Abstract
Objective:
Despite marked improvements in the accessibility of childhood vaccines, knowledge gaps remain about the vaccination of children in special risk groups (SRG). This study aimed to analyze the clinical data of children vaccinated in SRG in a single-center unit to contribute to the clinical evidence for the specific planning of immunization of children in SRG. The secondary aim is to present institutional consensus on the vaccination of children in SRG.
Materials and Methods:
This retrospective study was conducted at a single-center pediatric vaccination clinic. Patient charts between 2018 and 2021 were retrospectively reviewed, and clinical and laboratory data were extracted. Serial joint meetings with multiple healthcare professionals were performed to develop an institutional protocol for vaccination.
Results:
There were 479 children vaccinated between 2018 and 2021 for reasons such as postchemotherapy, after hematopoietic stem cell transplantation, before/after solid organ transplantation, allergies, and chronic diseases. Of these, 298 (62.2%) children vaccinated in the unit due to a history of food or vaccine allergies were excluded. One hundred eighty-one children were vaccinated at a median age of 11 [7-15] years. Most children were vaccinated after treatment for malignancies. Solid tumors were the most frequent malignancy (67%), followed by acute lymphoblastic leukemia (29.0%) and acute myeloid leukemia (4.0%). Institutional vaccination protocols for cancer survivors, hematopoietic stem cells, and solid organ recipient children were developed and presented.
Conclusion:
There is a need to prepare national guidelines for vaccinating children with altered immunocompetence. Sharing vaccination practices by multidisciplinary vaccination units might increase and provide knowledge to develop national policies.
Keywords: Cancer, children, hematopoietic stem cell transplantation, solid organ transplantation, vaccine
What is already known on this topic?
Vaccination of children in special risk groups, including children with chronic diseases, immunodeficiencies, solid organ/hematopoietic stem cell transplantation, and cancer survivors, requires specific planning.
There is no specific evidence-based national guideline for vaccinating children in special risk groups in Turkey.
What this study adds on this topic?
A wide variety of children need specific planning for vaccination, such as postchemotherapy, posttransplantation (bone marrow or solid organ), or chronic diseases.
Children receiving chemotherapy demonstrated that they were serologically highly negative against vaccine-preventable illnesses.
We present our institutional consensus on the vaccination of children in special risk groups to provide knowledge to develop future national guidelines.
Introduction
Global efforts to vaccinate children significantly improved child survival, but vaccine-preventable infections (VPI) still cause 21.7% of under-5 child mortality.1 Each country has a uniquely designed vaccination schedule. As such, Turkey leads the Expanded National Immunization Program since 2008, which covers vaccination against 13 antigens.2 Despite marked improvements in the accessibility of childhood vaccines, knowledge gaps remain about the vaccination of children in special risk groups (SRG). Risk groups include children with chronic diseases, immunodeficiencies, solid organ/hematopoietic stem cell transplantation (SOT/HSCT), and cancer survivors. Infection poses a significant but avoidable risk for these children as they have an altered immunity.3-5 Depending on the underlying condition, they may need extra doses, avoid specific vaccines such as live vaccines, or have an increased risk of VPI or complications.
It is noted that vaccine hesitancy is an increasingly common problem in many countries as in Turkey.6 Weakened herd immunity may expose children in SRG to an increased risk of VPI. For example, cancer survivors are at increased risk for VPI, such as invasive pneumococcal, Haemophilus influenzae infections, or fatal Varicella infections.7-10 Being previously fully immunized may not protect children, as waning immunity after chemotherapy has already been demonstrated.11-13 Although local guidelines exist in some countries for children in SRG, the ideal regimen or timing for a standard schedule is an area of future research.14
There is no specific evidence-based national guideline for vaccinating children in SRG in Turkey. The National Turkish health ministry published recommendations for vaccinating people in risk groups in 2017. Still, it is not explicitly designed for children, even though it includes sections related to children. Vaccination of these children requires expertise and collaborative work. This study aimed to analyze the clinical data of children vaccinated in SRG in a single-center unit to contribute to the clinical evidence for the specific planning of immunization of children in SRG. The secondary aim is to present institutional consensus on the vaccination of children in SRG and discuss the consensus in line with previous publications.
Materials and Methods
Study Design and Participants
This retrospective descriptive study was conducted at a single-center pediatric vaccination clinic at University Hospital in İstanbul. İstanbul is located in the western region of Turkey, where the vaccine coverage in the national immunization schedule was between 68 and 95% depending on the vaccine in the 2018 Turkey Demographic and Health Survey.15 The clinic is directed by the Social Pediatrics Department of the Marmara University, and vaccination of children in SRG was performed in the unit according to the institutional consensus.
Patient charts presented to the vaccination unit between 2018 and 2021 were retrospectively reviewed, and clinical and laboratory data were extracted. All patients vaccinated for specific reasons, such as for SOT/HSCT, postchemotherapy, or chronic diseases, were included. Patients vaccinated due to allergies were excluded. Consensus protocols for SOT/HSCT and postchemotherapy were updated and schematized after analysis of the data of patients between 2018 and 2021.
The consensus was produced by the collaborative work of pediatric infectious diseases and social pediatrics departments. A comprehensive literature search was performed, and findings were discussed with academics from these departments in serial joint meetings. All childhood vaccines in the national immunization schedule are freely available in the unit. In Turkey, routine childhood vaccines include BCG, hepatitis B, oral polio vaccine, pentavalent vaccine (DTaP, IPV, and H. influenzae type b), Streptococcus pneumonia, and measles–mumps–rubella (MMR), varicella, and hepatitis A (Table 1).16 Vaccines not covered in the national immunization schedule such as group B meningococcus (MenB), quadrivalent meningococcal, polysaccharide pneumococcal, human papillomavirus (HPV), and influenza vaccines purchased by the families were also administered in SRG.
Table 1.
National Immunization Schedule in Turkey
| Birth | First month | Second month | Fourth month | Sixth month | Ninth month | Twelfth month | Eighteenth month | Twenty-fourth month | Forty-eighth month | Thirteenth year | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatitis B | x | x | x | ||||||||
| BCG | x | ||||||||||
| PCV13 | x | x | x | ||||||||
| DTaP-Hib-IPV | x | x | x | x | |||||||
| OPV | x | x | |||||||||
| Varicella | x | ||||||||||
| MMR | x* | x | x | ||||||||
| Hepatitis A | x | x | |||||||||
| DTaP-IPV | x | ||||||||||
| Td | x |
DTaP-Hib-IPV, diphtheria/tetanus/acellular pertussis; Haemophilus influenzae b conjugate, inactivated polio virus; MMR, measles/mumps/rubella; PCV13, 13-valent pneumococcal conjugate.
*Administered as an additional dose in regions with a high risk of measles.
Ethics Committee Approval
The study was approved by the University Ethics Committee of Marmara University School of Medicine (03.02.2023.258). Due to the study’s retrospective nature, no written informed consent has been obtained from families. To ensure data confidentiality, none of the personal identifying data of the participants were handled nor accessed.
Statistical Analyses
Statistical analyses were performed using Statistical Package for the Social Sciences version 28.0 (IBM Inc., Armonk, NY, USA). Descriptive data were presented as median [interquartile range (IQR)] or n (%) with significance at the P < .05 level. Comparison of vaccine seropositivity among different groups (hematologic vs. nonhematologic malignancies) was performed by chi-square test.
Results
Characteristics of the Study Sample
There were 479 children vaccinated between 2018 and 2021 for special reasons such as postchemotherapy, after HSCT, before/after SOT, allergies, and chronic diseases requiring specific planning. Of these, 298 (62.2%) children vaccinated in the unit due to a history of food or vaccine allergies were excluded since the corresponding data was previously published.17 The final study sample included 181 children vaccinated at a median age of 11 [7-15] years (Table 2). Most children were vaccinated after treatment for malignancies. Other reasons were cochlear implantation, before/after SOT, HSCT, splenectomy, and other chronic diseases.
Table 2.
Characteristics of the Participants
| Characteristics | Variables |
|---|---|
| Age at vaccination, median [IQR] years | 11 [7-15] |
| Female, n (%) | 75 (41.4) |
| Reason for vaccination, n (%) | 181 (100.0) |
| Postchemotherapy | 100 (55.2) |
| Cochlear implantation | 15 (8.3) |
| Before solid organ transplantation | 10 (5.5) |
| Children incompletely vaccinated | 10 (5.5) |
| After hematopoietic stem cell transplantation | 7 (3.9) |
| After solid organ transplantation | 5 (2.8) |
| Before splenectomy | 5 (2.8) |
| Before chemotherapy | 3 (1.7) |
| Other chronic diseases | 26 (14.3) |
IQR, interquartile range.
Solid tumors were the most frequent malignancy (67%), followed by acute lymphoblastic leukemia (ALL) (29.0%) and acute myeloid leukemia (AML) (4.0%) (Table 2).
Timing of Vaccinations
Children were vaccinated with inactive antigens at a median of 7 [6-8] months and with live viral vaccines at a median of 13 [12-15] months following cessation of chemotherapy. Inactive vaccines were administered to bone marrow recipients at a median of 12 [10-13] months following transplantation, and live vaccines were administered at 24 months.
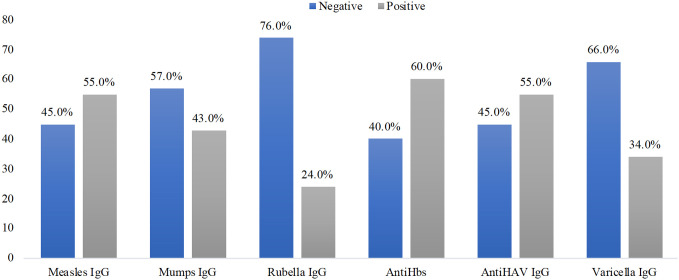
Seropositivity
Children who received chemotherapy for malignancies were routinely screened for seroprotection at a minimum of 6 months postchemotherapy before planning individual vaccination schedules. Accordingly, seroprotection against VPI was 24-60% (Figure 1). Rubella IgG showed the highest negativity, whereas antiHbs showed the highest seropositivity. When comparing children with solid tumors and hematological malignancies, significant differences in the seropositivity of vaccine antigens after chemotherapy were observed only for mumps IgG (61.2% vs. 34.4%, P = .006) and rubella IgG (82.1% vs. 50.0%, P = .003), which were more frequently negative in children with solid tumors.
Figure 1.
Seroprotection against VPI in children post chemotherapy. VPI, vaccine-preventable infections.
Discussion
This study’s findings indicate that a wide variety of children need specific planning for vaccination, such as postchemotherapy, posttransplantation (bone marrow or solid organ), or chronic diseases. Children receiving chemotherapy demonstrated that they were mainly unprotected against VPI concerning serologic evidence. These findings underline the need to evaluate children in SRG by the multidisciplinary team in vaccination units and the importance of systematic revaccination. We will present our institutional consensus and discuss the current literature in line with these findings.
Postchemotherapy Vaccination of Children
Postchemotherapy titers for VPI were negative in 40%-74% of children depending on the antigen, which indicates an obvious need for systematic vaccination of children who survived malignancies.
Data supporting the revaccination of survivors of childhood malignancies are scarce with variable quality.14 In many studies, a decline in the antibody titers to vaccine antigens has been demonstrated for both hematologic and solid tumors.11,18,19 Existing literature provides evidence for diminished seroprotection against S. pneumoniae, pertussis toxin, tetanus toxoid, varicella, and MMR, independent of vaccination history in former patients with childhood cancer.18,20 After revaccination, these children are capable of producing protective antibodies.18,20
Regional recommendations differ in timing or doses of postchemotherapy vaccination. Australian guideline recommends booster vaccination at 6 months postchemotherapy for people who completed primary vaccination without checking for seroprotection.21 The ECIL-7 (European Conference on Infections in Leukemia-7) guideline suggests booster vaccination according to the age and country guidelines at 3-6 months postchemotherapy irrespective of antibody titers in fully vaccinated children.22 For Varicella, an alternative option is to check seroprotection and vaccinate only seronegative children. This guideline covers only people with hematologic malignancies but not solid tumors. United Kingdom guidelines on vaccination for pediatric patients treated with standard-dose chemotherapy suggest booster vaccination doses at 6 months postchemotherapy.23 The Canadian guideline on immunizations of immunocompromised people recommends administering the routine inactivated vaccines to people with solid or hematologic malignancies according to the schedule, preferably 2 weeks before the start of chemotherapy or when the immunosuppression is at the lowest level.24 Doses administered during immunosuppressive therapy should be repeated once the patient is no longer immunocompromised.24 Live vaccines are only administered 3-6 months after chemotherapy.24 The need for booster doses for previously vaccinated children after chemotherapy is not specified. According to the US guidelines, fully vaccinated children before chemotherapy are expected to retain immune memory, and revaccination is not recommended.25
Decision-making for the revaccination of children after chemotherapy requires center-specific and patient-tailored planning depending on the available resources, such as titer measurements.14 Our suggested institutional protocol for postchemotherapy vaccination incorporates time after chemotherapy discontinuation, previous vaccination history, and serologic evidence of immune protection. Six months postchemotherapy, seroprotection for VPI (measles, mumps, rubella, hepatitis A, hepatitis B, and varicella) are checked through a blood test. After controlling the previous vaccine records, a schedule is arranged as in Table 3. Inactive vaccines are introduced in the schedule starting from 6 months postchemotherapy. Vaccination is planned 1 year after the last chemotherapy for the live vaccines. Lymphocyte counts were confirmed to be above the lower limit of age-matched reference data before live vaccination.26 For all vaccinations, in case of absent seroprotection, unknown history of immunization, or interrupted schedule, all age-appropriate vaccine doses are given: 2 doses of MMR and varicella, 3 doses of hepatitis B, and 2 doses of hepatitis A. In the routine Turkish NIP, the varicella vaccine is given in 1 dose. However, in case of negative protection, 2 varicella vaccines are given concerning the severe course of Varicella infections in children receiving chemotherapy. For the vaccines for which no serologic testing is available, all age-appropriate doses are given if not previously immunized, but only booster doses are given in case of a completed schedule before chemotherapy.
Table 3.
Recommendations for Children Treated with Chemotherapy for Malignancies
| Vaccines | Recommendationsa | ||
|---|---|---|---|
| Vaccines with no available serologic tests | |||
| Incomplete schedule | Complete schedule | ||
| DTaP-Hib-IPV/Tdap/Td | Age-appropriate complete schedule | Booster dose recommended | |
| PCV13 | Age-appropriate complete schedule | Booster dose recommended | |
| MenACWY | Age-appropriate complete schedule | Booster dose recommended | |
| Men B | Age-appropriate complete schedule | Booster dose recommended | |
| HPV | Age-appropriate complete schedule | Booster dose recommended | |
| Influenza | Yearly | Yearly | |
| Vaccines with available serologic tests | |||
| Hepatitis A | Seropositive but incomplete schedule: complete the schedule | ||
| Seronegative: 2 doses (0 and 6 months) | |||
| Hepatitis B | Seropositive but incomplete schedule: complete the schedule | ||
| Seropositive, complete schedule: Booster dose recommended | |||
| Seronegative: 3 doses (0.,1. and 6 months) | |||
| MMRb | Seronegative: | Measles negative: 2 doses (3 months apart) Mumps negative: 1 dose Rubella negative: 1 dose |
|
| Varicellab | Seronegative: 2 doses (3 months apart) | ||
HPV, human papillomavirus; MMR, measles-mumps-rubella.
aSeroprotection is checked for hepatitis A and B, measles, mumps, rubella, and varicella at 6 months after completion of chemotherapy. Previous history of vaccination is checked in national vaccination record system.
bLive vaccines are given at 12 months postchemotherapy after checking lymphocyte counts.
Vaccination After Hematopoietic Stem Cell Transplantation in children
Children with HSCT differ from patients with cancer because they are considered as if they were never vaccinated. Antibody decline after HSCT is demonstrated, contributing to the mortality resulting from infections.27,28 Influenza, varicella, and invasive pneumococcal infections are frequent reasons among the VPI for hospitalization.29 Guidelines suggest the revaccination of these children aligned with the national vaccine schedule regardless of the previous vaccination history or serology check.21,23-25,30-32
Inactive antigens were given at a median of 12 months, whereas live vaccines were administered 24 months post-HSCT. The optimal timing for the revaccination of hematopoietic stem cell recipients has yet to be established.32 Deferring revaccination may predispose the children to infections such as influenza and pneumococcal diseases.33,34 On the other hand, children may show variable or short-lasting immune responses depending on the time from transplantation to vaccination.35,36 Timing for inactive vaccines may differ between 3 and 12 months, while most guidelines suggest live vaccines 24 months after HSCT.32 Delay in starting vaccination with inactive antigens in our patients may result from a delayed referral or individual clinical conditions requiring deferral of immunization, such as graft versus host disease (GVHD) or other acute diseases. Vaccination at the earliest opportunity is recommended to protect children with HSCT from VPI.32
The following institutional vaccination schedule is developed for children after HSCT to comply with current national and international recommendations (Table 4). Use of any immunosuppressive medicines, intravenous immunoglobulin, history of GVHD, engraftment, or transfusion should be questioned before vaccination, as in regular vaccination. Baseline antibody titers are not routinely measured before immunization. The dosing and timing of each vaccine are determined by existing recommendations of the Ministry of Health, but international recommendations are also implemented.30-32 These include the administration of the DTaP vaccine regardless of age instead of low-dose diphtheria and pertussis vaccines since low-dose vaccines are poorly immunogenic post-HSCT when used in primary vaccination. In addition, supplementary vaccines (MenACWY, MenB, and HPV) are offered, and a booster dose of pneumococcal conjugate vaccine 13 (PCV13) instead of a 23-valent pneumococcal vaccine in case of GVHD is administered. Inactive vaccines are introduced in the schedule starting from 6 months. Live vaccines are administered 24 months posttransplant, provided the patients have no GVHD or neutropenia/lymphopenia and are not on immunosuppressive therapy.
Table 4.
Vaccination Schedule for Hematopoietic Stem Cell Recipient Children
| Time after Hematopoietic Stem Cell Transplantation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccines | Six months | Seven months | Eight months | Ten months | Twelve months | Thirteen months | Fourteen months | Eighteen months | Twenty-four months | Twenty-seven months | Seven years |
| DTaP-Hib-IPV | x | x | x | x | |||||||
| Hepatitis B | x | x | x | ||||||||
| Influenza | x | x | |||||||||
| Hepatitis A | x | x | |||||||||
| PCV13 | x | x | x | x* | |||||||
| PPSV23 | x | x | |||||||||
| MenACWY | x | x | |||||||||
| Men B | x | x | |||||||||
| HPV (>9 years) | x | x | x | ||||||||
| MMR | x | x | |||||||||
| Varicella | x | x | |||||||||
DTaP-Hib-IPV, diphtheria/tetanus/acellular pertussis; Haemophilus influenzae b conjugate, inactivated polio virus; HPV, human papillomavirus; MenACWY, menincococcal ACWY conjugate; Men B, meningococcal B conjugate; MMR, measles/mumps/rubella; PCV13, 13-valent pneumococcal conjugate; PPSV23: 23 valent pneumococcal polysaccharide.
*Administered if the patient has graft versus host disease instead of the PPSV23 vaccine.
Vaccination Before and After Solid Organ Transplantation in Children
Vaccine-preventable illnesses pose a significant but avoidable risk for children with SOT. Optimal pretransplantation vaccination is a priority since posttransplantation lifelong immunosuppression may oppose live vaccination or attenuate immune response. A recent study showed that pediatric solid organ recipients were hospitalized 87 times more often than the general population due to VPI in the first 5 years after transplant, frequently due to influenza, pneumococcus, varicella, and rotavirus infections.37 Pediatric transplant candidates should receive all age-appropriate vaccines to provide ideal posttransplantation outcomes.30 Despite these recommendations, the under-immunization of pediatric transplant patients is a common problem.38
Communication between multiple healthcare providers of the transplant candidate is crucial for proper and timely immunization before the transplant. Any child with organ failure that is expected to require transplantation should be checked for age-appropriate vaccination even before listing, and an accelerated schedule should be offered to eligible children. These include the administration of MMR and Varicella vaccines at 6-11 months of age for the pretransplant optimization of live vaccine uptake.30,39
Given the importance of pretransplant vaccination, the following vaccination principles are applied in our vaccination clinic for pediatric transplant candidates (Table 5):
Table 5.
Vaccination Recommendations for Pediatric Solid Organ Candidates Before Transplantation
| Vaccines | Recommendations* | |||
|---|---|---|---|---|
| DTaP-Hib-IPV | Complete schedule according to the age of the child | |||
| Hepatitis A | Incomplete schedule: complete the schedule | |||
| Seronegative: 2 doses | ||||
| Hepatitis B | Incomplete schedule: complete the schedule | |||
| Evaluation of serologic response 1 month after the last dose | If Anti-Hbs | <100→ apply booster dose | ||
| <10→ primary serial vaccination (3-dose series) | ||||
| Accelerated schedule if required: 20 µg at 0, 1, and 2 months | ||||
| HPV | After the age of 9, 3 doses are administered to all boys and girls at 0, 1-2 , and 6 months | |||
| Influenza | Yearly | |||
| MenACWY | Complete according to the age of the child. After 2 years of age, 2 doses are given regardless of age. | |||
| Men B | Complete according to the age of the child. | |||
| PCV13 | 24-71 months of age | |||
| Complete schedule with at least 2 doses of PCV13 | 1 dose PPSV23 | |||
| Unvaccinated or vaccinated with ≤ 3 doses of PCV 7 | 2 doses of PCV13 administered 8 weeks apart. | |||
| 4 doses of PCV7 or incomplete schedule of PCV7 | 1 dose of PCV13 administered at least 8 weeks after the last PCV7. | |||
| 72 months to 18 years | ||||
| Not vaccinated with PCV13 and PPSV23 | 1 dose of PCV13 followed by PPSV23 at least 8 weeks later | |||
| Completely vaccinated with PCV13 | PPSV23 at least 8 weeks after the last PCV13 | |||
| PPSV23 administered but not vaccinated with PCV13 | PCV13 at least 8 weeks after the last PPSV23 dose | |||
| PPSV23 | 1 dose is recommended 8 weeks after the last PCV13 dose. | |||
| A booster dose is recommended 5 years after vaccination. | ||||
| MMR | Seronegative: | 6-12 months: 1 dose (this dose is considered as zero dose), after the 12th month, 2 doses are administered 1-3 months apart depending on the estimated urgency of transplantation | ||
| >1 year: 2 doses (1-3 months apart) | ||||
| No seroconversion 4 weeks after 2 doses → an additional dose is administered | ||||
| Seropositive and the usual dose at 48 months is not administered yet→ The usual dose is given earlier than 48 months | ||||
| Not transplant at the time of the usual 48 months dose →1 dose of MMR is administered | ||||
| Varicella | 2 doses, 1-3 months apart (starting from 6 months of age) | |||
| First dose given before the age of 12 months (0 dose) → 1 dose of vaccination is performed and seroconversion is checked (4 weeks later) | ||||
HPV, human papillomavirus; MMR, measles, mumps, and rubella.
*Seroprotection against available vaccine antigens should be checked before vaccination planning.
All age-appropriate vaccines in the national vaccination schedule should be given to pediatric transplant candidates at the usual time.
MMR and Varicella vaccinations should be initiated for young transplant candidates starting from 6 months of age. If the child is not transplanted at 12 months, 2 doses of MMR and Varicella vaccines should be administered 1-3 months apart.
An accelerated schedule should be considered for hepatitis B vaccination with double dosing.
If the transplant candidate is already vaccinated according to the national vaccination schedule, serologic titers should be checked for measles, mumps, rubella, hepatitis A, hepatitis B, and varicella.
In children between 1 and 4 years of age, the usual booster dose of MMR at 4 years of age should be given earlier even though the titers are positive.
Live vaccines should be administrated at least 1 month before transplant, whereas inactive vaccines from 2 weeks before the transplant. Ideally, serologic titers should be checked 1 month after the last vaccination, and vaccines should be repeated if necessary.
Supplementary vaccines (MenB, MenACWY, and HPV) should be commenced age appropriately.
Pneumococcal vaccination is performed according to the previously published recommendations of Turkish Ministry of Health.
After SOT, vaccination is generally withheld in the earliest period, when the immunosuppression is maximum.30 Exceptionally, inactive influenza is recommended as early as 1-month posttransplant in the influenza season.30 Most centers restart vaccination at 3-12 months posttransplantation.40,41 However, there is no consensus on the timing and administration of other inactive vaccines posttransplant since it is still to be determined which level of immunosuppression does permit adequate response to vaccines. Likewise, the practice of checking serologic titers shows wide variation.40-42 Any interrupted schedule should be completed after the transplant, but the need for booster doses needs to be better defined. Given the waning antibody titers, we follow the following principles for vaccination after SOT:
Serologic titers for hepatitis A and B are measured, and complete series are administered if negative, regardless of the previous vaccination history.
Booster doses of all other inactive vaccines are recommended.
Inactive vaccines are administered 1-year posttransplant after a clinical evaluation.
Seasonal influenza vaccine is given starting from 1-month posttransplant in the flu season.
Although there is a safety concern, evidence supports the administration of live vaccines in specific solid organ recipients. Safe and effective administration of live vaccines after transplant should be planned in the area of increasing vaccine refusal that causes weakened herd immunity.43 Specific criteria for low-level immunosuppression for kidney and liver transplant recipients have been defined for the safe administration of MMR and Varicella vaccines.39,44 A comprehensive clinical and immunological evaluation should be warranted before administering live vaccines. We follow the previously defined clinical and immunological criteria for live vaccination of pediatric transplant recipients. 12,44
Conclusion
In conclusion, vaccination of children in SRG requires expertise and a multidisciplinary approach. There is a need to prepare national guidelines for vaccinating children after chemotherapy, bone marrow transplantation, before/after solid organ transplantation, and other children with altered immunocompetence, such as chronic diseases or using immunomodulatory drugs. Sharing vaccination practices by multidisciplinary vaccination units might increase awareness about the vaccination of these children and provide knowledge to develop future national guidelines.
Footnotes
Ethics Committee Approval: This study was approved by Ethics Committee of Marmara University School of Medicine (approval no: 09.2023.258, date: 03.02.2023).
Informed Consent: Due to the study’s retrospective nature, no written informed consent has been obtained from families. To ensure data confidentiality, none of the personal identifying data of the participants were handled nor accessed.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – H.E.B., E.K., P.B.; Design – H.E.B., E.K., P.B.; Supervision – A.K., A.G.T., E.K., E.K.A., P.B.; Resources – F.İ.A.S., P.Ş., B.Ü., Z.E.; Materials – F.İ.A.S., P.Ş., B.Ü., Z.E., H.E.B.; Data Collection and/or Processing – F.İ.A.S., P.Ş., B.Ü., Z.E., H.E.B.; Analysis and/or Interpretation – H.E.B., E.K., P.B., Z.E., E.K.A., A.G.T, A.K.; Literature Search – H.E.B., E.K., P.B., Z.E., E.K.A., A.G.T, A.K.; Writing – H.E.B., B.Ü.; Critical Review – P.B., E.K.A., A.G.T, A.K.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: This study received no funding.
This research was previously presented as an oral presentation at the 56th Turkish Pediatric Congress, October 17-21, 2020, in Antalya.
References
- 1. Perin J, Mulick A, Yeung D, et al. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc Health. 2022;6(2):106 115. ( 10.1016/S2352-4642(21)00311-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Available at: https://dosyasb.saglik.gov.tr/Eklenti/1117,gbpgenelge2008pdf.pdf?0. Accessed July 02, 2023. [Google Scholar]
- 3. Perkins JL, Chen Y, Harris A, et al. Infections among long-term survivors of childhood and adolescent cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2014;120(16):2514 2521. ( 10.1002/cncr.28763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zajac-Spychala O, Kampmeier S, Lehrnbecher T, Groll AH. Infectious complications in paediatric haematopoetic cell transplantation for acute lymphoblastic leukemia: current status. Front Pediatr. 2021;9:782530. ( 10.3389/fped.2021.782530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Delden C, Stampf S, Hirsch HH, et al. Burden and timeline of infectious diseases in the first year after solid organ transplantation in the Swiss transplant cohort study. Clin Infect Dis. 2020;71(7):e159 e169. ( 10.1093/cid/ciz1113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gür E. Vaccine hesitancy - vaccine refusal. Turk Pediatr Ars. 2019;54(1):1 2. ( 10.14744/TurkPediatriArs.2019.79990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meisel R, Toschke AM, Heiligensetzer C, Dilloo D, Laws HJ, von Kries RV. Increased risk for invasive pneumococcal diseases in children with acute lymphoblastic leukaemia. Br J Haematol. 2007;137(5):457 460. ( 10.1111/j.1365-2141.2007.06601.x) [DOI] [PubMed] [Google Scholar]
- 8. Lehrnbecher T, Schubert R, Behl M, et al. Impaired pneumococcal immunity in children after treatment for acute lymphoblastic leukaemia. Br J Haematol. 2009;147(5):700 705. ( 10.1111/j.1365-2141.2009.07903.x) [DOI] [PubMed] [Google Scholar]
- 9. Kriner P, Lopez K, Leung J, Harpaz R, Bialek SR, Centers for Disease Control and Prevention (CDC). Notes from the field: varicella-associated death of a vaccinated child with leukemia—California, 2012. MMWR Morb Mortal Wkly Rep. 2014;63(7):161. [PMC free article] [PubMed] [Google Scholar]
- 10. Zignol M, Peracchi M, Tridello G, et al. Assessment of humoral immunity to poliomyelitis, tetanus, hepatitis B, measles, rubella, and mumps in children after chemotherapy. Cancer. 2004;101(3):635 641. ( 10.1002/cncr.20384) [DOI] [PubMed] [Google Scholar]
- 11. Bochennek K, Allwinn R, Langer R, et al. Differential loss of humoral immunity against measles, mumps, rubella and varicella-zoster virus in children treated for cancer. Vaccine. 2014;32(27):3357 3361. ( 10.1016/j.vaccine.2014.04.042) [DOI] [PubMed] [Google Scholar]
- 12. Toret E, Yel SE, Suman M, et al. Immunization status and re-immunization of childhood acute lymphoblastic leukemia survivors. Hum Vaccin Immunother. 2021;17(4):1132 1135. ( 10.1080/21645515.2020.1802975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sewnarine M, Rajan S, Redner A, Rubin LG. Varicella in a previously immune patient with leukemia. J Pediatr Infect Dis Soc. 2017;6(2):e4 e6. ( 10.1093/jpids/piw078) [DOI] [PubMed] [Google Scholar]
- 14. Guilcher GMT, Rivard L, Huang JT, et al. Immune function in childhood cancer survivors: a Children’s Oncology Group review. Lancet Child Adolesc Health. 2021;5(4):284 294. ( 10.1016/S2352-4642(20)30312-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Available at: http://www.sck.gov.tr/wp-content/uploads/2020/08/TNSA2018_ana_Rapor.pdf. Accessed July 02, 2023. [Google Scholar]
- 16.Available at: https://immunizationdata.who.int/pages/schedule-by-country/tur.html?DISEASECODE=&TARGETPOP_GENERAL. Accessed July 02, 2023. [Google Scholar]
- 17. Barış HE, Boran P, Kıykım A, Barış S, Özen A, Aydıner EK. Immunization practices in children with a history of allergies. Turk Pediatr Ars. 2020;55(3):244 250. ( 10.14744/TurkPediatriArs.2020.96636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Top KA, Vaudry W, Morris SK, et al. Waning vaccine immunity and vaccination responses in children treated for acute lymphoblastic leukemia: a Canadian immunization research network study. Clin Infect Dis. 2020;71(9):e439 e448. ( 10.1093/cid/ciaa163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paulides M, Stöhr W, Laws HJ, et al. Antibody levels against tetanus and diphtheria after polychemotherapy for childhood sarcoma: a report from the Late Effects Surveillance System. Vaccine. 2011;29(8):1565 1568. ( 10.1016/j.vaccine.2010.12.084) [DOI] [PubMed] [Google Scholar]
- 20. Speckhart SA. MMR vaccination timing and long-term immunity among childhood cancer survivors. Pediatr Blood Cancer. 2023;70(4):e30133. ( 10.1002/pbc.30133) [DOI] [PubMed] [Google Scholar]
- 21.Available at: https://immunisationhandbook.health.gov.au/contents/vaccination-for-special-risk-groups/vaccination-for-people-who-are-immunocompromised. Accessed July 02, 2023. [Google Scholar]
- 22. Mikulska M, Cesaro S, de Lavallade H, et al. Vaccination of patients with haematological malignancies who did not have transplantations: guidelines from the 2017 European Conference on Infections in leukaemia (ECIL 7). Lancet Infect Dis. 2019;19(6):e188 e199. ( 10.1016/S1473-3099(18)30601-7) [DOI] [PubMed] [Google Scholar]
- 23. Patel SR, Skinner R, Heath P. Vaccinations for paediatric patients treated with standard-dose chemotherapy and haematopoietic stem cell transplantation (HSCT) recipients. Available at: https://www.cclg.org.uk/write/MediaUploads/Member%20area/Treatment%20guidelines/Vaccination_CCLG_April2020.pdf. Accessed July 02, 2023. [Google Scholar]
- 24.Available at: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-3-vaccination-specific-populations/page-8-immunization-immunocompromised-persons.html#a15. Accessed July 02, 2023. [Google Scholar]
- 25.Available at: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/immunocompetence.pdf. Accessed July 02, 2023. [Google Scholar]
- 26. Besci Ö, Başer D, Öğülür İ, et al. Reference values for T and B lymphocyte subpopulations in Turkish children and adults. Turk J Med Sci. 2021;51(4):1814 1824. ( 10.3906/sag-2010-176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Colton H, Greenfield DM, Snowden JA, et al. Long-term survivors following autologous haematopoetic stem cell transplantation have significant defects in their humoral immunity against vaccine preventable diseases, years on from transplant. Vaccine. 2021;39(34):4778 4783. ( 10.1016/j.vaccine.2021.07.022) [DOI] [PubMed] [Google Scholar]
- 28. Styczyński J, Tridello G, Koster L, et al. Death after hematopoietic stem cell transplantation: changes over calendar year time, infections and associated factors. Bone Marrow Transplant. 2020;55(1):126 136. ( 10.1038/s41409-019-0624-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Danino D, Stanek JR, Rangarajan H, Ardura MI. Hospitalizations for vaccine-preventable infections among pediatric hematopoietic cell transplantation recipients in the first 5 years after transplantation. Bone Marrow Transplant. 2021;56(11):2656 2663. ( 10.1038/s41409-021-01373-z) [DOI] [PubMed] [Google Scholar]
- 30. Rubin LG, Levin MJ, Ljungman P, et al. 2014 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2013;58:1–57. [DOI] [PubMed] [Google Scholar]
- 31. Cordonnier C, Einarsdottir S, Cesaro S, et al. Vaccination of haemopoietic stem cell transplant recipients: guidelines of the 2017 European conference on infections in leukaemia (ECIL 7). Lancet Infect Dis. 2019;19(6):e200 e212. ( 10.1016/S1473-3099(18)30600-5) [DOI] [PubMed] [Google Scholar]
- 32. Miller P, Patel SR, Skinner R, et al. Joint consensus statement on the vaccination of adult and paediatric haematopoietic stem cell transplant recipients: prepared on behalf of the British society of blood and marrow transplantation and cellular therapy (BSBMTCT), the Children’s cancer and Leukaemia Group (CCLG), and British Infection Association (BIA). J Infect. 2023;86(1):1 8. [DOI] [PubMed] [Google Scholar]
- 33. Nichols WG, Guthrie KA, Corey L, Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39(9):1300 1306. ( 10.1086/425004) [DOI] [PubMed] [Google Scholar]
- 34. Engelhard D, Cordonnier C, Shaw PJ, et al. Early and late invasive pneumococcal infection following stem cell transplantation: a European bone marrow transplantation survey. Br J Haematol. 2002;117(2):444 450. ( 10.1046/j.1365-2141.2002.03457.x) [DOI] [PubMed] [Google Scholar]
- 35. Karras NA, Weeres M, Sessions W, et al. A randomized trial of one versus two doses of influenza vaccine after allogeneic transplantation. Biol Blood Marrow Transplant. 2013;19(1):109 116. ( 10.1016/j.bbmt.2012.08.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cordonnier C, Labopin M, Chesnel V, et al. Randomized study of early versus late immunization with Pneumococcal Conjugate Vaccine after allogeneic stem cell transplantation. Clin Infect Dis. 2009;48(10):1392 1401. ( 10.1086/598324) [DOI] [PubMed] [Google Scholar]
- 37. Feldman AG, Beaty BL, Curtis D, Juarez-Colunga E, Kempe A. Incidence of hospitalization for vaccine-preventable infections in children following solid organ transplant and associated morbidity, mortality, and costs. JAMA Pediatr. 2019;173(3):260 268. ( 10.1001/jamapediatrics.2018.4954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Feldman AG, Sundaram SS, Beaty BL, et al. Immunization rates at the time of pediatric liver transplant: a prospective multicenter study through the study of liver diseases. Hepatology. 2018;68(suppl 1):149A. [Google Scholar]
- 39. Suresh S, Upton J, Green M, et al. Live vaccines after pediatric solid organ transplant: proceedings of a consensus meeting, 2018. Proceedings of a Consensus Meeting. Pediatr Transplant. 2019;23(7):e13571. ( 10.1111/petr.13571) [DOI] [PubMed] [Google Scholar]
- 40. Danziger-Isakov L, Kumar D, AST ID Community of Practice ID Community of Practice. Vaccination of solid organ transplant candidates and recipients: guidelines from the American Society of Transplantation infectious diseases community of practice. Clin Transplant. 2019;33(9):e13563. ( 10.1111/ctr.13563) [DOI] [PubMed] [Google Scholar]
- 41. Miyairi I, Funaki T, Saitoh A. Immunization practices in solid organ transplant recipients. Vaccine. 2016;34(16):1958 1964. ( 10.1016/j.vaccine.2016.03.001) [DOI] [PubMed] [Google Scholar]
- 42. Groeneweg L, Loeffen YGT, Versluys AB, Wolfs TFW. Safety and efficacy of early vaccination with live attenuated measles vaccine for hematopoietic stem cell transplant recipients and solid organ transplant recipients. Vaccine. 2021;39(25):3338 3345. ( 10.1016/j.vaccine.2021.04.049) [DOI] [PubMed] [Google Scholar]
- 43. Feldman AG, Hsu EK, Mack CL. The importance of prioritizing Pre and posttransplant immunizations in an era of vaccine refusal and epidemic outbreaks. Transplantation. 2020;104(1):33 38. ( 10.1097/TP.0000000000002936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shinjoh M, Hoshino K, Takahashi T, Nakayama T. Updated data on effective and safe immunizations with live-attenuated vaccines for children after living donor liver transplantation. Vaccine. 2015;33(5):701 707. ( 10.1016/j.vaccine.2014.11.052) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a