Abstract
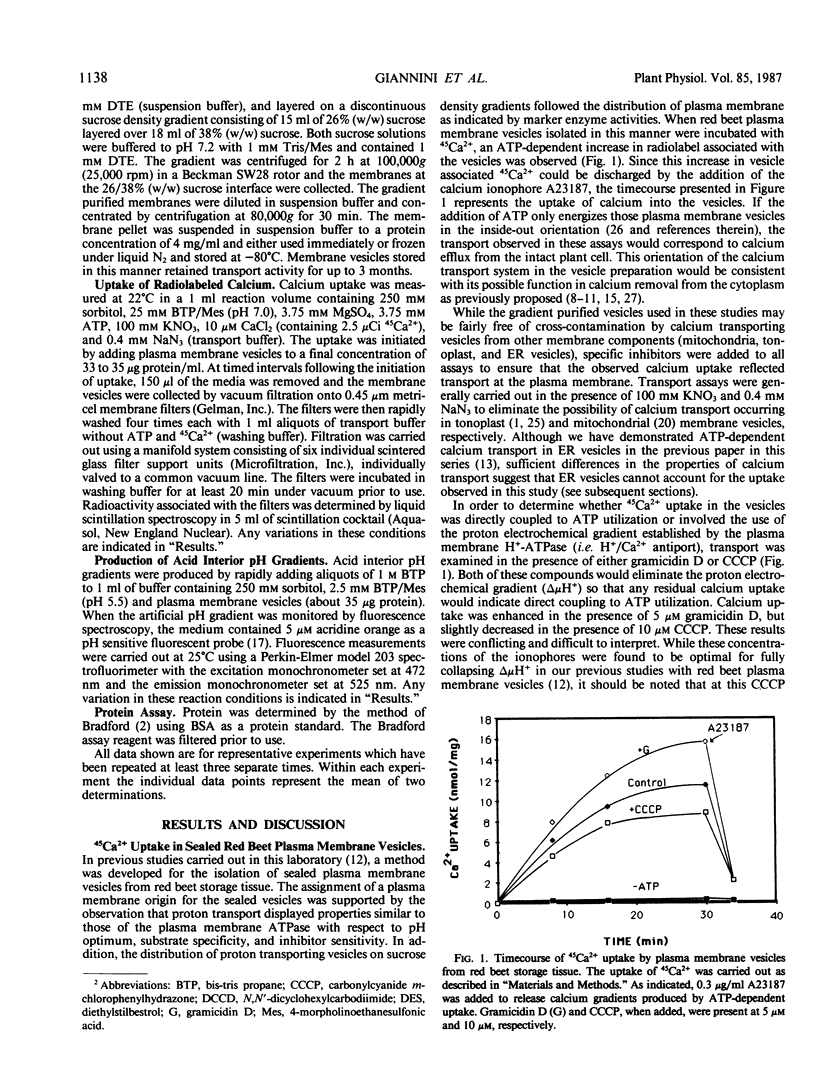
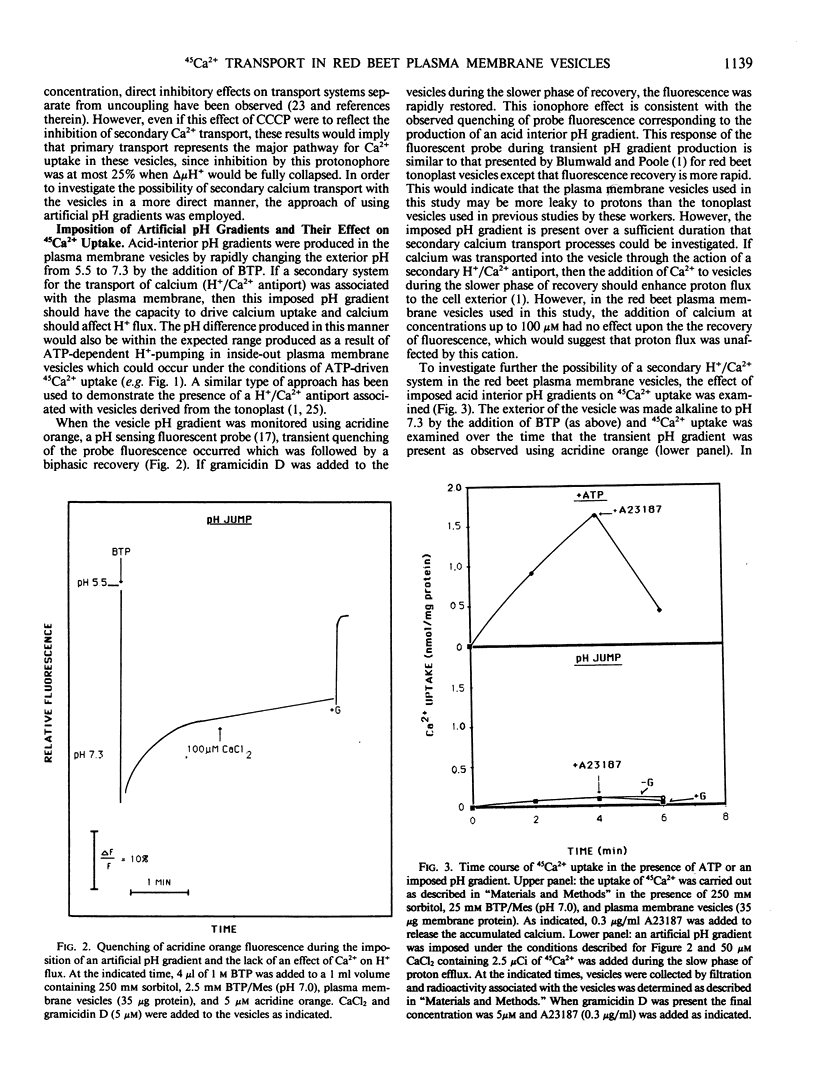
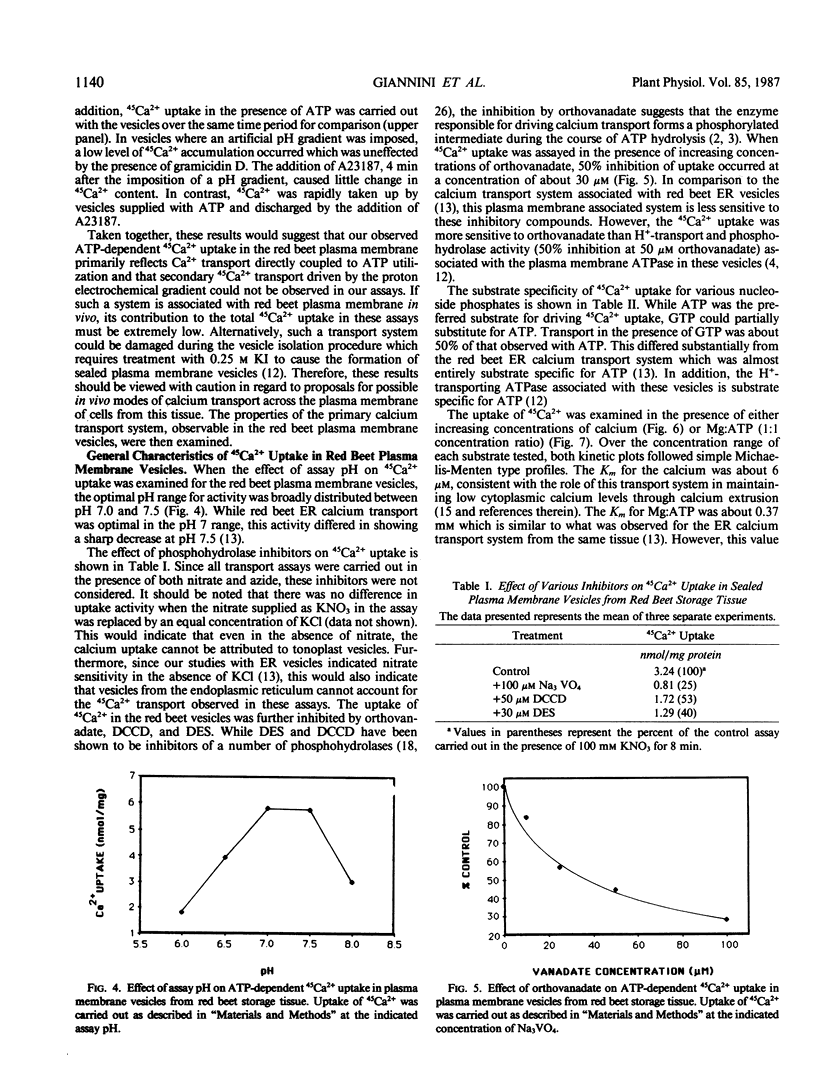
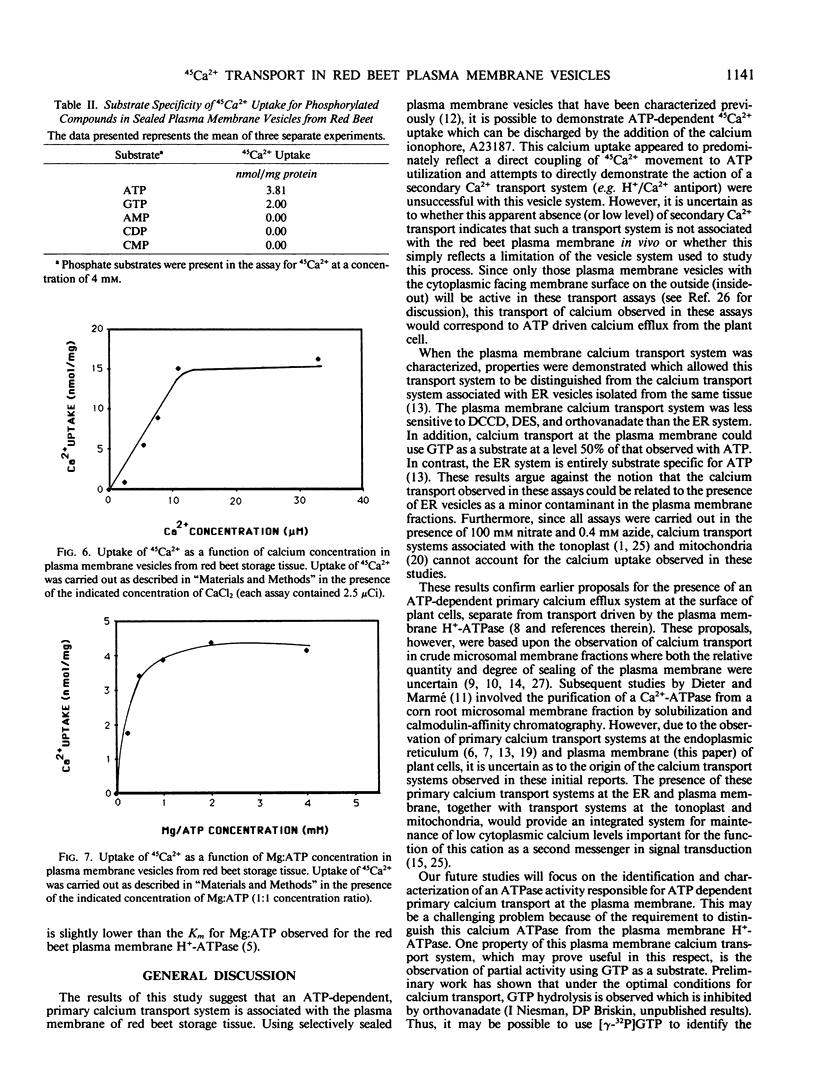
Calcium uptake was examined in sealed plasma membrane vesicles isolated from red beet (Beta vulgaris L.) storage tissue using 45Ca2+. Uptake of 45Ca2+ by the vesicles was ATP-dependent and radiotracer accumulated by the vesicles could be released by the addition of the calcium ionophore A23187. The uptake was stimulated by gramicidin D but slightly inhibited by carbonylcyanide m-chlorophenylhydrazone. Although the latter result might suggest some degree of indirect coupling of 45Ca2+ uptake to ATP utilization via δμH+, no evidence for a secondary H+/Ca2+ antiport in this vesicle system could be found. Following the imposition of an acid-interior pH gradient, proton efflux from the vesicle was not enhanced by the addition of Ca2+ and an imposed pH gradient could not drive 45Ca2+ uptake. Optimal uptake of 45Ca2+ occurred broadly between pH 7.0 and 7.5 and the transport was inhibited by orthovanadate, N,N′-dicyclohexylcarbodiimide, and diethylstilbestrol but insensitive to nitrate and azide. The dependence of 45Ca2+ uptake on both calcium and Mg:ATP concentration demonstrated saturation kinetics with Km values of 6 micromolar and 0.37 millimolar, respectively. While ATP was the preferred substrate for driving 45Ca2+ uptake, GTP could drive transport at about 50% of the level observed for ATP. The results of this study demonstrate the presence of a unique primary calcium transport system associated with the plasma membrane which could drive calcium efflux from the plant cell.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumwald E., Poole R. J. Kinetics of Ca/H Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris L. Plant Physiol. 1986 Mar;80(3):727–731. doi: 10.1104/pp.80.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Briskin D. P., Poole R. J. Characterization of a k-stimulated adenosine triphosphatase associated with the plasma membrane of red beet. Plant Physiol. 1983 Feb;71(2):350–355. doi: 10.1104/pp.71.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckhout T. J. Characterization of Ca Transport in Purified Endoplasmic Reticulum Membrane Vesicles from Lepidium sativum L. Roots. Plant Physiol. 1984 Dec;76(4):962–967. doi: 10.1104/pp.76.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieter P., Marmé D. Calmodulin activation of plant microsomal Ca uptake. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7311–7314. doi: 10.1073/pnas.77.12.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini J. L., Gildensoph L. H., Briskin D. P. Selective production of sealed plasma membrane vesicles from red beet (Beta vulgaris L.) storage tissue. Arch Biochem Biophys. 1987 May 1;254(2):621–630. doi: 10.1016/0003-9861(87)90145-7. [DOI] [PubMed] [Google Scholar]
- Gross J., Marmé D. ATP-dependent Ca uptake into plant membrane vesicles. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1232–1236. doi: 10.1073/pnas.75.3.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew R. R., Briskin D. P., Wyse R. E. Ca uptake by endoplasmic reticulum from zucchini hypocotyls : the use of chlorotetracycline as a probe for ca uptake. Plant Physiol. 1986 Sep;82(1):47–53. doi: 10.1104/pp.82.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller I. M., Kay C. J., Palmer J. M. Chlortetracycline and the transmembrane potential of the inner membrane of plant mitochondria. Biochem J. 1986 Aug 1;237(3):765–771. doi: 10.1042/bj2370765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penniston J. T. Plasma membrane Ca2+-pumping ATPases. Ann N Y Acad Sci. 1982;402:296–303. doi: 10.1111/j.1749-6632.1982.tb25751.x. [DOI] [PubMed] [Google Scholar]
- Poole R. J., Briskin D. P., Krátký Z., Johnstone R. M. Density gradient localization of plasma membrane and tonoplast from storage tissue of growing and dormant red beet : characterization of proton-transport and ATPase in tonoplast vesicles. Plant Physiol. 1984 Mar;74(3):549–556. doi: 10.1104/pp.74.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes J., Benos D. J. Changes in interfacial potentials induced by carbonylcyanide phenylhydrazone uncouplers: possible role in inhibition of mitochondrial oxygen consumption and other transport processes. Membr Biochem. 1984;5(3):243–268. doi: 10.3109/09687688409150281. [DOI] [PubMed] [Google Scholar]
- Schumaker K. S., Sze H. A Ca/H Antiport System Driven by the Proton Electrochemical Gradient of a Tonoplast H-ATPase from Oat Roots. Plant Physiol. 1985 Dec;79(4):1111–1117. doi: 10.1104/pp.79.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]


