrinsically disordered regions regulates phase separation. Nat. Chem. Biol. 2019, 15 (1), 51–61. 10.1038/s41589-018-0180-7. [DOI] [PubMed] [Google Scholar]
- Fetahaj Z.; Ostermeier L.; Cinar H.; Oliva R.; Winter R. Biomolecular Condensates under Extreme Martian Salt Conditions. J. Am. Chem. Soc. 2021, 143 (13), 5247–5259. 10.1021/jacs.1c01832. [DOI] [PubMed] [Google Scholar]
- Krainer G.; Welsh T. J.; Joseph J. A.; Espinosa J. R.; Wittmann S.; de Csillery E.; Sridhar A.; Toprakcioglu Z.; Gudiskyte G.; Czekalska M. A.; et al. Reentrant liquid condensate phase of proteins is stabilized by hydrophobic and non-ionic interactions. Nat. Commun. 2021, 12 (1), 1085. 10.1038/s41467-021-21181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y.; Najafi S.; Casey T.; Shea J. E.; Han S. I.; Hwang D. S. Hydrophobicity of arginine leads to reentrant liquid-liquid phase separation behaviors of arginine-rich proteins. Nat. Commun. 2022, 13 (1), 7326. 10.1038/s41467-022-35001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch Leshem A.; Sloan-Dennison S.; Massarano T.; Ben-David S.; Graham D.; Faulds K.; Gottlieb H. E.; Chill J. H.; Lampel A. Biomolecular condensates formed by designer minimalistic peptides. Nat. Commun. 2023, 14 (1), 421. 10.1038/s41467-023-36060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qamar S.; Wang G. Z.; Randle S. J.; Ruggeri F. S.; Varela J. A.; Lin J. Q.; Phillips E. C.; Miyashita A.; Williams D.; Ströhl F.; et al. FUS Phase Separation Is Modulated by a Molecular Chaperone and Methylation of Arginine Cation-π Interactions. Cell 2018, 173 (3), 720–734. 10.1016/j.cell.2018.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R. K.; Pappu R. V. Conformations of intrinsically disordered proteins are influenced by linear sequence distributions of oppositely charged residues. P Natl. Acad. Sci. USA 2013, 110 (33), 13392–13397. 10.1073/pnas.1304749110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawle L.; Ghosh K. A theoretical method to compute sequence dependent configurational properties in charged polymers and proteins. J. Chem. Phys. 2015, 143 (8), 085101. 10.1063/1.4929391. [DOI] [PubMed] [Google Scholar]
- Givaty O.; Levy Y. Protein Sliding along DNA: Dynamics and Structural Characterization. J. Mol. Biol. 2009, 385 (4), 1087–1097. 10.1016/j.jmb.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Azia A.; Levy Y. Nonnative Electrostatic Interactions Can Modulate Protein Folding: Molecular Dynamics with a Grain of Salt. J. Mol. Biol. 2009, 393, 527. 10.1016/j.jmb.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Salonen L. M.; Ellermann M.; Diederich F. Aromatic rings in chemical and biological recognition: energetics and structures. Angew. Chem., Int. Ed. Engl. 2011, 50 (21), 4808–4842. 10.1002/anie.201007560. [DOI] [PubMed] [Google Scholar]
- Liao S. M.; Du Q. S.; Meng J. Z.; Pang Z. W.; Huang R. B. The multiple roles of histidine in protein interactions. Chem. Cent J. 2013, 7 (1), 44. 10.1186/1752-153X-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onuchic P. L.; Milin A. N.; Alshareedah I.; Deniz A. A.; Banerjee P. R. Divalent cations can control a switch-like behavior in heterotypic and homotypic RNA coacervates. Sci. Rep 2019, 9 (1), 12161. 10.1038/s41598-019-48457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.; Peng X.; Li Y.; Tang W.; Zhu J.; Huang J.; Qi Y.; Zhang Z. LLPSDB: a database of proteins undergoing liquid-liquid phase separation in vitro. Nucleic Acids Res. 2020, 48 (D1), D320–D327. 10.1093/nar/gkz778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigman L. S.; Levy Y. Proteins: molecules defined by their trade-offs. Curr. Opin Struc Biol. 2020, 60, 50–56. 10.1016/j.sbi.2019.11.005. [DOI] [PubMed] [Google Scholar]
- Lin Y. H.; Forman-Kay J. D.; Chan H. S. Theories for Sequence-Dependent Phase Behaviors of Biomolecular Condensates. Biochemistry, American Chemical Society 2018, 57, 2499–2508. 10.1021/acs.biochem.8b00058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






