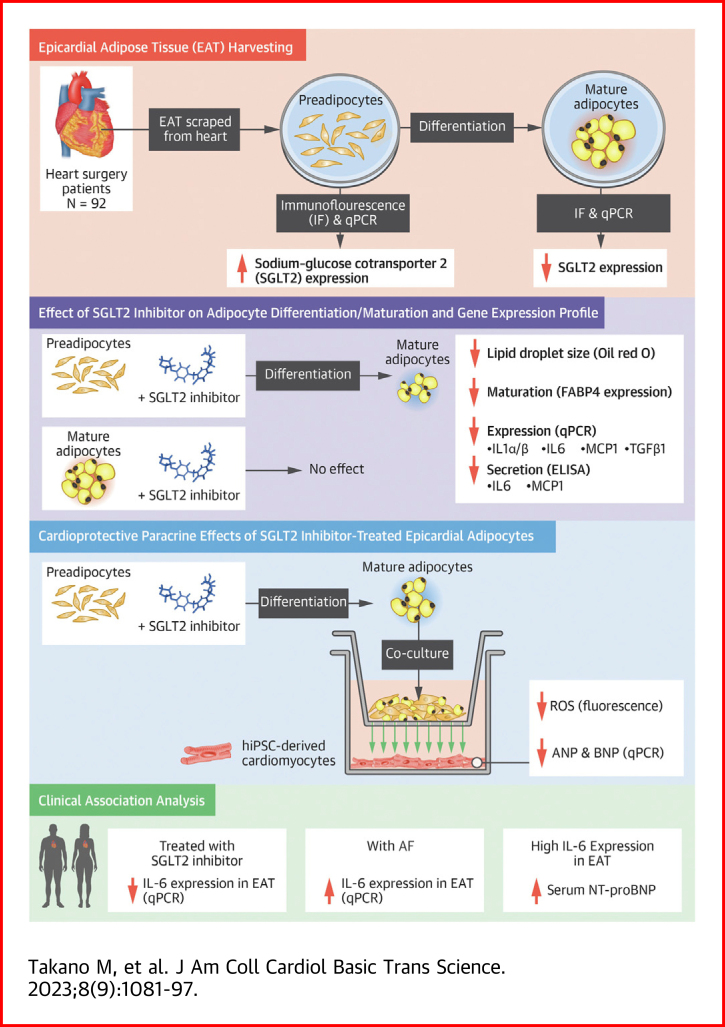
Visual Abstract
Key Words: epicardial adipose tissue, interleukin 6, paracrine effect, preadipocyte, sodium-glucose cotransporter 2 inhibitor
Highlights
-
•
SGLT2 is primarily expressed in human preadipocytes in the epicardial adipose tissue.
-
•
The expression levels of SGLT2 diminish as the preadipocytes are differentiated to mature adipocytes.
-
•
The SGLT2 inhibitor empagliflozin improves the paracrine secretome profiles, potentially leading to the cardioprotective effect.
-
•
IL6 may be a critical adipokine that is regulated by SGLT2 inhibitors.
Summary
Sodium-glucose cotransporter 2 (SGLT2) inhibitors reduce epicardial adipose tissue (EAT) in humans, enhancing cardioprotective effects on heart failure and atrial fibrillation. We investigated the direct effect of the SGLT2 inhibitor empagliflozin on human primary epicardial adipocytes and preadipocytes. SGLT2 is primarily expressed in human preadipocytes in the EAT. The expression levels of SGLT2 significantly diminished when the preadipocytes were terminally differentiated. Adipogenesis of preadipocytes was attenuated by empagliflozin treatment without affecting cell proliferation. The messenger RNA levels and secreted protein levels of interleukin 6 and monocyte chemoattractant protein 1 were significantly decreased in empagliflozin-treated adipocytes. Coculture of human induced pluripotent stem cell–derived atrial cardiomyocytes and adipocytes pretreated with or without empagliflozin revealed that empagliflozin significantly suppressed reactive oxygen species. IL6 messenger RNA expression in human EAT showed significant clinically relevant associations. Empagliflozin suppresses human epicardial preadipocyte differentiation/maturation, likely inhibiting epicardial adipogenesis and improving the paracrine secretome profile of EAT, particularly by regulating IL6 expression.
Several large clinical trials consecutively demonstrated the cardioprotective effects of sodium-glucose cotransporter 2 (SGLT2) inhibitors.1, 2, 3, 4, 5, 6 Although several potential direct and indirect effects of SGLT2 inhibitors are reported,7 a recent clinical trial revealed that empagliflozin significantly reduced epicardial adipose tissue (EAT) volume.8 The effect of SGLT2 inhibitors in reducing EAT volume may be one of the primary mechanisms for preventing cardiovascular diseases. Moreover, SGLT2 inhibitors could favorably affect the secretome profile of EAT.9,10
A subanalysis of the DECLARE TIMI-58 (The Dapagliflozin Effect on Cardiovascular Events Thrombolysis In Myocardial Infarction-58) clinical study revealed that SGLT2 inhibitors are potentially promising drugs for preventing atrial fibrillation (AF).11 Consistent with heart failure, AF is also closely associated with the quantity of EAT. The EAT volume was significantly increased in patients with AF.12 Moreover, the periatrial EAT volume index was significantly correlated with the left atrial volume index. It could predict the prevalence of new AF in patients with coronary artery disease.13 In terms of the quality of EAT, a study demonstrated that human EAT induces fibrosis of the atrial myocardium through the secretion of adipofibrokines.14 However, the specific mechanism of EAT reduction by SGLT2 inhibitors remains unclear. In addition, it has never been demonstrated whether SGLT2 inhibitor–treated epicardial adipocytes could favorably affect human cardiomyocytes via the paracrine effect.
Based on these findings, we investigated the direct effect of the SGLT2 inhibitor empagliflozin on human epicardial preadipocytes and mature adipocytes derived from human EAT of patients undergoing cardiac surgery in terms of maturation, differentiation, and the paracrine secretome of adipocytes. In addition, a coculture experiment using adipocytes and human induced pluripotent stem cell–derived atrial cardiomyocytes (iPS-ACM) was performed to demonstrate the effect of SGLT2 inhibitors on the paracrine effect of adipocytes. Finally, we verified the validity of the findings obtained from basic research from a clinical perspective and clarified that interleukin (IL)-6 is an important EAT adipokine regulated by SGLT2 inhibitors.
Methods
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Samples and study population
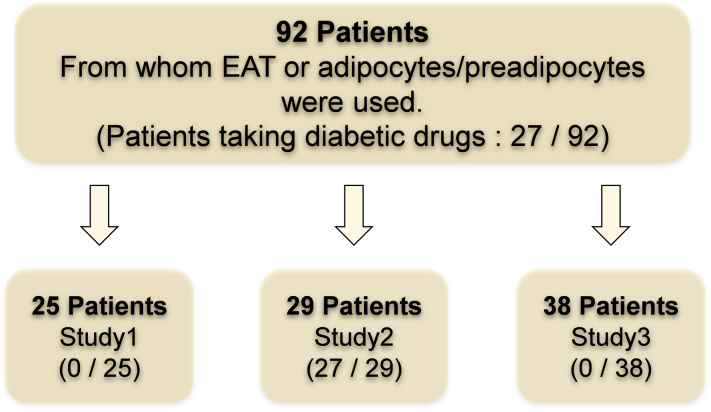
A total of 162 patients who underwent open heart surgery because of severe valvular heart disease, ischemic heart disease, and aortic disease at Oita University Hospital and Oita Oka Hospital were enrolled in the multicenter cohort (Oita Adipo-Cardiovascular Cohort Study) between May 2020 and April 2022. Patients undergoing dialysis or reoperation, those with systemic inflammatory disease (active collagen disease, infectious endocarditis, and severe infection), and those using steroids were excluded. The study protocol is illustrated in Figure 1. EAT or adipocytes/preadipocytes from 92 patients were used. Of these, SGLT2 expression in human EAT and preadipocytes/adipocytes was evaluated in 25 nondiabetic patients (study 1). The patient demographics, indications for surgery, and medications are presented in Table 1. In vitro incubation studies or coculture experiments using human adipocytes/preadipocytes and human iPS–derived cardiomyocytes included the same nondiabetic 25 patients to confirm the pure effect of SGLT2 inhibitors on preadipocytes/adipocytes.
Figure 1.
Flowchart of 92 Consecutive Patients Who Underwent Open Heart Surgery and Were Enrolled in the Multicenter Cohort
SGLT2 expression levels in human EAT and preadipocytes/adipocytes were evaluated in 25 nondiabetic patients. In vitro incubation studies or coculture experiments using human adipocytes/preadipocytes and human induced-pluripotent stem cell–derived cardiomyocytes included the same nondiabetic 25 patients to confirm the pure effect of SGLT2 inhibitors on preadipocytes/adipocytes (study 1). A cohort-wide association study to clinically validate the effect of SGLT2 inhibitors on EAT included 29 diabetic patients treated with SGLT2 inhibitors or other antidiabetic drugs (study 2). In addition, the gene expression levels of inflammatory adipocytokines were statistically compared using samples from 19 patients with AF and 19 patients without AF (study 3). EAT = epicardial adipose tissue; SGLT2 = sodium-glucose cotransporter.
Table 1.
Demographic Characteristics of the Study Participants in Study 1 (N = 25)
| Age, y | 72 (54-75) |
| Male | 17 (68) |
| BMI, kg/m2 | 22.67 ± 4.16 |
| Surgery (combined) | |
| AVR/AS, AR | 16 |
| MVR/MS, MR | 8 |
| CABG/AP, OMI | 6 |
| Other/TAA, AAA | 8 |
| Hypertension | 15 (60.0) |
| Dyslipidemia | 12 (48.0) |
| T2DM | 0 |
| NYHA functional class | |
| I | 10 (40.0) |
| II | 9 (36.0) |
| III | 5 (20.0) |
| IV | 1 (4.0) |
| Laboratory data | |
| TG, mg/dL | 111.04 ± 55.76 |
| HDL-C, mg/dL | 54.77 ± 14.32 |
| LDL-C, mg/dL | 113.55 ± 27.24 |
| FBS, mg/dL | 99.25 ± 19.89 |
| HbA1c, % | 5.64 ± 0.38 |
| Ccr, mL/min/1.73 m2 | 75.65 ± 48.82 |
| NT-proBNP, pg/mL | 432 (148-1,488) |
| Medications | |
| ACE inhibitor/ARB | 12 (48.0) |
| Beta-blocker | 8 (32.0) |
| CCB | 10 (40.0) |
| ARN inhibitor | 0 |
| Statin | 9 (36.0) |
| Diuretic | 3 (12.0) |
| SGLT2 inhibitor | 0 |
| DPP4 inhibitor | 0 |
| Insulin | 0 |
| Other antidiabetics | 0 |
| Steroid | 0 |
Values are median (IQR), n (%), or mean ± SD.
AAA = abdominal aortic aneurysm; ACE = angiotensin-converting enzyme; AP = angina pectoris; AR = aortic regurgitation; ARB = angiotensin receptor blocker; ARN = angiotensin receptor neprilysin; AS = aortic stenosis; AVR = aortic valve replacement; BMI = body mass index; CABG = coronary artery bypass grafting; CCB = calcium-channel blocker; Ccr = creatinine clearance; DPP4 = dipeptidyl peptidase 4; FBS = fasting blood sugar; HbA1c = hemoglobin A1c; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; MR = mitral regurgitation; MS = mitral stenosis; MVR = mitral valve replacement; NT-proBNP = N-terminal pro–B-type natriuretic peptide; OMI = old myocardial infarction; SGLT2 = sodium-glucose cotransporter 2; T2DM = type 2 diabetes mellitus; TAA = thoracic aortic aneurysm; TG = triglyceride.
In contrast, a cohort-wide association study to clinically validate the effect of SGLT2 inhibitors on EAT included 29 diabetic patients treated with SGLT2 inhibitors or other antidiabetic drugs (study 2). The demographic characteristics of the participants are presented in Supplemental Table 1. In addition, the gene expression levels of inflammatory adipocytokines were statistically compared using samples from 19 patients with AF and 19 patients without AF (study 3) (Supplemental Table 2).
Risk factor definition and ethics
Dyslipidemia and hypertension were defined according to the current European Society of Cardiology guidelines.15,16 Diabetes mellitus was defined according to the American Diabetes Association guidelines.17 The research protocol is based on the Declaration of Helsinki, and all patients provided informed consent. The study protocol was approved by the Ethics Committee of the Oita University Hospital (approval no. 1797). Written informed consent was obtained from all patients. This study was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN000042229) as “Analysis of Human Adipose and Cardiovascular Tissue Profile and Prognosis in Patients With Cardiovascular Diseases.” All procedures were conducted according to institutional guidelines.
Human EAT harvesting
Human EAT segments were collected from the atrioventricular/interventricular groove of the heart during surgery in ice-cold Krebs HEPES buffer (NaCl 99 mmol/L, KCl 4.7 mmol/L, MgSO4 1.2 mmol/L, KH2PO4 1 mmol/L, CaCl2 1.9 mmol/L, NaHCO3 25 mmol/L, glucose 25 mmol/L, HEPES 20 mmol/L; pH 7.35) and immediately transferred to the laboratory on ice. The EAT was washed in ice-cold Krebs HEPES buffer and cut into thin strips. The strips were snap-frozen and stored at –80 °C for baseline phenotyping and evaluation of SGLT2 expression.
Extraction of preadipocytes and cell culture
Fresh EAT was shredded in a sterile environment and digested with collagenase II solution (2 mg/mL) for 15 minutes at 37 °C to degrade fibrous tissue. Digested samples were centrifuged at 1,000 revolutions/min for 5 minutes and resuspended in Dulbecco’s Modified Eagle Medium Nutrient Mixture F-12 (catalog no. 11320-033, Gibco/Thermo Fisher Scientific) mixed with 10% fetal bovine serum and 0.2 ng/mL human epidermal growth factor (Thermo Fisher Scientific). When the isolated preadipocytes reached 100% confluence, the medium was replaced with a preadipocyte differentiation medium (catalog no. C-27437, PromoCell) and cultured for 5 days. They were then cultured in an adipocyte nutrition medium (catalog no. C-27439, PromoCell) for 14 days, successfully leading to mature/differentiated adipocytes. Empagliflozin (Cayman Chemical) was dissolved in sterile dimethyl sulfoxide (Tocris Bioscience/Bio-Techne) and adjusted to concentrations of 0 μmol/L (control), 1 μmol/L, 5 μmol/L, 10 μmol/L, and 100 μmol/L in the culture medium.
Fluorescent immunohistochemistry
EATs and cultured cells were stained using a rabbit anti-SGLT2 antibody (1:200 dilution; ab85626, Abcam) and a mouse anti-CD105 antibody (1:500 dilution; ab11414, Abcam). CD105 is expressed in mesenchymal stem cells and is a preadipocyte marker. Goat anti-mouse immunoglobulin G H&L Alexa Fluor 488 (1:200 dilution; ab150113, Abcam) and goat anti-rabbit immunoglobulin G H&L Alexa Fluor 594 (1:200 dilution; ab150080, Abcam) were used as fluorescently labeled secondary antibodies. Stained specimens were encapsulated with Antifade Mounting Medium with 4’,6-diamidino-2-phenylindole (DAPI) (VECTASHIELD Vibrance, Vector Laboratories) and observed under a fluorescence microscope (BZ-9000, KEYENCE) as previously described.18 The fluorescence intensity of the obtained image was measured using ImageJ software, version 1.53a (National Institutes of Health).
Oil Red O staining and measurement of lipid droplets
Differentiated adipocytes were rinsed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde. Staining was performed with Oil Red O (Fujifilm) stock solution (0.3% Oil Red O in isopropanol) for 20 minutes. After washing with water, counterstaining was performed with hematoxylin. Images were obtained using an inverted phase-contrast microscope. The cells were then harvested with 60% isopropanol and centrifuged to measure the Oil Red O dye eluted in the solvent using a spectrophotometer with an absorbance of 520 nm. The number and size of lipid droplets were quantitatively analyzed by ImageJ software, version 1.53a.
RNA isolation and quantitative real-time polymerase chain reaction
Total RNA was isolated using RNAlater stabilization solution (Invitrogen/Thermo Fisher Scientific), followed by a spin column–based RNA purification method using the QIA shredder and RNeasy Micro or Mini Kit (Qiagen). As previously described, RNA concentration and integrity were assessed spectrophotometrically using a NanoDrop 2000 spectrophotometer.19 RNA was reverse-transcribed to complementary DNA using the SuperScript VILO Master Mix (Thermo Fisher Scientific) with the ezDNase Enzyme Kit (Invitrogen/Thermo Fisher Scientific) following the manufacturer’s instructions. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using TaqMan chemistry, using the standard universal TaqMan protocol as indicated by the manufacturer, on a LightCycler 96 (Roche Diagnostics). All samples were duplicated using 5 ng of complementary DNA as the starting mass. Peptidylprolyl isomerase A (PPIA) was used as the housekeeping gene for human EAT and adipocytes, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for iPS-ACM. The IDs of the TaqMan probes (Thermo Fisher Scientific) used are PPIA: Hs99999904_m1; GAPDH: Hs02758991_g1; SGLT1: Hs01573793_m1; SGLT2: Hs00894642_m1; SGLT4: Hs01597600_m1; SGLT5: Hs00738326_m1; SGLT6: Hs01086559_m1; CD105: Hs00923996_m1; peroxisome proliferator activated receptor gamma (PPARγ): Hs01115513_m1; CCAAT enhancer binding protein alpha (CEBPA): Hs00269972_s1; fatty acid binding protein 4 (FABP4): Hs01086177_m1; perilipin 2 (PLIN2): Hs00605340_m1; uncoupling protein (UCP1): Hs00222453_m1; IL1α: Hs00174092_m1; IL1β: Hs01555410_m1; IL-6: Hs00174131_m1; monocyte chemoattractant protein 1 (MCP1): Hs00234140_m1; transforming growth factor-beta 1 (TGF-β1): Hs00998133_m1; tumor necrosis factor (TNF): Hs00174128_m1; IL10: Hs00961622m1; leptin (LEP): Hs00174877_m1; adiponectin (ADIPOQ): Hs00605917_m1; natriuretic peptide A (NPPA): Hs00383230_g1; and natriuretic peptide B (NPPB): Hs00173590_m1.
In our cohort-wide gene expression analyses, target gene and housekeeping gene cycle threshold (Ct) values were quantified by using qRT-PCR. Furthermore, the same standards were run on every quantitative PCR (qPCR) plate to extrapolate the amplification efficiency per plate. Target gene and housekeeping gene Ct values were quantified and further used to quantify target copy numbers with endogenous housekeeping correction (efficiency – ΔCt). This corrected value was then normalized to the highest standard of the standard curve, providing a corrected expression value relative to the highest standard (efficiency – ΔΔCt). Hence, the provided values are housekeeping-normalized expressions relative to a single reference sample (the highest standard of the standard curve), allowing for accurate interpolate analysis. The cutoffs for low and high IL6 and MCP1 levels in EAT are the medians.
Measurement of adipocyte-secreted cytokines in the supernatant
Enzyme-linked immunosorbent assay kits were used to determine the supernatant levels of IL-1α (Invitrogen/Thermo Fisher Scientific), IL-1β (Invitrogen/Thermo Fisher Scientific), IL-6 (Invitrogen/Thermo Fisher Scientific), TGF-β1 (Abcam), and MCP1 (Abcam) according to the manufacturer’s protocol.
Coculture of iPS-ACM with human adipocytes
Preadipocytes were cultured on Nunc Polycarbonate Cell Culture Inserts (Thermo Fisher Scientific) with a 0.4-μm polycarbonate membrane filter and differentiated under each empagliflozin concentration (0 μmol/L, 10 μmol/L, and 100 μmol/L). After 19 days of incubation, differentiated adipocytes were treated with a new adipocyte nutrition medium without empagliflozin. iPS-ACM (Axol Bioscience) was cultured with Cardiomyocyte Maintenance Medium (Axol Bioscience) in a 6-well dish coated with fibronectin (37 °C, 5% CO2). Culture inserts in which adipocytes were cultured were combined into 6-well dishes in which cardiomyocytes were cultured, enabling the coculture of iPS-ACM with adipocytes treated with or without empagliflozin for 2 days (37 °C, 5% CO2).
Incubation of iPS-ACM with IL6
iPS-ACM (Axol Bioscience) was incubated with human recombinant IL-6 (1 ng/mL, Abcam) for 2 days.
Superoxide quantification
Following the in vitro experiments, cells were scraped and sonicated in ice-cold Krebs HEPES buffer (pH 7.35) in the presence of a protease inhibitor (Roche Applied Science). O2.– production was measured in cell lysates using lucigenin (5 μmol/L)–enhanced chemiluminescence, as previously described.20 The protein concentration was quantified and unified to 0.2 mg before an experiment. Contribution of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity to cardiomyocyte O2.– production was quantified in the presence of NADPH at 100 μmol/L.
Oxidative fluorescent microtopography
Incubated iPS-ACM was directly incubated with CellROX Oxidative Stress Reagent (Life Technologies/Thermo Fisher Scientific) (5 μmol/L for 30 minutes) and washed with phosphate-buffered saline 3 times. Fluorescence image acquisition and analysis were performed similarly as for adipocytes, as described earlier.
Statistical analysis
Baseline clinical characteristics are presented as mean with SD, median with IQR, or frequencies with percentages, as appropriate. For continuous variables, the normality of the distribution was tested using the Shapiro-Wilk test. For continuous variables, Student’s t-test or Mann-Whitney U test (for 2 groups, as appropriate) or analysis of variance (for >2 groups) was used, followed by Bonferroni correction for multiple comparisons. The Fisher exact test was used to analyze categorical variables. The correlation of 2 continuous variables was evaluated with Pearson’s r, as appropriate. Statistical significance was set at a P value of <0.05. All computations were performed using GraphPad Prism 9 (GraphPad Software) and SPSS statistical software (version 26.0), running on Windows 11 (Microsoft).
Results
SGLT2 expression in human EAT (study 1-I)
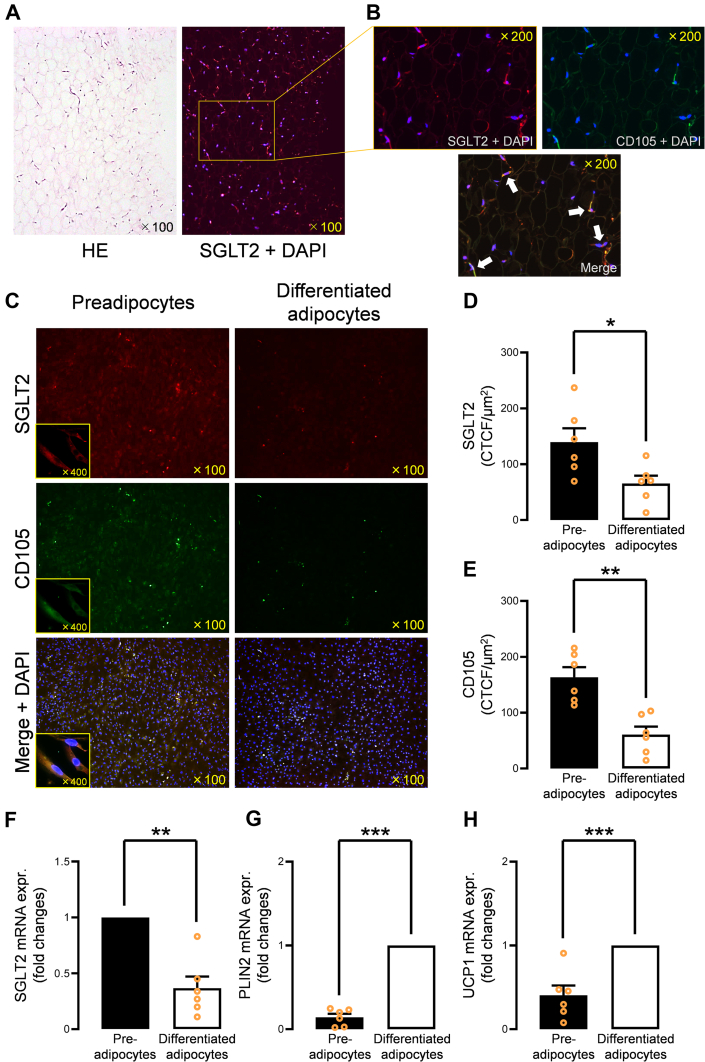
The expression of SGLT2 was evaluated by immunofluorescent staining of SGLT2 using human EAT samples from 25 patients (Table 1). SGLT2 was abundantly expressed in the human EAT (Figure 2A, right). There was not a significant association between SGLT2 gene expression levels in EAT and glycosylated hemoglobin levels (rP = 0.041, P = 0.84) (Supplemental Figure 1, Supplemental Table 3), indicating that SGLT2 expression may not be affected by diabetes status. Double immunofluorescent staining of CD105, a marker of mesenchymal stromal cells, and SGLT2 revealed that CD105-positive cells, defined as preadipocytes in our study, primarily expressed SGLT2 in the human EAT (Figure 2B). Further, we explored the alteration in SGLT2 gene expression in human primary cultured preadipocytes isolated from EAT. Interestingly, SGLT2 expression in preadipocytes diminished as the preadipocytes differentiated (Figure 2C). The fluorescence intensity of SGLT2 (Figure 2D) and CD105 (Figure 2E), calculated as corrected total cell fluorescence, decreased in differentiated adipocytes. The down-regulated gene expression level of SGLT2 in differentiated adipocytes was confirmed using qPCR (Figure 2F). In contrast, time-course–dependent down-regulation of SGLT2 was not observed in the preadipocytes without the induction of differentiation (Supplemental Figures 2A to 2C). Then, we evaluated the expression levels of PLIN2 and UCP1 to confirm whether adipocytes have features of EAT adipocytes. PLIN2 and UCP1 messenger RNA (mRNA) expression levels were up-regulated in the differentiated adipocytes compared with the preadipocytes (Figures 2G and 2H). We investigated the expression levels of SGLT1, SGLT4, SGLT5, and SGLT6 in the preadipocytes and differentiated adipocytes. The expression levels were not significantly reduced in the differentiated adipocytes (Supplemental Figures 2D to 2G).
Figure 2.
SGLT2 Expression in Human EAT
(A) SGLT2 was abundantly expressed in the human EAT. (B)SGLT2 was primarily expressed in CD105-positive preadipocytes in human EAT. (C) SGLT2 expression was predominantly expressed in human epicardial preadipocytes, and SGLT2 expression was greatly diminished in the differentiated and mature adipocytes. (D) Fluorescence intensity of SGLT2 was significantly decreased in differentiated adipocytes. (E) Fluorescence intensity of CD105 was significantly decreased in differentiated adipocytes. (F) mRNA SGLT2 expression was significantly down-regulated in the differentiated adipocytes. (G)PLIN2 and (H)UCP1 mRNA expression levels were up-regulated in the differentiated adipocytes compared to the preadipocytes. Data are presented as mean ± SD and compared using the Student’s t-test or Mann-Whitney U test. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. CTCF = corrected total cell fluorescence; DAPI = 4’,6-diamidino-2-phenylindole (DAPI); expr. = expression; HE = hematoxylin and eosin; mRNA = messenger RNA; other abbreviations as in Figure 1.
Direct effect of empagliflozin in differentiation/maturation on human EAT–derived preadipocytes (study 1-II)
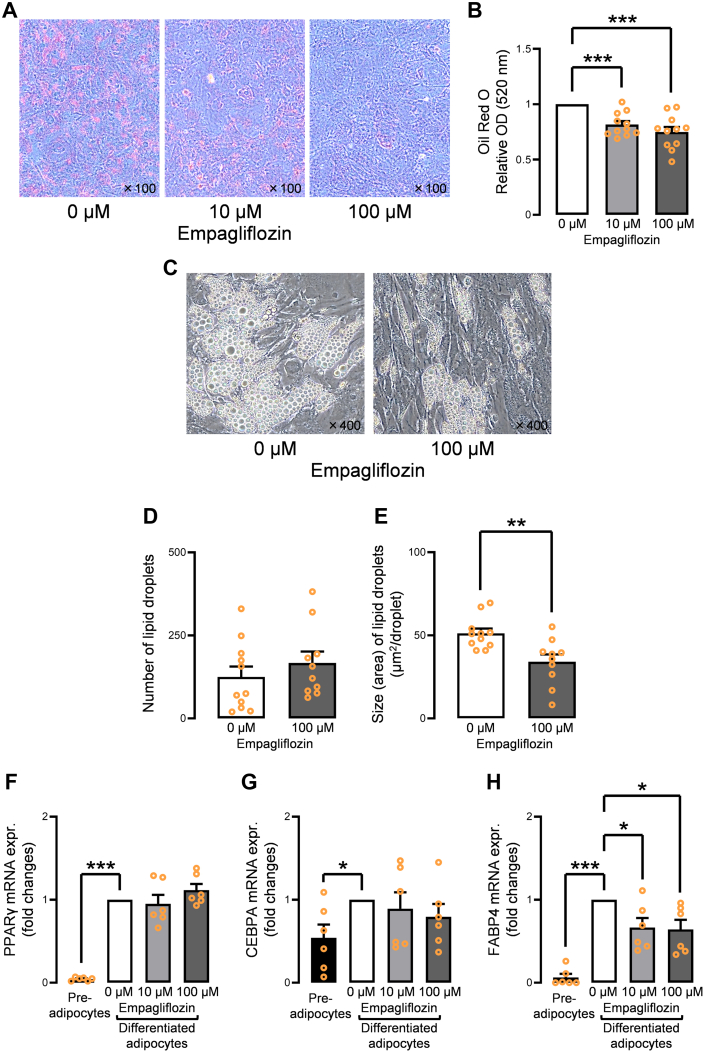
First, empagliflozin’s effect on the proliferation of preadipocytes was evaluated to confirm the nontoxicity of empagliflozin to cells, demonstrating no dose-dependent cytotoxicity (Supplemental Figure 3A). In addition, the expression of CD105 in the preadipocytes was not affected by empagliflozin (Supplemental Figure 3B). Next, the effect of empagliflozin on the differentiation and maturation of human preadipocytes was investigated using Oil Red O staining. Preadipocytes incubated with empagliflozin (10 μmol/L and 100 μmol/L) showed poor differentiation in a dose-dependent manner despite adequate induction of differentiation (Figures 3A and 3B); 0 μmol/L was used as the control. The number of lipid droplets incubated with 100 μmol/L empagliflozin was not significantly decreased, and the size of the lipid droplets was significantly smaller than those of control (Figures 3C to 3E). The expression of adipose differentiation-related genes (PPARγ, CEBPA, and FABP4) was evaluated using qPCR. The expression levels of PPARγ and CEBPA were not significantly affected by incubation with empagliflozin (Figures 3F and 3G). However, FABP4, the final differentiation/maturation marker, was significantly suppressed by incubation with empagliflozin (Figure 3H). FABP4 mRNA expression was significantly lower in the human EAT in patients receiving SGLT2 inhibitors than in patients receiving other antidiabetic agents (Supplemental Figures 4A to 4C).
Figure 3.
Effect of Empagliflozin on the Differentiation/Maturation of Human Epicardial Preadipocytes
(A) Incubation with empagliflozin (10 μmol/L, 100 μmol/L) attenuated the Oil Red O–stained area. (B) Oil Red O dye measured using spectrophotometer with an absorbance of 520 nm was significantly decreased by incubation with empagliflozin (10 μmol/L, 100 μmol/L). (C) Representative images show that the size of lipid droplets incubated with 100 μmol/L empagliflozin were apparently smaller than in the control. (D) The number of lipid droplets incubated with 100 μmol/L empagliflozin was not significantly decreased, whereas (E) the size of lipid droplets was significantly smaller than in the control. (F) Gene expression levels of PPARγ were not significantly suppressed by empagliflozin incubation. (G)CEBPA gene expression was not significantly suppressed by empagliflozin incubation. (H)FABP4 gene expression was significantly reduced by empagliflozin incubation. Data are presented as mean ± SD and compared using the Student’s t-test, Mann-Whitney U test, or analysis of variance. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. M = mol/L; OD = optical density.
Direct effect of empagliflozin on adipokine gene expression profiles of human EAT–derived adipocytes (study 1-III)
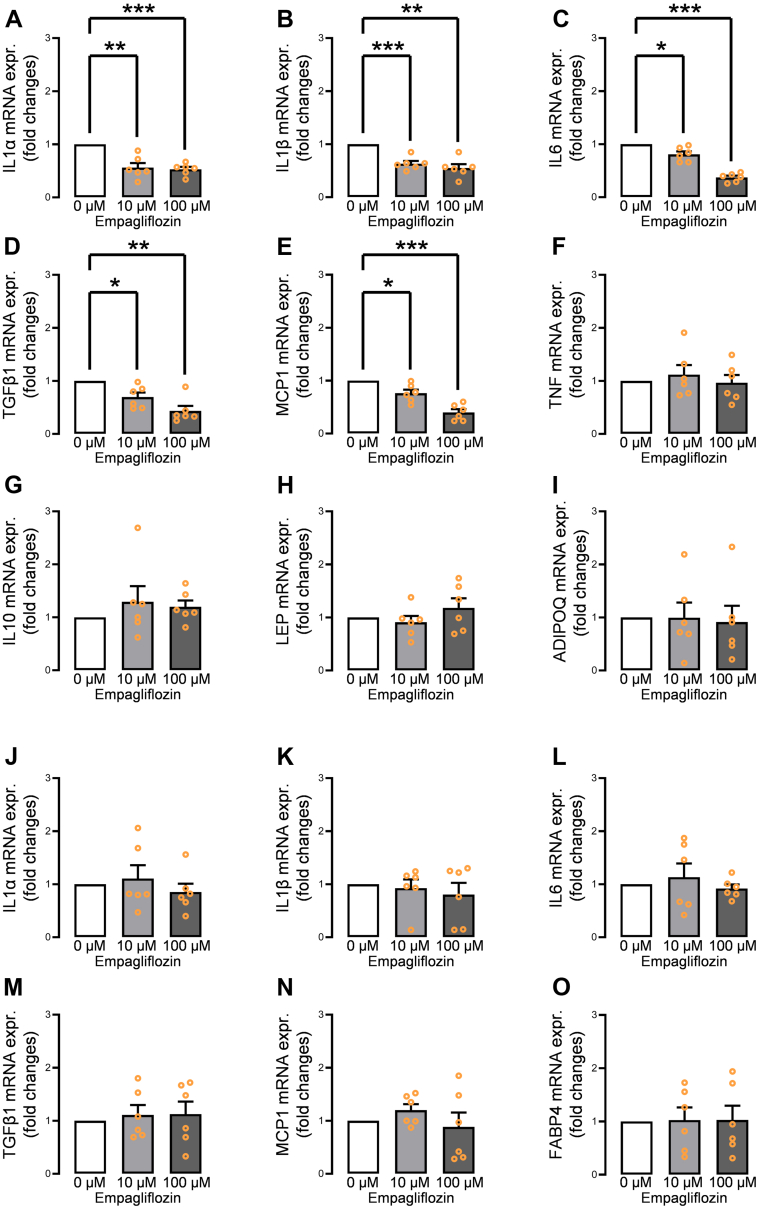
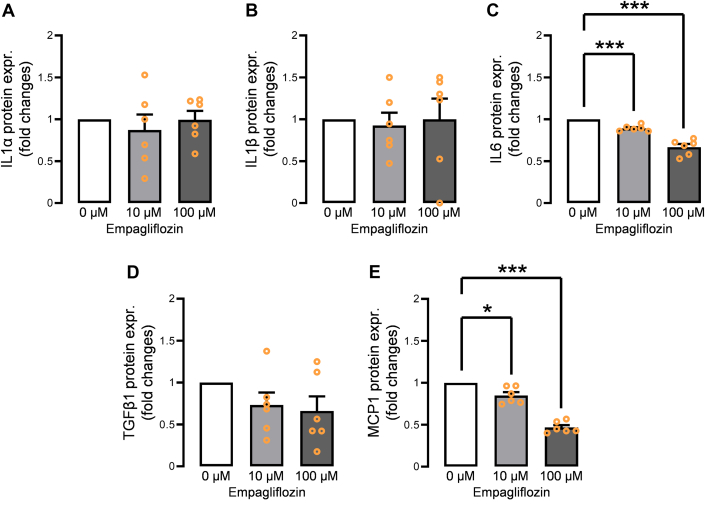
The gene expression levels of several adipocytokines in human EAT–derived differentiated adipocytes incubated with empagliflozin were examined by qPCR. Adipocytes incubated with empagliflozin (10 μmol/L, 100 μmol/L) before differentiation showed dose-dependent down-regulation of IL1α, IL1β, IL6, TGFβ1, and MCP1 (Figures 4A to 4E). However, TNF, IL10, LEP, and ADIPOQ were not affected (Figures 4F to 4I). In contrast, adipocytes incubated with empagliflozin (10 μmol/L, 100 μmol/L) after the differentiation presented no significant gene expression down-regulations of IL1α, IL1β, IL6, TGFβ1, and MCP1 (Figures 4J to 4N), indicating that empagliflozin exerts its effect through preadipocyte-expressed SGLT2. In addition, FABP4 expression did not decrease after incubation with empagliflozin postdifferentiation (Figure 4O). To measure the actual secreted protein levels of IL-1α, IL-1β, IL-6, TGF-β1, and MCP-1, the supernatant was evaluated using enzyme-linked immunosorbent assay. Of these, the protein levels of IL-6 and MCP-1 were significantly decreased in a dose-dependent manner (Figures 5A to 5E). To confirm whether the incubation with a lower range of empagliflozin could exert the effect on premature/mature adipocytes, we tested 1 μmol/L and 5 μmol/L of empagliflozin. The incubation with 5 μmol/L empagliflozin significantly reduced IL-6, MCP-1, and FABP4 mRNA expression (Supplemental Figure 5).
Figure 4.
Effect of Empagliflozin on Adipokine Gene Expression Profiles of Human Epicardial Adipocyte
Incubation of human preadipocytes with empagliflozin (10 μmol/L, 100 μmol/L) from the beginning of the differentiation significantly suppressed (A)IL1α, (B)IL1β, (C)IL6, (D)TGFβ1, and (E)MCP1; however, (F)TNF, (G)IL10, (H)LEP, and (I)ADIPOQ gene expression levels were not affected. In contrast, mature human epicardial adipocytes incubated with empagliflozin (10 μmol/L, 100 μmol/L) showed no significant down-regulation of (J)IL1α, (K)IL1β, (L)IL6, (M)TGFβ1, (N)MCP1, and (O)FABP4. Data are presented as mean ± SD and compared using Student’s t-test. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. IL = interleukin; other abbreviations as in Figures 2 and 3.
Figure 5.
Secreted Protein Levels From the Empagliflozin-Treated Adipocytes
Adipocyte-secreted protein levels of (A) IL-6 and (B) MCP-1 significantly decreased in a dose-dependent manner upon incubation with empagliflozin, whereas those of (C) IL-1α, (D) IL-1β, and (E) TGF-β1 did not decrease. Data are presented as mean ± SD and compared using Student’s t-test. ∗P < 0.05 and ∗∗∗P < 0.001. Abbreviations as in Figures 2 and 3.
Furthermore, we investigated whether there was a difference in the gene expression levels of proinflammatory cytokines between the preadipocytes and mature adipocytes without empagliflozin. There were no significant differences in IL-6, MCP-1, and TGF-β between the preadipocytes and mature adipocytes (Supplemental Figures 6A to 6C), whereas the IL-1α and IL-1β gene expression levels were significantly reduced in the mature adipocytes (Supplemental Figures 6D and 6E).
Coculture of human EAT–derived differentiated adipocytes and human iPS-ACM (study 1-IV)
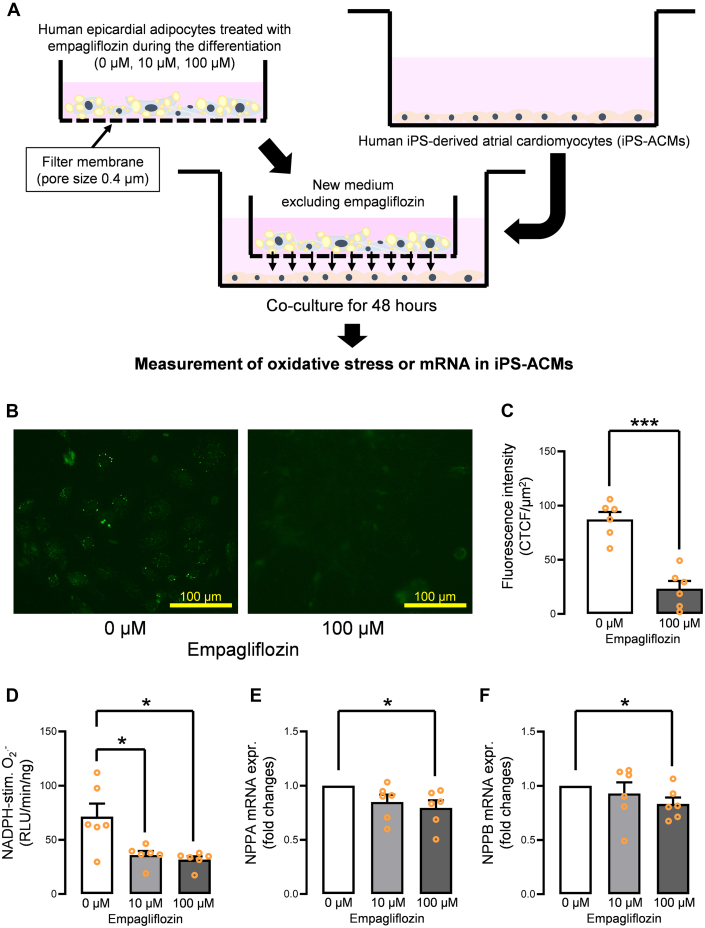
Coculture of human epicardial adipocytes treated with empagliflozin and human iPS-ACM for 48 hours was performed, as shown in Figure 6A. Oxidative fluorescent microtopography revealed that coculture with 100 μmol/L empagliflozin–treated adipocytes significantly reduced the fluorescence intensity evaluated using the CellROX kit (Figures 6B and 6C), indicating suppression of all reactive oxygen species in human iPS-ACM. Lucigenin-enhanced chemiluminescence also showed that coculture with empagliflozin-treated adipocytes reduced basal (Supplemental Figure 7A) and NADPH-stimulated (Figure 6D) O2.– level generation in a dose-dependent manner. Furthermore, we investigated whether the mRNA levels of natriuretic peptide A (NPPA) and natriuretic peptide B (NPPB), which are common markers of cardiomyocyte stress, were changed. NPPA and NPPB gene expression levels were suppressed in a dose-dependent manner (Figures 6E and 6F). In addition, we investigated whether coculture with iPS-ACM could affect the adipocyte secretome profile by measuring the IL6 protein levels in the supernatant from the adipocytes with or without the coculture treatment with iPS-ACM. There were no significant differences between the 2 groups (Supplemental Figure 7B). The morphology and lipid content of the mature adipocytes after coincubation with cardiomyocytes were also assessed, showing that these were not changed (Supplemental Figures 7C and 7D).
Figure 6.
Confirmation of Improved Paracrine Effect Using Coculture of Human Epicardial Adipocytes Treated With Empagliflozin and Human iPS-ACM
(A) Schematic of the coculture experiment. (B) Coincubation of iPS-ACM with empagliflozin (10 μmol/L, 100 μmol/L)–treated adipocytes suppressed the whole reactive oxygen species evaluated by the CellROX system. (C) Fluorescence intensity of reactive oxygen species was significantly decreased in the iPS-ACM coincubated with the adipocytes treated with 100 μmol/L empagliflozin. (D) NADPH-stimulated superoxide generation was significantly suppressed in the iPS-ACM coincubated with the adipocytes treated with 10 μmol/L and 100 μmol/L empagliflozin. (E)NPPA and (F)NPPB gene expression levels were suppressed in a dose-dependent manner. Data are presented as mean ± SD and compared using the Student’s t-test or Mann-Whitney U test. ∗P < 0.05 and ∗∗∗P < 0.001. iPS-ACM = induced pluripotent stem cell–derived atrial cardiomyocytes; RLU = relative light unit; stim. = stimulated; other abbreviations as in Figures 2 and 3.
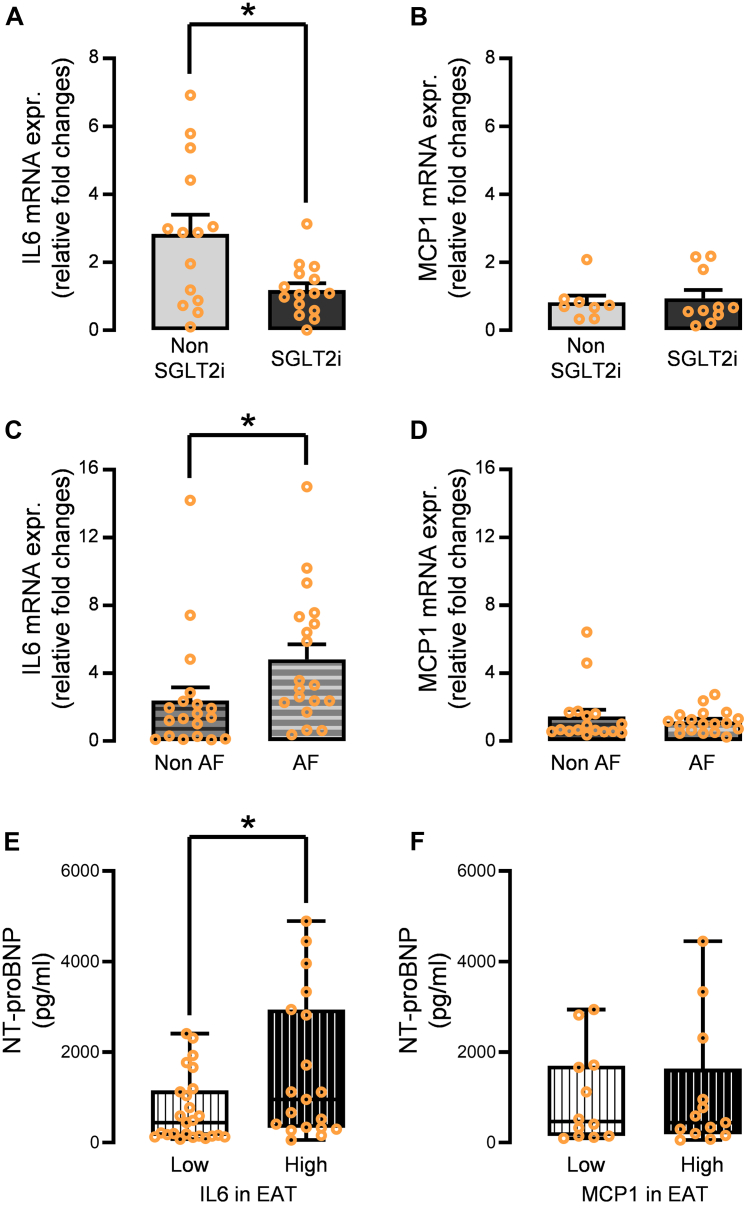
Clinical validation of the impact of IL-6 and MCP-1 in human EAT (studies 2 and 3)
Finally, the validity of the obtained findings in study 1 was verified from a clinical perspective. First, the gene expression levels of IL6 in the EAT were significantly decreased in diabetic patients treated with SGLT2 inhibitors compared to those without; however, MCP1 expression was not significantly reduced (Figures 7A and 7B). Consistent with these findings, only IL6 mRNA levels in the EAT were significantly increased in patients with AF compared with those without AF (Figures 7C and 7D). IL6 expression, but not MCP1 expression, in EAT was also strongly correlated with markers of wall stretch and N-terminal pro–B-type natriuretic peptide (Figures 7E and 7F). It was validated that the incubation of human iPS-ACM with recombinant IL-6 showed increased O2.– measured using luminometry compared with the control (Supplemental Figures 7E and 7F).
Figure 7.
Clinical Validation of the Impact of IL-6 and MCP-1 Using Data of the Cohort Study
(A) Gene expression levels of IL6 in the EAT were significantly attenuated in the diabetic patients treated with SGLT2 inhibitor compared to those treated with other antidiabetic drugs. (B) Those of MCP1 was not significantly affected. (C)IL6 mRNA levels in the EAT were significantly increased in the patients with atrial fibrillation compared to those without atrial fibrillation. (D) No significant difference was observed in MCP1 between the 2 groups. (E) Patients with high IL6 expression in EAT have higher plasma NT-proBNP levels. (F) No significant difference was observed in NT-proBNP levels between the patients with high MCP1 expression in EAT and low MCP1 expression in EAT. Data are presented as mean ± SD and compared using the Mann-Whitney U test. ∗P < 0.05. NT-proBNP = N-terminal pro–B-type natriuretic peptide; other abbreviations as in Figures 1, 2, 3, and 4.
Discussion
The significant findings from this study, involving studies 1 through 3, are as follows:
-
•
SGLT2 was substantially expressed in the human EATs, particularly in the preadipocytes.
-
•
SGLT2 expression levels of the human preadipocytes were attenuated along with the differentiation process.
-
•
Empagliflozin significantly suppressed the differentiation and maturation of human preadipocytes.
-
•
The incubation of preadipocytes with empagliflozin before the differentiation dose-dependently down-regulated proinflammatory adipocytokines, particularly MCP-1 and IL-6, in the adipocytes and their secretome.
-
•
Coculture experiments using human iPS-ACM and adipocytes showed that empagliflozin had cardioprotective paracrine effects on cardiomyocytes regarding oxidative stress.
-
•
IL6 was an essential adverse adipocytokine secreted from the human epicardial adipocytes or EAT that can be suppressed by medication with SGLT2 inhibitors from a clinical perspective.
Although the role of SGLT isoforms in myocardial physiology remains controversial, several studies have demonstrated that SGLT2 is barely detectable in the human myocardium.20,21 In contrast, a previous study reported that SGLT2 is expressed in human EAT.9 Díaz-Rodriguez et al9 demonstrated that SGLT2 was expressed in EAT; however, they did not show the expression in a particular cell type.9 It has not been elucidated whether SGLT2 is expressed in epicardial adipocytes or other types of cells, such as preadipocytes, immune cells, and fibroblasts, which are components of EAT. Using a large number of EATs and isolated human preadipocytes, we definitively demonstrated that SGLT2 is primarily and abundantly expressed in human preadipocytes compared to mature adipocytes; thus, empagliflozin could suppress the differentiation and maturation of human preadipocytes by blocking glucose uptake through SGLT2 inhibition, thereby reducing human EAT volume.
In addition, Díaz-Rodriguez et al9 showed an improvement of adipocyte differentiation in EAT after incubation with dapagliflozin, which is not consistent with our results. However, the discrepancies in results can be attributed to the use of different SGLT2 inhibitors (empagliflozin vs dapagliflozin) or different participant characteristics. The blocking selectivity to SGLT2 is different between empagliflozin and dapagliflozin. Empagliflozin displays higher SGLT2 affinity than dapagliflozin.22 Furthermore, dapagliflozin increased the protein expression levels of glucose transporter type 4 (GLUT4) in EAT,9 whereas empagliflozin did not alter the GLUT4 expression level.23 It has been reported that the overexpression of GLUT4 in adipocytes has elevated adipose tissue lipogenesis in mice.24 These different characteristics of the 2 drugs might explain the opposite effects. Empagliflozin may suppress the maturation more strongly than the differentiation because PPARγ and CEBPA, involved in adipogenesis, are not affected by empagliflozin in our study. FABP4, which is the final differentiation/maturation marker, was significantly suppressed upon incubation with empagliflozin, indicating that adipocyte stores fewer triglycerides because of reduced energy intake. In addition, the facts that the number of lipid droplets incubated with empagliflozin was not significantly decreased, whereas the size of lipid droplets was significantly smaller than that of the control, and that mRNA CD105 expression was not detected in mature adipocytes pretreated with or without empagliflozin also support this speculation. Only the SGLT2 expression level was significantly reduced in mature adipocytes, and the incubation with empagliflozin after differentiation did not affect the adipocyte secretome profile, potentially indicating that empagliflozin mainly affects the preadipocytes by inhibiting SGLT2. Even though the expression levels of the other SGLT members were not affected by maturation, we cannot exclude the possibility that some effects are mediated through a broader inhibition of other SGLT family members. Clinically available SGLT2 inhibitors have variable SGLT2 affinities. Empagliflozin is considered the most selective SGLT2 inhibitor among other SGLT2 inhibitors,22 potentially enhancing the modulatory effect on epicardial preadipocytes. However, in our study, IL6 gene expression was down-regulated in diabetic patients who were administered SGLT2 inhibitors, including empagliflozin, dapagliflozin, and ipragliflozin, indicating the class effect of SGLT2 inhibitors.
The quality deterioration of EAT and its paracrine secretome is significantly associated with cardiac inflammation and fibrosis.25, 26, 27 SGLT2 inhibitors are the most promising candidates for improving the quantity and quality, as demonstrated in our in vitro and clinical association studies in studies 1-3. In particular, coculture experiments using human iPS-derived cardiomyocytes and empagliflozin-treated human epicardial adipocytes revealed an improvement in the paracrine effect of epicardial adipocytes on cardiomyocytes, which is the novelty of our study. However, there is a discrepancy in these findings. MCP-1 and IL-6 were important adipocyte-secreted adipokines regulated by empagliflozin in vitro; nevertheless, only IL-6 gene expression levels in human EAT were significantly affected by the administration of SGLT2 inhibitors. As mentioned previously, EAT comprises adipocytes, fibroblasts, stromovascular cells, and immune cells, which could explain this mismatch. MCP-1 is mainly secreted from the immune cells, such as macrophages and monocytes; therefore, SGLT2 inhibitors may have poor MCP-1-suppressive effects on these cell types compared with adipocytes, presumably leading to the insignificance of the study using EAT.
IL-6 is a proinflammatory cytokine that induces myocardial inflammatory fibrosis and vascular inflammation.28,29 It also causes excess production of reactive oxygen species like superoxide,30,31 leading to further exacerbation of inflammation and fibrosis. Considering the potential efficacy of IL-6 inhibition in recent clinical studies,32 SGLT2 inhibitor–mediated IL-6 suppression in the EAT secretome would ameliorate cardiac inflammation and fibrosis, which could contribute to the prevention of the progression of heart failure and AF pathogenesis, because we clearly show significant IL-6 up-regulation in the EAT of AF patients and the positive correlation of plasma N-terminal pro–B-type natriuretic peptide level and IL6 expression in EAT.
We have shown the acute or subacute effects of empagliflozin (incubation for 19 days) on human epicardial preadipocytes in culture. Empagliflozin suppressed the differentiation/maturation and improved the paracrine secretome profile of mature epicardial adipocytes (decrease of IL-6 production). This modification of the paracrine effect suppressed myocardial oxidative stress. Based on these acute/subacute effects in the culture, longer-term exposure of empagliflozin in the clinical setting can lead to the reduction of EAT and the suppression of cardiac inflammatory fibrosis.
The mechanism of SGLT2-mediated deterioration of the paracrine secretome in EAT remains unclear. Further mechanistic in vitro studies are required to explore complex intracellular signaling in human epicardial adipocytes, which could be the next phase of our research.
Study limitations
Because of the nature of our study (non–SGLT2 inhibitor vs SGLT2 inhibitor or non-AF vs AF), there is a lack of randomization among the samples/studies and inherent confounders that complicate the interpretation of the results.
Conclusions
We demonstrated, for the first time to our knowledge, that SGLT2 is expressed dominantly in epicardial preadipocytes in human EAT and that empagliflozin suppresses the maturation/differentiation of epicardial preadipocytes via the inhibition of SGLT2 while suppressing the expression and secretion of several proinflammatory adipokines in the EAT. IL-6 may be one of the crucial adipokines secreted from human EAT that SGLT2 inhibitors can modify.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: SGLT2 inhibitors contribute to the reduction in epicardial adipose tissue in the clinical setting, potentially being one of the mechanisms for the cardioprotective effect of this drug class. This study clarified the direct impact of this drug on the adipogenesis of epicardium and the paracrine secretome gene expression profiles of epicardial adipocytes using isolated human epicardial preadipocytes from patients undergoing cardiac surgery.
TRANSLATIONAL OUTLOOK: This study demonstrated that the secretome profile modification of human epicardial adipocytes by SGLT2 inhibitors suppresses oxidative stress in human cardiomyocytes. Intervention in the quantity and quality of epicardial adipose tissue may be a new therapeutic target for preventing cardiovascular diseases.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Masae Hayashi, Ikuko Morisaki, Moe Hamada, Tomomi Shuto, and Shota Kobayashi for their excellent technical assistance.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Zinman B., Wanner C., Lachin J.M., et al. EMPA-REG OUTCOME Investigators Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 2.Neal B., Perkovic V., Mahaffey K.W., et al. CANVAS Program Collaborative Group Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 3.Wiviott S.D., Raz I., Bonaca M.P., et al. DECLARE–TIMI 58 Investigators Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 4.McMurray J.J.V., Solomon S.D., Inzucchi S.E., et al. DAPA-HF Trial Committees and Investigators Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 5.Packer M., Anker S.D., Butler J., et al. EMPEROR-Reduced Trial Investigators Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 6.Anker S.D., Butler J., Filippatos G., et al. EMPEROR-Preserved Trial Investigators Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 7.Lopaschuk G.D., Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. J Am Coll Cardiol Basic Trans Science. 2020;5:632–644. doi: 10.1016/j.jacbts.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Requena-Ibáñez J.A., Santos-Gallego C.G., Rodriguez-Cordero A., et al. Mechanistic insights of empagliflozin in nondiabetic patients with HFrEF: from the EMPA-TROPISM study. J Am Coll Cardiol HF. 2021;9:578–589. doi: 10.1016/j.jchf.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Díaz-Rodríguez E., Agra R.M., Fernández Á.L. Effects of dapagliflozin on human epicardial adipose tissue: modulation of insulin resistance, inflammatory chemokine production, and differentiation ability. Cardiovasc Res. 2018;114:336–346. doi: 10.1093/cvr/cvx186. [DOI] [PubMed] [Google Scholar]
- 10.Kondo H., Takahashi N. Reduced hospitalization for heart failure using anti-diabetic drug dapagliflozin: implications of DECLARE-TIMI 58 for the basic science community. Cardiovasc Res. 2019;115:e54–e57. doi: 10.1093/cvr/cvz073. [DOI] [PubMed] [Google Scholar]
- 11.Zelniker T.A., Bonaca M.P., Furtado R.H.M., et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE-TIMI 58 trial. Circulation. 2020;141:1227–1234. doi: 10.1161/CIRCULATIONAHA.119.044183. [DOI] [PubMed] [Google Scholar]
- 12.Nagashima K., Okumura Y., Watanabe I., et al. Association between epicardial adipose tissue volumes on 3-dimensional reconstructed CT images and recurrence of atrial fibrillation after catheter ablation. Circ J. 2011;75:2559–2565. doi: 10.1253/circj.cj-11-0554. [DOI] [PubMed] [Google Scholar]
- 13.Nakanishi K., Fukuda S., Tanaka A., et al. Peri-atrial epicardial adipose tissue is associated with new-onset nonvalvular atrial fibrillation. Circ J. 2012;76:2748–2754. doi: 10.1253/circj.cj-12-0637. [DOI] [PubMed] [Google Scholar]
- 14.Venteclef N., Guglielmi V., Balse E., et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J. 2015;36:795–805a. doi: 10.1093/eurheartj/eht099. [DOI] [PubMed] [Google Scholar]
- 15.Catapano A.L., Graham I., De Backer G., et al. ESC Scientific Document Group 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 16.Williams B., Mancia G., Spiering W., et al. ESC Scientific Document Group 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 17.Garza L., Dols J., Gillespie M. An initiative to improve primary prevention of cardiovascular disease in adults with type II diabetes based on the ACC/AHA (2013) and ADA (2016) guidelines. J Am Assoc Nurse Pract. 2017;29:606–611. doi: 10.1002/2327-6924.12492. [DOI] [PubMed] [Google Scholar]
- 18.Kondo H., Kira S., Oniki T., et al. Interleukin-10 treatment attenuates sinus node dysfunction caused by streptozotocin-induced hyperglycaemia in mice. Cardiovasc Res. 2019;115:57–70. doi: 10.1093/cvr/cvy162. [DOI] [PubMed] [Google Scholar]
- 19.Uemura K., Kondo H., Ishii Y., et al. Mast cells play an important role in the pathogenesis of hyperglycemia-induced atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27:981–989. doi: 10.1111/jce.12995. [DOI] [PubMed] [Google Scholar]
- 20.Kondo H., Akoumianakis I., Badi I., et al. Effects of canagliflozin on human myocardial redox signalling: clinical implications. Eur Heart J. 2021;42:4947–4960. doi: 10.1093/eurheartj/ehab420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Franco A., Cantini G., Tani A., et al. Sodium-dependent glucose transporters (SGLT) in human ischemic heart: a new potential pharmacological target. Int J Cardiol. 2017;243:86–90. doi: 10.1016/j.ijcard.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 22.Anker S.D., Butler J. Empagliflozin, calcium, and SGLT1/2 receptor affinity: another piece of the puzzle. ESC Heart Fail. 2018;5:549–551. doi: 10.1002/ehf2.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mustroph J., Lücht C.M., Wagemann O., et al. Empagliflozin enhances human and murine cardiomyocyte glucose uptake by increased expression of GLUT1. Diabetologia. 2019;62:726–729. doi: 10.1007/s00125-019-4819-z. [DOI] [PubMed] [Google Scholar]
- 24.Moraes-Vieira P.M., Saghatelian A., Kahn B.B. GLUT4 expression in adipocytes regulates de novo lipogenesis and levels of a novel class of lipids with antidiabetic and anti-inflammatory effects. Diabetes. 2016;65:1808–1815. doi: 10.2337/db16-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kondo H., Abe I., Gotoh K., et al. Interleukin 10 treatment ameliorates high-fat diet-induced inflammatory atrial remodeling and fibrillation. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.117.006040. [DOI] [PubMed] [Google Scholar]
- 26.Abe I., Teshima Y., Kondo H., et al. Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart Rhythm. 2018;15:1717–1727. doi: 10.1016/j.hrthm.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Wong C.X., Ganesan A.N., Selvanayagam J.B. Epicardial fat and atrial fibrillation: current evidence, potential mechanisms, clinical implications, and future directions. Eur Heart J. 2017;38:1294–1302. doi: 10.1093/eurheartj/ehw045. [DOI] [PubMed] [Google Scholar]
- 28.Frangogiannis N.G. Cardiac fibrosis. Cardiovasc Res. 2021;117:1450–1488. doi: 10.1093/cvr/cvaa324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brasier A.R. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86:211–218. doi: 10.1093/cvr/cvq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ge W., Guo X., Song X., et al. The role of immunoglobulin E and mast cells in hypertension. Cardiovasc Res. 2022;118(14):2985–2999. doi: 10.1093/cvr/cvac010. [DOI] [PubMed] [Google Scholar]
- 31.Ten Cate H., Guzik T.J., Eikelboom J., Spronk H.M.H. Pleiotropic actions of factor Xa inhibition in cardiovascular prevention: mechanistic insights and implications for anti-thrombotic treatment. Cardiovasc Res. 2021;117:2030–2044. doi: 10.1093/cvr/cvaa263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ridker P.M. From RESCUE to ZEUS: will interleukin-6 inhibition with ziltivekimab prove effective for cardiovascular event reduction? Cardiovasc Res. 2021;117:e138–e140. doi: 10.1093/cvr/cvab231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.