Abstract
A 59-year-old male patient with history of rheumatic heart disease with 3 previous surgical aortic valve replacements with the last one being homograft followed by transcatheter aortic valve implantation in failed homograft presented with severe aortic regurgitation and cardiogenic shock requiring urgent TAV-in-TAV-in homograft. (Level of Difficulty: Advanced.)
Key Words: cardiogenic shock, homograft, TAV-in-TAV, TAVI
Central Illustration
History of Presentation
A 59-year-old male presented to our emergency department with progressive dyspnea and cough productive of blood-tinged sputum. On physical examination, he was afebrile, normotensive (124/82 mm Hg), tachycardic (heart rate 120 beats/min) with mild respiratory distress (respiratory rate 29/min) requiring oxygen 4 L/min by nasal cannula. Diffuse crackles were heard on lung auscultation. He also had a 4/6 diastolic murmur.
Learning Objectives
-
•
To demonstrate that urgent TAV-in-TAV in homograft can be a safe option in patients with multiple prior sternotomies if the anatomy is low risk for coronary obstruction.
-
•
To demonstrate that multidetector computed tomography planning is critical in establishing the feasibility of TAV-in-TAV, particularly in patients who are graft dependent.
Past Medical History
The patient had a history of rheumatic heart disease and 3 sternotomies. The first was at 20 years of age for surgical aortic valve replacement (SAVR) with mechanical valve, and the second was approximately 15 years later for redo SAVR with mechanical valve. The third was 20 years before the current presentation for aortic valve endocarditis with valve dehiscence, which required redo SAVR with 24-mm homograft and saphenous vein graft (SVG) to right coronary artery and SVG to left main coronary artery performed at our institution. Subsequently, he had a transcatheter aortic valve implantation (TAVI) procedure performed elsewhere with 34-mm Evolut R (Medtronic) 4 years prior for homograft failure with severe aortic regurgitation (AR). Other comorbidities included type 2 diabetes, hypertension, and hyperlipidemia.
Differential Diagnosis
Differential diagnoses included acute heart failure exacerbation secondary to valve dysfunction, infective endocarditis, and pneumonia.
Investigations
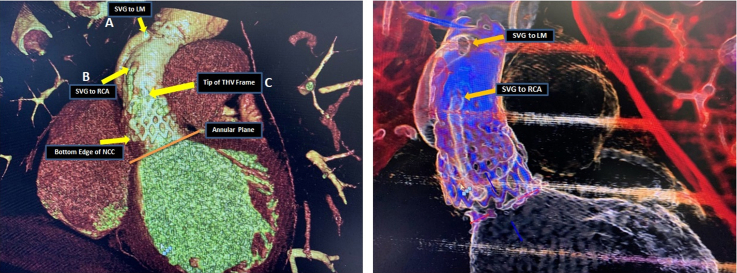
Chest computed tomography (CT) ruled out pulmonary embolism; however, it revealed multifocal infiltrates concerning for multilobed pneumonia. He had mild leukocytosis at 11.83 k/μL (normal range: 4.5-11 k/μL). Troponin levels were 29 and 27 ng/L at 0 and 3 hours, respectively (normal range: 0-19 ng/L). B-type natriuretic peptide levels were elevated with 589 pg/mL (normal range 0-100 pg/mL). Clinically the patient was in decompensated heart failure. Transthoracic echocardiography on admission showed left ventricular ejection fraction 20%-24% with severe prosthetic AR (Video 1). Transesophageal echocardiography (TEE) showed severe transvalvular AR without clear evidence of vegetations (Video 2). Multiple sets of blood cultures remained negative. Cardiac gated positron emission tomography showed no uptake of fluorodeoxyglucose. Given prior history of endocarditis, we attributed severe prosthetic AR to possibly another episode of endocarditis (healed), although cultures and positron emission tomography were negative. We could not rule out noninfective structural valve deterioration of the previous TAVI after 4 years. Society of Thoracic Surgeons–predicted risk of mortality was 2.4%. After extensive heart team discussions, the patient was deemed very high risk for fourth sternotomy, TAVI explant, and redo root replacement. He underwent CT TAVI protocol for anatomic feasibility for a transcatheter aortic valve (TAV)-in-TAV procedure. CT TAVI revealed that the leaflets of the transcatheter heart valve (THV) were mildly calcified, with no thrombosis. Both coronaries were sewn in the ascending aortic homograft with end-to-side vein graft anastomoses without significant stenosis. The THV frame was below the origin of both SVGs, with the patient being entirely graft dependent and at a low risk for coronary obstruction with TAV-in-TAV (Figure 1).
Figure 1.
Cardiac Gated CT Showing the Aortic Homograft
(A) Saphenous vein graft (SVG) to LM. (B) SVG to right coronary artery (RCA). (C) Tip of the previous transcatheter heart valve (THV). CT = computed tomography; LM = left main coronary artery; NCC = non-coronary cusp.
Management
The patient was initially managed with antibiotics and intravenous diuretics. He developed rapid atrial fibrillation, which was treated with beta blocker and amiodarone. Shortly after the CT, he deteriorated rapidly, with increased oxygen requirements, worsening tachycardia, hypotension, decreased peripheral perfusion, and cardiogenic shock. Considering feasibility of TAV-in-TAV with low risk of coronary obstruction, the patient was urgently brought to the cardiac catheterization laboratory for TAV-in-TAV. We chose a supra-annular self-expanding THV platform for this patient who had multiple aortic valve reinterventions, in view of superior hemodynamics and the lower rate of structural valve deterioration.
A 34-mm Evolut FX was advanced via transfemoral route in usual fashion and positioned such that the inflow of the previously placed valve was aligned with the inflow of the new THV. Once good position was confirmed by fluoroscopy and TEE, it was deployed in the usual manner. Echocardiography showed the valve in good position with no perivalvular leak. Ascending aortogram showed good alignment of inflows of both valves no perivalvular leak (Video 3). Aortic pressure increased from 76/54 mm Hg to 108/78 mm Hg. Later, the patient developed some hemodynamic instability and pressor requirement. Echocardiography showed no pericardial effusion and angiography showed no annular rupture or coronary obstruction. A left femoral intra-aortic balloon pump with 1:1 configuration was subsequently placed along with a right internal jugular Swan-Ganz catheter for hemodynamic monitoring. Patient was extubated on the second day. The intra-aortic balloon pump was removed on postoperative day (POD) 3 and Swan-Ganz catheter on day 4. Oxygen requirements decreased after the procedure, and on POD 4, the patient was doing well on room air. Guideline-directed medical therapy for heart failure therapy was initiated. After initiating oral apixaban, the patient was successfully cardioverted on POD 4 after TEE confirmed no thrombus in the left atrium or left atrial appendage.
Discussion
Valve-in-valve (ViV) implantation can be a viable option for the management of bioprosthetic aortic valve failure in patients at high risk for surgery.1 Redo TAVI appears to be safe and is linked with positive short- and long-term outcomes for the treatment of postprocedural and late-incidence paravalvular regurgitation and TAVI prosthesis failure.2 There have been reports of recurrent TAV-in-TAV studies for the treatment of failed surgically implanted valves; however, these consecutive TAVIs were completed directly during the original ViV procedure after deployment failure of the first valve.3,4 Few studies exist for repeated TAV-in-TAV in failed homograft.5,6 In these studies, TAVI was shown to serve as a practical and reliable substitute for high-risk surgical reintervention for failed ViV for aortic homografts.
To our knowledge, there are no published reports of patients with failed homograft and a previous TAVI requiring urgent TAV-in-TAV in the setting of severe AR and cardiogenic shock.
The main potential challenges with TAV-in-TAV or ViV are high postprocedural gradients and coronary obstruction. After ViV TAVI, high postprocedural gradients, defined as gradient >20 mm Hg, occurred in approximately 28% of cases.1 Our patient had acceptable gradients with mean gradient of 6.6 mm Hg and peak gradient of 11 mm Hg. The risk of coronary ostial obstruction may be high in TAV-in-TAV procedures, especially in patients with previous commissural misalignment particularly with a previously implanted Evolut valve.7
In our case, fortunately both coronaries, anastomosed with SVG to the ascending aorta high above the prior self-expanding valve, rendering the risk of coronary obstruction with TAV-in-TAV essentially close to zero. We chose a self-expanding valve because of superior hemodynamics and the lower rate of structural valve deterioration, particularly in this patient who had multiple aortic valve reinterventions.8 Urgent/emergent TAVI has been described to be feasible with acceptable outcomes in select group of patients with severe aortic stenosis from the STS/ACC TVT Registry.9 Our patient was in cardiogenic shock, was deemed to be very high risk for fourth time sternotomy, and fortunately was at low risk for coronary obstruction with TAV-in-TAV in a failing homograft. After heart team discussion, we decided to proceed with urgent TAVI, which was performed uneventfully. A balloon pump was placed as anticipated at the end of the procedure and successfully weaned off in the perioperative period.
Follow-up
The patient was discharged home 1 week after the procedure. He was doing well at his 1-month and 3-month follow-up appointments. Transthoracic echocardiography at the latest follow-up showed trivial AR with mean gradient of 4 mm Hg and peak gradient of 8 mm Hg (Video 4). He will continue to follow up in the valve clinic.
Conclusions
We describe the case of a 59-year-old male patient with 3 previous SAVRs who presented with severe AR and cardiogenic shock secondary to failed TAV-in-homograft. Multidetector CT planning was critical in establishing the feasibility of emergent TAV-in-TAV in this patient who was entirely graft dependent and at low risk of coronary obstruction.
Funding Support and Author Disclosures
Dr Atkins is a consultant for W L Gore & Associates. Dr Reardon is a consultant for Medtronic, Boston Scientific, Abbott, and W L Gore & Associates. Dr Kleiman is a local principal investigator in trials sponsored by Boston Scientific, Medtronic, Abbott, and Edwards Lifesciences. Dr Goel is a consultant for Medtronic and W L Gore & Associates; and is on the Speakers Bureau for Abbott Structural Heart. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Transesophageal Echocardiography
Transthoracic echocardiography on admission showing severe prosthetic aortic regurgitation.
Transthoracic Echocardiography
Transthoracic echocardiography on admission showing severe transvalvular aortic regurgitation.
Procedural Details
(A) Valve crossed using a pigtail. (B) Valve delivery system advanced over the extra small safari wire positioned in the left ventricle. (C) Valve positioning in fluoroscopic view with no parallax in transcatheter heart valve frame. (D) Valve deployment under ventricular pacing (rate 140/min). (E) Optimal transcatheter heart valve positioning with frames lined up. (F) Hemostasis successfully achieved using proglide.
Follow-Up Transthoracic Echocardiography
Follow-up transthoracic echocardiography showing trivial aortic regurgitation.
References
- 1.Dvir D., Webb J., Brecker S., et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve-in-valve registry. Circulation. 2012;126:2335–2344. doi: 10.1161/CIRCULATIONAHA.112.104505. [DOI] [PubMed] [Google Scholar]
- 2.Barbanti M., Webb J.G., Tamburino C., et al. Outcomes of redo transcatheter aortic valve replacement for the treatment of postprocedural and late occurrence of paravalvular regurgitation and transcatheter valve failure. Circ Cardiovasc Interv. 2016;9(9) doi: 10.1161/CIRCINTERVENTIONS.116.003930. [DOI] [PubMed] [Google Scholar]
- 3.Bagur R., Dumont É., Doyle D., et al. Transcatheter aortic valve-in-valve-in-valve implantation for a failed xenograft. Ann Thorac Surg. 2012;93:647–650. doi: 10.1016/j.athoracsur.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Nuis R.J., Benitez L.M., Nader C.A., et al. Valve-in-valve-in-valve transcatheter aortic valve implantation to treat a degenerated surgical bioprosthesis in a subaortic position. Tex Heart Inst J. 2013;40:323–325. [PMC free article] [PubMed] [Google Scholar]
- 5.Hollander K.N., Montealegre-Gallegos M., Mahmood F. Valve-in-valve-in homograft: a case of a repeat transcatheter aortic valve replacement in a patient with an aortic homograft. Ann Card Anaesth. 2016;19(4):737–739. doi: 10.4103/0971-9784.191575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Díez J.G., Schechter M., Dougherty K.G., et al. Transcatheter aortic valve-in-valve replacement instead of a 4th sternotomy in a 21-year-old woman with aortic homograft failure. Tex Heart Inst J. 2016;43(4):334–337. doi: 10.14503/THIJ-15-5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang G.H.L., Zaid S., Fuchs A., et al. Alignment of transcatheter aortic-valve neo-commissures (ALIGN TAVR): impact on final valve orientation and coronary artery overlap. J Am Coll Cardiol Intv. 2020;13(9):1030–1042. doi: 10.1016/j.jcin.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 8.O'Hair D., Yakubov S.J., Grubb K.J., et al. Structural valve deterioration after self-expanding transcatheter or surgical aortic valve implantation in patients at intermediate or high risk. JAMA Cardiol. 2023;8(2):111–119. doi: 10.1001/jamacardio.2022.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolte D., Khera S., Vemulapalli S., et al. Outcomes following urgent/emergent transcatheter aortic valve replacement: insights from the STS/ACC TVT Registry. J Am Coll Cardiol Intv. 2018;11(12):1175–1185. doi: 10.1016/j.jcin.2018.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transesophageal Echocardiography
Transthoracic echocardiography on admission showing severe prosthetic aortic regurgitation.
Transthoracic Echocardiography
Transthoracic echocardiography on admission showing severe transvalvular aortic regurgitation.
Procedural Details
(A) Valve crossed using a pigtail. (B) Valve delivery system advanced over the extra small safari wire positioned in the left ventricle. (C) Valve positioning in fluoroscopic view with no parallax in transcatheter heart valve frame. (D) Valve deployment under ventricular pacing (rate 140/min). (E) Optimal transcatheter heart valve positioning with frames lined up. (F) Hemostasis successfully achieved using proglide.
Follow-Up Transthoracic Echocardiography
Follow-up transthoracic echocardiography showing trivial aortic regurgitation.