Central Illustration
Key words: anti-inflammatory agents, blood coagulation, embolism and thrombosis, inflammation, interleukin-1, post-thrombotic syndrome
Highlights
-
•
Inflammation is intricately intertwined with coagulation and plays a critical role in venous thrombogenesis.
-
•
NLRP3 inflammasome activation, pyroptosis, and IL-1 are emerging contributors to thromboinflammation and VTE.
-
•
This review provides an overview of preclinical and clinical evidence supporting IL-1 and the NLRP3 inflammasome as potential therapeutic targets in VTE.
-
•
Studies exploring inflammasome signaling blockade for the prevention and management of VTE and ensuing complications are warranted.
Summary
Venous thromboembolism (VTE) remains a major health burden despite anticoagulation advances, suggesting incomplete management of pathogenic mechanisms. The NLRP3 (NACHT–, LRR- and pyrin domain–containing protein 3) inflammasome, interleukin (IL)-1, and pyroptosis are emerging contributors to the inflammatory pathogenesis of VTE. Inflammasome pathway activation occurs in patients with VTE. In preclinical models, inflammasome signaling blockade reduces venous thrombogenesis and vascular injury, suggesting that this therapeutic approach may potentially maximize anticoagulation benefits, protecting from VTE occurrence, recurrence, and ensuing post-thrombotic syndrome. The nonselective NLRP3 inhibitor colchicine and the anti–IL-1β agent canakinumab reduce atherothrombosis without increasing bleeding. Rosuvastatin reduces primary venous thrombotic events at least in part through lipid-lowering independent mechanisms, paving the way to targeted anti-inflammatory strategies in VTE. This review outlines recent preclinical and clinical evidence supporting a role for inflammasome pathway activation in venous thrombosis, and discusses the, yet unexplored, therapeutic potential of modulating inflammasome signaling to prevent and manage VTE.
Inflammation triggers coagulation to limit pathogen dissemination.1,2 During infection, this physiological, protective host response—immunothrombosis—is the result of the interplay between innate immunity, platelets, and the endothelium that synergistically trigger blood coagulation leading to thrombosis.1,2 Excessive immunothrombosis results in pathologic hypercoagulability.2,3 This paradigm has come to the forefront during the severe acute respiratory syndrome coronavirus 2 pandemic, as the clinical phenotype of patients with severe COVID-19 is often characterized by hyperinflammation and hypercoagulability.3,4 Aberrant activation of innate immunity and coagulation may also occur in the absence of invading pathogens and is referred to as thromboinflammation. During the last decade, thromboinflammation has emerged as a key contributor to sterile thrombosis (venous, arterial, microvascular).1,2
NLRP3 (NACHT-, LRR-, and pyrin domain–containing protein 3) is an intracellular innate immune receptor that serves as a sensor.5, 6, 7 In response to cellular stress or damage, NLRP3 forms a macromolecular complex—the NLRP3 inflammasome—which produces active interleukin (IL)-1β and IL-18, 2 potent proinflammatory, procoagulant cytokines of the IL-1 family, and induces a form of inflammatory cell death termed pyroptosis.5, 6, 7 NLRP3 inflammasome activation and downstream molecular events can enhance recruitment and activation of leukocytes and platelets, increase vascular permeability, and favor a prothrombotic endothelial phenotype.4,6, 7, 8 Inflammasome activation also promotes thromboinflammation by inducing release of tissue factor (TF) by monocytes and macrophages, and generation of neutrophil extracellular traps (NETs).9, 10, 11 These vascular processes prime a prothrombotic milieu, eventually leading to formation and propagation of venous thrombosis. Recent preclinical studies show that inhibition of the NLRP3 inflammasome and IL-1 signaling reduces venous thrombogenesis, and may favor vein wall healing, suggesting that these therapeutic approaches may have the potential to reduce the occurrence and recurrence of venous thromboembolism (VTE), as well as long-term complications such as the post-thrombotic syndrome (PTS).11, 12, 13, 14, 15
Landmark trials in patients with coronary artery disease have demonstrated a reduction in atherothrombosis associated with pharmacologic agents that target the NLRP3 inflammasome pathway, thereby reinforcing the inflammatory hypothesis of atherothrombosis, and shedding light on the potential, yet largely unexplored, of inflammasome pathway-targeted therapeutics across a larger spectrum of thrombotic disorders including VTE.16
In this review, we summarize the preclinical and clinical evidence on the emerging role of the NLRP3 inflammasome and IL-1 in the pathogenesis of venous thrombosis. We also provide insights on the therapeutic potential of targeting inflammasome signaling to prevent and manage VTE.
Venous Thromboembolism
VTE, comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), affects approximately 10 million people every year worldwide, and is the third leading vascular disease after acute myocardial infarction and stroke.17,18 Despite therapeutic advances, the incidence of VTE continues to rise with increased life expectancy and prevalence of conditions that predispose to VTE.17,18 VTE remains the first cause of preventable hospital death, and is associated with short- and long-term morbidity, disability, and health care system costs.17 Up to 10% and 25% of patients experience recurrent VTE at 1 and 5 years, respectively, with an associated case fatality rate of 5%.19 PTS is a highly morbid complication of VTE that occurs in 20% to 50% of patients with DVT. Patients with PTS experience persistent inflammation and chronic venous dysfunction ranging from leg swelling and pain, to ulceration and gangrene.20 Larger thrombi, incomplete thrombus clearance, and vein scarring have been linked to the development of PTS after DVT.20 Similarly, the post-PE syndrome, comprising chronic thromboembolic pulmonary hypertension, complicates up to 16% of PEs, impacting prognosis and quality of life.17
VTE as an Inflammatory Disease
In the pathogenesis of VTE, multiple factors can interact to reach the so-called thrombosis threshold. Active cancer, major trauma and surgery, and restricted mobility are frequently associated with provoked VTE.17,18 However, an apparent risk factor is not identified in approximately one third of VTE episodes, which are classified as unprovoked.17,18
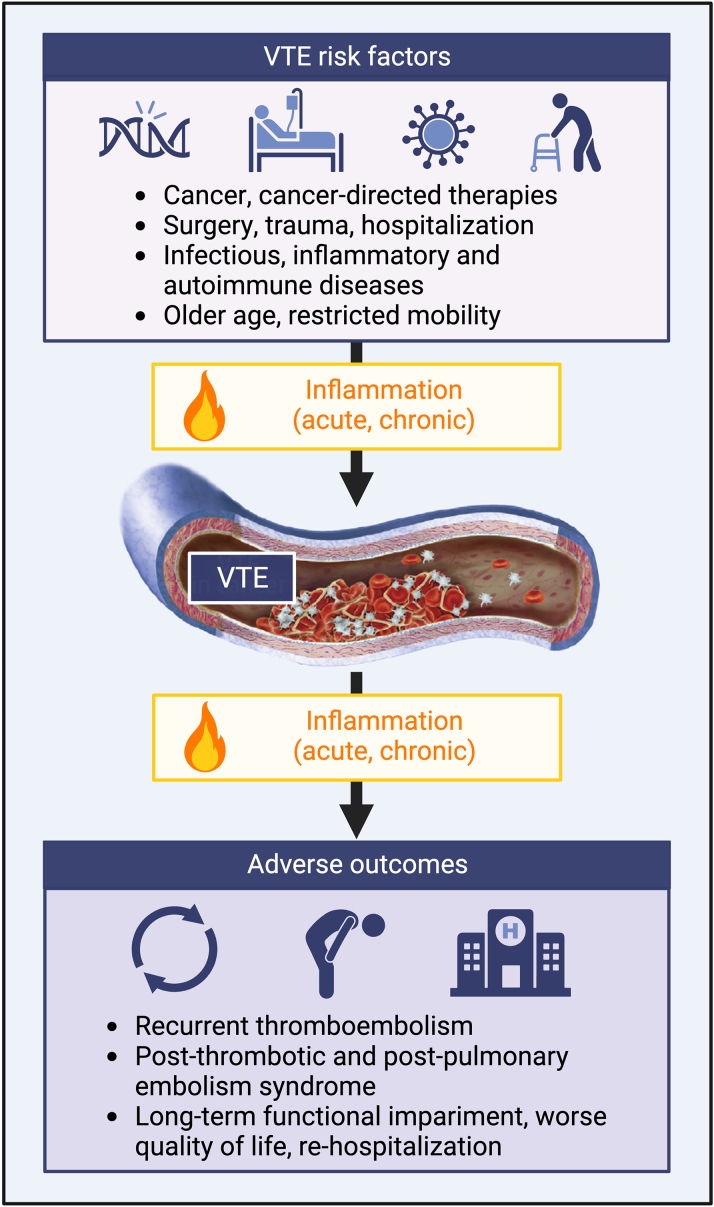
Inflammation could be regarded to as a pathogenic substrate common to several VTE-predisposing conditions (Figure 1).21,22 Surgery is a potent proinflammatory stimulus associated with increased risk of VTE that extends for several months after the procedure.23 Acute infections account for a 15-fold increased VTE incidence, with a legacy effect lasting for up to 1 year.24,25 Low-grade, chronic inflammation also provides a strong prothrombotic milieu promoting VTE as observed in patients with cancer, obesity, heart failure, chronic kidney disease, or autoimmune disorders.26, 27, 28, 29, 30
Figure 1.
Inflammation as a Substrate for the Development, Progression, and Complications of VTE
Created with BioRender.com. VTE = venous thromboembolism.
C-reactive protein (CRP), an acute-phase protein induced by IL-6 downstream of IL-1β, is an inflammatory biomarker widely used for cardiovascular risk stratification.31 In COVID-19, CRP predicts in-hospital VTE and mortality, with residually elevated CRP at discharge predicting postdischarge VTE.32,33 In hospitalized patients, CRP independently correlates with the 90-day risk of VTE regardless of the cause of hospitalization.34 Chronically elevated CRP, as it may occur in cancer and obesity, is associated with VTE.26,27 A positive correlation between CRP and VTE is also found among apparently healthy individuals. CRP >3 mg/L vs <1 mg/L doubles VTE risk.35 A meta-analysis comprising 81,635 subjects from 8 population-based prospective cohorts estimated a 23% increased risk of VTE per 5 mg/L increase in CRP.36 Taken together, clinical and subclinical inflammation may mark a population of patients at higher risk of VTE.
Venous thrombus formation and vein wall injury are capable of inducing a robust inflammatory response, reflected by systemic elevations in leukocytes and proinflammatory cytokines shortly after VTE.37,38 In patients with DVT, IL-6 and CRP levels correlate with thrombus extension, residual venous obstruction, and severity of clinical manifestations.38, 39, 40, 41 A proinflammatory state can persist in the subacute and remote post-thrombotic phases, with sustained increases in CRP independently associating with long-term venous dysfunction.42,43 Excessive inflammation is found to parallel with persistent alterations in the markers of hypercoagulability (factor VIII, von Willebrand factor [VWF]), fibrinolysis (fibrinogen, D-dimer), endothelial activation (E-selectin, intercellular adhesion molecule 1 [ICAM-1]), and vascular proteolysis.44 Unopposed inflammation might, therefore, simultaneously affect coagulation and fibrinolysis, sustain vascular dysfunction, and impair vein wall healing after VTE. Patients with PTS exhibit higher IL-6, IL-8, and ICAM-1 when compared with those without PTS.45 In the BioSOX study (Biomarker Substudy of the Compression Stockings to Prevent the Post-Thrombotic Syndrome [SOX] trial) including including 803 participants followed for 24 months after DVT, elevated CRP at 1 month and IL-6 at 1 and 6 months associated with higher incidence of PTS.46 CRP >5 mg/L at 12 months after unprovoked DVT has been independently linked to an 8-fold increased risk of developing PTS within the following year, regardless of anticoagulant regimen.47 Inflammation may also play a role in VTE recurrence.48 High-sensitivity CRP >4.5 mg/L, measured after withdrawal of anticoagulant therapy, independently predicted a 10-fold increased risk of recurrence in patients with cancer.49 Increased levels of proinflammatory cytokines are also found after acute PE,50 and appear to correlate with right ventricular dysfunction and worse prognosis.51 These observations may collectively suggest that exaggerated inflammation after VTE may be associated with, and perhaps contribute to, the development of ensuing complications.
Overview of the NLRP3 Inflammasome Pathway
The NLRP3 inflammasome pathway plays a key role in the development and progression of several cardiovascular diseases.6,7,52 Innate immunity is mediated by evolutionarily conserved receptors, the pattern recognition receptors (PRRs), that sense tissue injury by recognizing “danger” molecules sharing similar chemical properties.53 These include pathogen-associated molecular-patterns (PAMPs), generated during infection, and damage-associated molecular-patterns (DAMPs), generated in sterile injury. Binding of DAMPs or PAMPs to PRRs initiates an innate immune response.53 The most characterized PRRs are NOD-like receptors (NLRs) and Toll-like receptors (TLRs; reviewed elsewhere).53 NLRs include proteins forming the NLRP3 inflammasome, an intracellular macromolecular complex that produces active cytokines of the IL-1 family and promotes pyroptosis.5, 6, 7 NLRP3 is the most studied and engaged in cardiovascular pathology, however, other inflammasomes such as NLRP1, NLRC4, and AIM2 have been identified.54
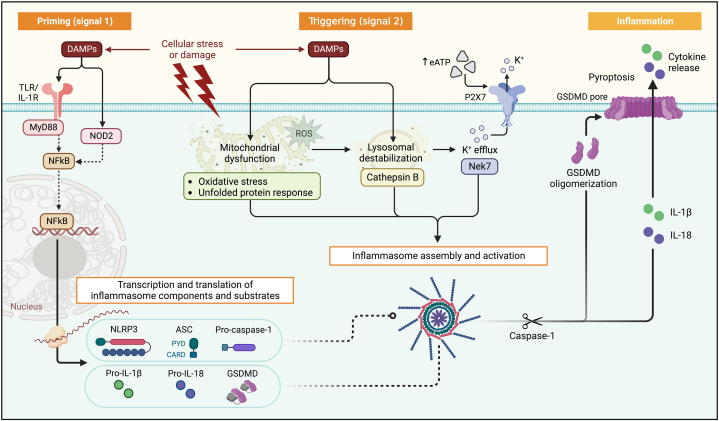
NLRP3 consists of 3 main domains: the C-terminal leucine-rich repeats (LRR) domain, the central NACHT domain (also known as NOD, nucleotide-binding oligomerization domain), and the pyrin domain (or PYD) serving as the N-terminal effector domain.5, 6, 7 Inflammasome assembly is finely regulated and, depending on the cytotype, it requires 2 distinct signals: priming and triggering. Priming induces the production of a reserve of inflammasome components in cells expressing low basal levels.5, 6, 7 Priming can be induced by PRRs (eg, TLRs), cytokine receptors, or other receptors not conventionally linked to inflammatory pathways such as angiotensin receptor type 1 receptor and β-adrenergic receptor (Figure 2).5, 6, 7 Nuclear factor-kappa B (NF-kB) regulates the transcription of inflammasome core components and substrates. When priming is completed, a variety of signals can trigger inflammasome activation. These include extracellular stimuli such as adenosine triphosphate (ATP)-mediated activation of the P2X purinoreceptor (P2X7R), and intracellular stimuli such as reactive oxygen species, mitochondrial or lysosomal damage, and disruption of autophagy.5, 6, 7 These perturbations induce K+ intracellular decline or lysosomal destabilization, which are sensed by NLRP3 that undergoes conformational changes, and forms a macromolecular effector platform, the NLRP3 inflammasome.5, 6, 7 NLRP3 interacts with the PYD domain of apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), leading to ASC polymerization into filaments. These star-like filamentous structures, through the caspase recruitment domain of ASC, engage pro-caspase-1, which undergoes autocatalytic activation to mature caspase-1.5, 6, 7 Within the inflammasome, caspase-1 cleaves pro–IL-1β and pro–IL-18 into active IL-1β and IL-18.5, 6, 7
Figure 2.
Schematic of the NLRP3 Inflammasome Pathway
NLRP3 inflammasome activation requires 2 signals. Priming (signal 1) is induced by DAMPs, including the IL-1β/(pro)–IL-1α or extracellular ATP released during tissue injury. DAMPs activate membrane receptors, including TLRs and IL-1R, leading to NF-κB translocation into the nucleus, and transcription and translation of inflammasome components and the precursors of IL-1β and IL-18, which accumulate in the cytoplasm. Triggering (signal 2) induces inflammasome assembly and activation, promoted by extracellular ATP (through purinergic receptor P2X7R), or other intracellular other DAMPs (eg, mitochondrial or lysosomal proteins, like ROS and cathepsin B). These induce K+ efflux with NLRP3 activation. In response, NLRP3 oligomerizes and binds ASC and procaspase-1, resulting in autocatalytic activation of procaspase-1 to caspase-1. Caspase-1 cleaves pro–IL-1β and pro–IL-18 into their active forms. Caspase-1 also cleaves GSDMD, producing N-terminal fragments that oligomerize and form cell membrane pores, which enable extracellular release of IL-1β and IL-18. Caspase-1 and GSDMD pores also mediate pyroptotic cell death. Created with BioRender.com. ASC = apoptosis-associated speck-like protein containing a caspase recruitment domain; ATP = adenosine triphosphate; CARD = caspase recruitment domain; DAMPs = damage-associated molecular-patterns; GSDMD = gasdermin D; IL = interleukin; IL-1RA = interleukin-1 receptor antagonist; IL-1R = interleukin-1 receptor; MyD88 = myeloid differentiation factor 88; NLRP3 = NACHT-, LRR-, and pyrin domain–containing protein 3; NF-kB = nuclear factor-kappa B; NOD2 = nucleotide-binding oligomerization domain-containing protein 2; P2X7R = P2X7 receptor.
Caspase-1 also cleaves gasdermin D (GSDMD) into N-terminal fragments, which migrate to the cell membrane and form pores that facilitate extracellular release of IL-1β and IL-18 (Figure 2).5, 6, 7 IL-1α is another proinflammatory cytokine of the IL-1 family and an inducer of IL-1β. Pro–IL-1α is constitutively expressed by mesenchymal cells (eg, endothelial cells), and is inducible in myeloid lineages.5, 6, 7 In contrast to IL-1β, pro–IL-1α lacks a caspase-1 processing site and is already active in its precursor form, which acts as an “alarmin” in an autocrine or paracrine fashion when released by damaged or dead cells.5, 6, 7 IL-1α can be also found membrane-anchored and signal through cell-cell interactions.5, 6, 7
Both IL-1β and IL-1α signal through the IL-1 receptor, a heterodimeric type I transmembrane receptor formed by the IL-1 receptor type I (IL-1RI) and the IL-1 receptor accessory protein.5, 6, 7 Binding of IL-1β or IL-1α triggers the heterodimer to recruit myeloid differentiation factor 88, resulting in the translocation of NF-kB to the nucleus, where it regulates the transcription of several proinflammatory genes (Figure 3).5, 6, 7 The IL-1 receptor antagonist (IL-1RA) is a decoy ligand that binds and sequesters IL-1RI, serving as an endogenous counterpoint to IL-1 signaling. IL-18 signals through the heterodimeric IL-18 receptor (IL-18R). IL-18 binding protein is a natural IL-18 antagonist that sequester IL-18 into a stable complex, preventing the interaction with IL-18R.5, 6, 7
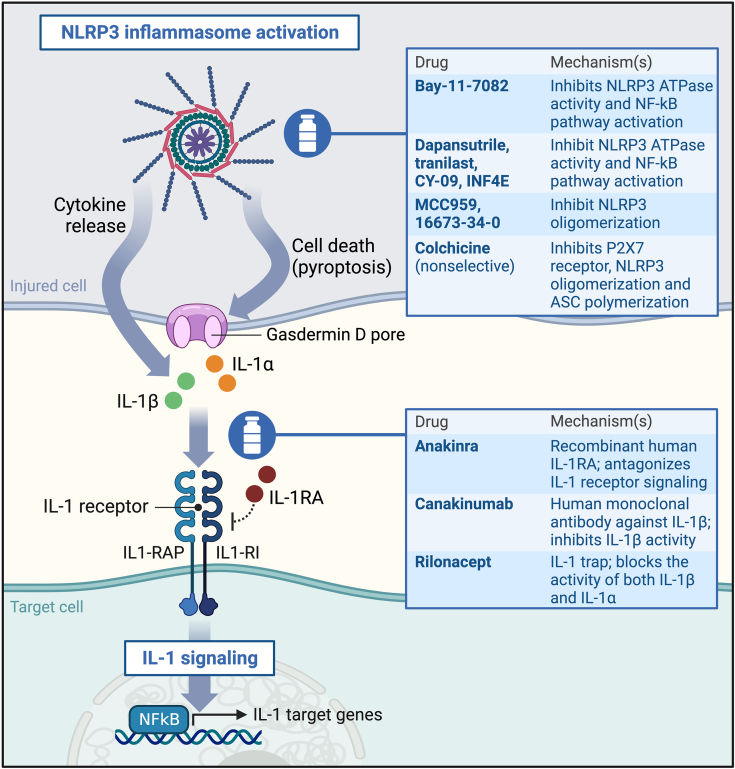
Figure 3.
Pharmacologic Agents Targeting the NLRP3 Inflammasome and IL-1, and Their Mechanism of Action
Created with BioRender.com. Abbreviations as in Figure 2.
The series of events that follow inflammasome activation, caspase-1 activation, and GSDMD pore formation may result in pyroptosis, which can also occur through noncanonical pathways (eg, caspase-4/-5 in humans, caspase-11 in mice).5, 6, 7 Unlike programmed cell death, pyroptosis is associated with cell rupture and release of proinflammatory molecules in the extracellular milieu, where they contribute to modulate thrombogenesis and cellular responses to vascular injury.52,55
Cell-Specific Thromboinflammatory Contributions of Inflammasome Signaling
Innate Immune Cells
Venous thrombus formation can be initiated by the extrinsic coagulation pathway via TF, which leads to proteolytic activation of coagulation factors VIIa, Xa, and IIa (thrombin). These sequential reactions ultimately result in thrombin-mediated fibrin formation.16,56 Under pathologic conditions, activated myeloid cells, primary circulating monocytes, and extracellular vesicles are critical sources of intravascular TF.56, 57, 58, 59 Inflammasome assembly and caspase-1 activation in monocyte-lineage cells induce robust TF exposure and release, triggering clotting in vitro.60 In mice, activation of canonical (caspase-1) and noncanonical (caspase-11) inflammasome pathways results in macrophage release of TF-rich extracellular vesicles and widespread thrombosis.9 Deletion of GSDMD, but not of IL-1/IL-18 receptors, was protective during endotoxemia-induced thrombosis.9 Although awaiting confirmation in sterile thrombosis models, this finding suggests that NLRP3-mediated TF release by monocytes may depend on pyroptosis, and supports a role for inflammasome-mediated, IL-1-independent thrombosis pathways. Tumor cells also release abundant TF-positive extracellular vesicles, which correlate with VTE in patients with cancer.61 Further investigation is, however, warranted to appraise the potential role of the NLRP3 inflammasome pathway in cancer-associated VTE.
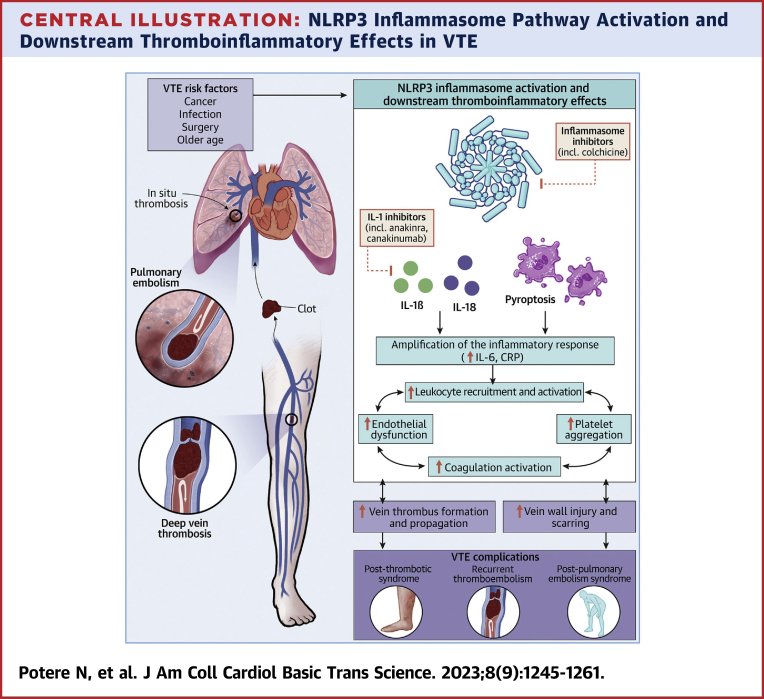
Macrophages, neutrophils, and platelets require minimal priming for inflammasome activation and rapid cytokine release after injury.5, 6, 7 Early in thrombogenesis, IL-1α derived from damaged cells (eg, vascular endothelial cells) can favor recruitment and activation of macrophages, neutrophils, and platelets at the site of thrombus formation.16,62 IL-1β and IL-18 released by recruited leukocytes and platelets work to increase vascular permeability, endothelial adhesion molecule expression, and additional release of proinflammatory mediators. These perturbations may in turn further promote leukocyte and platelet recruitment, endothelial dysfunction, amplification of tissue injury, and triggering of coagulation.16,62 This feed-forward cycle appears critical to the thromboinflammatory pathogenesis of VTE (Central Illustration).16,22,56
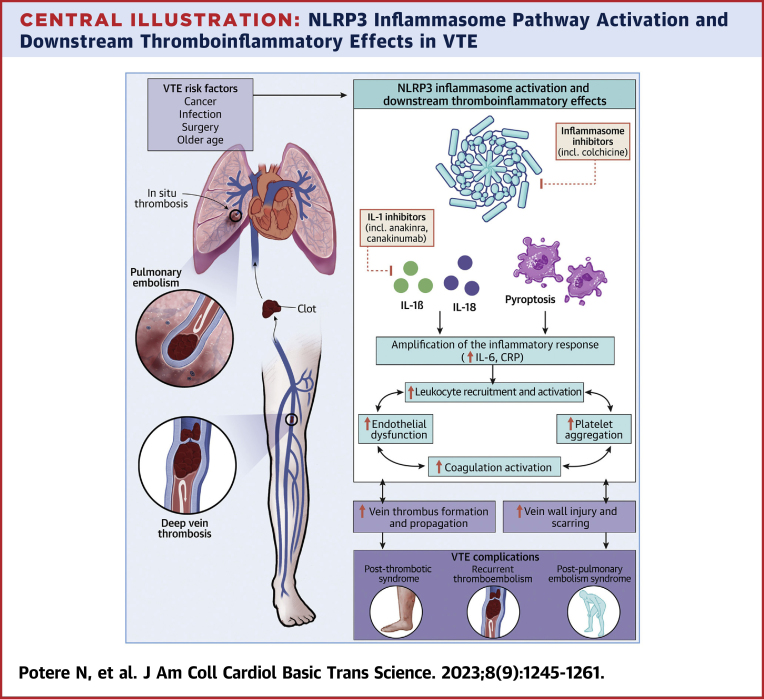
Central Illustration.
NLRP3 Inflammasome Pathway Activation and Downstream Thromboinflammatory Effects in VTE
Created with BioRender.com. CRP = C-reactive protein; IL = interleukin; NLRP3 = NOD-, LRR- and pyrin domain-containing protein 3; VTE = venous thromboembolism.
Venous thrombi are rich in fibrin, platelets, erythrocytes, and innate immune cells.59 Neutrophils expressing TF can bind to the injured endothelium and platelets, supporting platelet aggregation and thrombus formation.63 Neutrophils also sustain thrombogenesis through generation of reactive oxygen species and NETs.16,59 NET formation is an evolutionary defense mechanism that occurs when neutrophils encountering pathogens release their DNA as web-like structures, comprising a DNA core, histones, and proteases.16,56 Also under sterile conditions, NETs contribute to thrombogenesis. They can capture and present TF to blood, promote factor XII autoactivation, and proteolytically disrupt endogenous anticoagulant factors such as antithrombin and TF pathway inhibitor. These processes culminate in a procoagulant microenvironment that boosts thrombin and fibrin generation.16,56 The DNA core of NETs also interacts with VWF and fibrin, providing a fibrinolysis-resistant scaffold for thrombus stability and accretion.16,56 NETs are also implicated in cancer-associated VTE.64 Evidence suggests that inflammasome activation and NETosis are interconnected. NLRP3 inflammasome induction in neutrophils is requisite for some forms of NETosis.10,11 Vice versa, NETs can license NLRP3 inflammasome activation and subsequent release of IL-1β and IL-18, resulting in a feed-forward thromboinflammatory loop (Figure 4).10,11 Accordingly, genetic deletion or pharmacologic inhibition of NLRP3 suppresses NETosis in vitro, and mice lacking NLRP3 form smaller venous thrombi containing fewer NETs.11
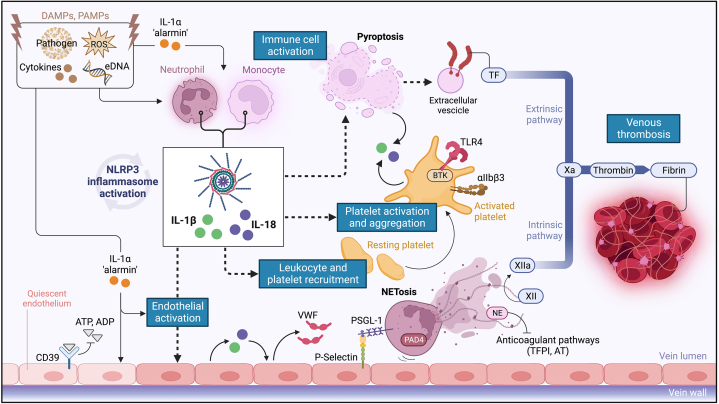
Figure 4.
Proposed Role of the NLRP3 Inflammasome and IL-1 in Venous Thrombogenesis
Early in thrombogenesis, multiple DAMPs and PAMPs (eg, extracellular FNA, ROS, proinflammatory cytokines), promote inflammasome activation in innate immune cells, primarily macrophages, and neutrophils. IL-1β and IL-18 released by infiltrating leukocytes induce endothelial IL-1 production and expression of adhesion molecules and prothrombotic factors, facilitating platelet recruitment. In a feed-forward loop, activation of platelet NLRP3 inflammasome, mediated by TLR4 and BKT, induces platelet IL-1 release and aggregation. IL-1α is abundantly released after vascular injury and, serving as an “alarmin,” sustains thromboinflammation. Macrophage NLRP3 activation leads to pyroptosis with release of TF, potently triggering coagulation with thrombin generation and fibrin formation. Neutrophil NLRP3 activation results, through PAD4, in NETosis. In turn, NETs sustain NLRP3 activation and IL-1 production. NETs and neutrophil-derived enzymes promote factor XII autoactivation and disrupt endogenous anticoagulant pathways (TFPI, AT), thus boosting thrombin generation. The DNA core of NETs interacts with VWF and fibrin providing a scaffold, resistant to fibrinolysis, for thrombus stability and growth. Created with BioRender.com. AT = antithrombin; BTK = Bruton's tyrosine kinase; eATP = extracellular ATP; GSDMD = gasdermin D; DAMPs = damage-associated molecular-patterns; eATP = extracellular ATP; IL = interleukin; NE = neutrophil elastase; NET = neutrophil extracellular traps; NLRP3 = NACHT-, LRR- and pyrin domain-containing protein 3; NF-kB = nuclear factor-kappa B; PAD4 = peptidylarginine deiminase 4; PAMPs = pathogen-associated molecular-patterns; PSGL-1 = P-selectin glycoprotein ligand-1; P2X7R = P2X7 receptor; ROS = reactive oxygen species; TF = tissue factor; TFPI = tissue factor pathway inhibitor; TLR4 = Toll-like receptor 4; VWF = von Willebrand factor; other abbreviations as in Figure 2.
Platelets
Activated platelets exhibit NLRP3 inflammasome activation and are capable of releasing IL-1β, IL-18, and IL-1α.65, 66, 67 Collagen and thrombin were shown to trigger, through TLR4 and Bruton’s tyrosine kinase, inflammasome activation, caspase-1 cleavage, and subsequent IL-1β release by platelets.65,68 Although representing a matter of debate and ongoing exploration, IL-1β release by platelets may also occur independently from inflammasome assembly, suggesting that these cells can store pools of mature IL-1β for rapid release upon activation.69 IL-1β was found to bind megakaryocyte IL-1RI activating NF-kB and mitogen-activated protein kinases to promote megakaryocyte maturation, and to enhance platelet aggregation and adhesion, with the latter being reduced in mice lacking IL-1RI.70 Systemic ablation of IL-1RI or IL-1β also reduced circulating levels of platelet aggregates in endotoxemic mice.70 Platelets derived from NLRP3 knock-out mice, and human platelets treated with NLRP3 or caspase-1 inhibitors, exhibit reduced IL-1β secretion, activation, and aggregation.65 The same inhibitors also reduce thrombus formation in vitro, with a similar effect observed when using NLRP3 knock-out blood.65 Although platelet NLRP3 deficiency did not result in altered expression of platelet-specific receptors such as αIIbβ3 integrin, GPIbα, and GPVI, transfusion of NLRP3-deficient platelets into wild-type mice prolonged tail-bleeding time and delayed arterial thrombus formation. Yet, NLRP3 deficiency was shown to reduce phosphorylation of c-Src/Syk tyrosine kinases downstream of αIIbβ3, decreasing platelet spreading and impairing clot retraction.71 Interestingly, treatment of wild-type platelets with an anti-IL-1β antibody elicited similar effects, which were reversed when exogenous recombinant IL-1β was added to NLRP3-deficient platelets.71 Treatment with a direct selective NLRP3 inhibitor significantly restrained aggregation of human platelets in response to collagen or ADP, and clot retraction.71 Although further research is necessary to establish the exact mechanisms and clinical relevance of such events, these findings suggest a role for NLRP3 activation and IL-1β in regulating platelet thromboinflammatory responses.
Platelets also support thromboinflammation by boosting NLRP3 inflammasome expression and activation in human innate immune cells, and are critical for optimal IL-1 responses in vivo.72 While the mechanisms underlying platelet-mediated modulation of IL-1 production and activity remain to be further elucidated, this seems to occur independently from cell-to-cell contact but rather through a constitutively expressed protein factor released by platelets that engages macrophage calcium-sensing receptors triggering inflammasome activation.72 Furthermore, activation of platelet NLRP3 inflammasome and subsequent shedding of platelet-derived, IL-1β–rich extracellular vesicles was shown to enhance endothelial permeability, providing an additional layer of platelet contributions fueling IL-1–driven thrombosis.73
Endothelial Cells
Endothelial NLRP3 inflammasome activation contributes to vascular dysfunction.74 Deletion of NLRP3 mitigated vascular inflammation and permeability in a mouse model of acute lung injury.75 Among multiple effectors, IL-1α and IL-1β can, in an autocrine or paracrine fashion, dramatically up-regulate the endothelial expression of adhesion molecules (P-selectin, ICAM-1, vascular cell adhesion molecule 1) and procoagulant factors (VWF, TF), and down-regulate endogenous anticoagulants (protein C, thrombomodulin) (Figure 4).76, 77, 78 NETs have been shown to induce, through concerted action of cathepsin G and IL-1α, TF expression and activity at the endothelial surface, thereby promoting thromboinflammation and vascular dysfunction after thrombosis.79 Of note, pretreatment of NETs with anti–IL-1α–neutralizing antibody or IL-1RA, but not with anti-IL-1β–antibody, suppressed the endothelial expression of ICAM-1, vascular cell adhesion molecule 1, and TF, thus establishing a central role for IL-1α in NET-mediated thrombogenicity.79 In response, TF and fibrin can exacerbate IL-1β–mediated endothelial activation in a thromboinflammatory circuit whereby IL-1 signaling induces a prothrombotic endothelial phenotype and, in turn, thrombosis sustains IL-1 production.80,81 Accordingly, IL-1RA treatment suppressed endothelial activation,77 and endothelial-specific deletion of IL-1RI alleviated vascular inflammation induced by intraperitoneal IL-1β injection in mice.81 Injection of IL-1β into rabbits altered the endothelial architecture resulting in platelet and fibrin deposition on vein walls, suggesting that IL-1β can prime the vascular environment for thrombus accretion.82
Endothelial cells also express IL-18 and IL-18R.83 IL-18 administration induced endothelial activation, VWF expression, and down-regulation of tissue plasminogen activator.84 IL-18 was also shown to amplify the inflammatory response through NF-kB activation, and increase the expression of matrix metalloproteases, which may contribute to adverse vein wall remodeling and vascular dysfunction after VTE.83,84
NLRP3 Inflammasome and IL-1 in Venous Thrombus Formation and Propagation
Single-nucleotide polymorphisms in the genes coding for IL-1β, IL-1RA, and IL-18 have been described to predispose to DVT.85, 86, 87 Small reports from patients with rare, monogenic autoinflammatory disorders involving genes coding for NLRP3 and IL-1RA resulting in unchecked IL-1β signaling noted high rates of venous thrombosis.88,89
In patients with altitude-induced DVT, inflammasome pathway component transcripts are highly expressed in peripheral blood mononuclear cells (PBMCs), accompanied by increases in caspase-1 activity and plasma levels of NLRP3, IL-1β, and IL-18.12,87 Similarly, PBMCs from patients with cancer-associated DVT exhibit enhanced NF-kB activity and IL-1β production corresponding to higher circulating levels of IL-1β compared to cancer patients without DVT.90
Multiple roles for engagement of the NLRP3 inflammasome pathway have been established in preclinical models of DVT (Table 1, Figure 5). After murine inferior vena cava stenosis, marked increases in NLRP3, IL-1β, and IL-18 transcripts were observed in the plasma.12 Thrombi and thrombosed vein segments were enriched in NLRP3 and IL-1β transcripts and proteins in parallel with enhanced caspase-1 activation.12 PBMCs and platelets from thrombotic mice also exhibited robust up-regulation of NLRP3 and IL-1β mRNA levels.12
Table 1.
Targeting of Inflammasome Signaling in Preclinical Models of Venous Thrombosis
| First Author (Ref. #) | Model | Inflammasome Signaling Inhibition Strategy |
Main Findings |
|---|---|---|---|
| Gupta et al12 | Rat IVC stenosis | NLRP3 siRNA Caspase-1 inhibition IL-1β inhibition |
siRNA against NLRP3 and treatment with the caspase-1 inhibitor ac-YVAD-cmk or anti–IL-1β antibody reduced thrombus size, prothrombin/D-dimer levels, and platelet aggregability |
| Yadav et al15 | Mouse IVC stenosis | IL-1 receptor antagonism IL-1β inhibition |
Administration of an anti–IL-1β antibody or the IL-1 receptor antagonist anakinra decreased thrombus incidence and size without prolonging tail bleeding time |
| Li et al84 | Rat IVC stenosis | IL-18 down-regulation | IL-18 down-regulation through lentiviral vectors suppressed venous thrombogenesis and the expression of NF-kB and vWF |
| Zhang et al13 | Mouse IVC stenosis | Caspase-1-/- Caspase-11-/- GSDMD-/- |
Deletion of caspase-1/GSDMD but not of caspase-11 reduced the incidence and extension of venous thrombosis |
| Campos et al14 | Mouse IVC stenosis | Caspase-1 inhibition | Administration of the caspase-1 inhibitor ac-YVAD-cmk reduced the incidence of thrombosis as well as thrombus size and NETosis |
| Munzer et al11 | Mouse IVC stenosis | NLRP3-/- Selective NLRP3 inhibition |
NLRP3 inhibition with MCC950 in either mouse or human neutrophils diminished NETosis; NLRP3 deficiency reduced NETosis and thrombus progression |
| Abu-Fanne et al97 | Mouse IVC stenosis | Nonselective NLRP3 inhibition | Colchicine treatment decreased clot size, and heparin coadministration reduced the heparin dose required to prevent thrombosis, with no discernable impact on hemostasis |
GSDMD = gasdermin D; IVC = inferior vena cava; IL = interleukin; NF-kB = nuclear factor-kappa B; NLRP3 = NOD-, LRR-, and pyrin domain–containing protein 3; NETs = neutrophil extracellular traps; siRNA = small-interfering RNA; VWF = von Willebrand Factor.
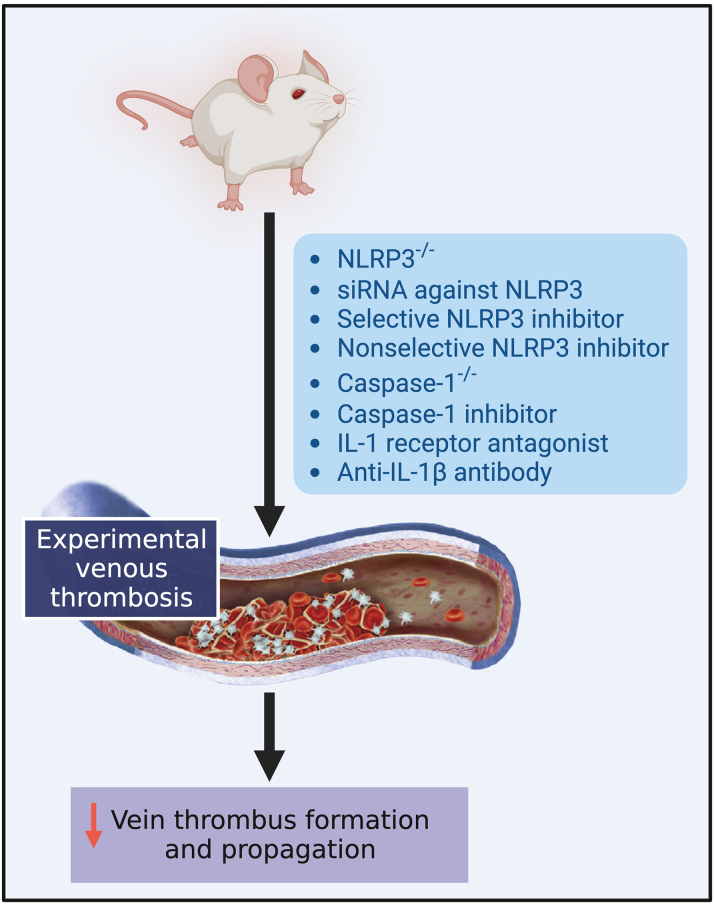
Figure 5.
Strategies Targeting Inflammasome Signaling Shown to Reduce Venous Thrombosis in Preclinical Models
Created with BioRender.com. NLRP3 = NACHT-, LRR- and pyrin domain-containing protein 3; DVT = deep vein thrombosis; siRNA = small-interfering RNA; other abbreviation as in Figure 2.
Exposure of rats to hypoxic, hypobaric conditions to mimic high-altitude conditions markedly up-regulates inflammasome signaling and aggravates venous thrombosis, while inhibiting hypoxia-inducible factor 1-alpha reverses these effects, indicating that hypoxia potentiates inflammasome activation and worsens thrombotic burden.12 Disruption of inflammasome signaling through small-interfering RNAs against NLRP3, a caspase-1 inhibitor or an anti–IL-1β antibody significantly reduces thrombus size, as well as prothrombin and D-dimer generation.12 Consistent with an upstream role for the inflammasome in platelet activation, inflammasome inhibition also decreases platelet aggregation in response to ADP.12
Mice genetically lacking caspase-1 or GSDMD but not caspase-11 are less prone to venous thrombosis, and form smaller thrombi compared with wild-type littermates.13 Monocyte depletion or TF deficiency were also shown to attenuate thrombogenesis.9,13 Taken together, these observations may suggest that canonical NLRP3 inflammasome activation in monocytes can induce pyroptosis, leading to TF release and thrombosis.9,13 Of note, caspase-1 or GSDMD deficiency did not affect platelet aggregation in response to thrombin or collagen ex vivo.13 Although abrogation of distinct inflammasome pathway components may impact platelet activity differently, in vitro and ex vivo assays may be subjected to intrinsic variability. Hence, additional in vivo studies are required to elucidate the impact of these interventions on platelet-mediated thromboinflammation and hemostasis.
A role for the inflammasome in the propagation of venous thrombosis has been recognized. Neutrophils from NLRP3-deficient mice exhibit reduced NETosis, with comparable effects obtained when treating human neutrophils with selective NLRP3 or caspase-1 inhibitors.11 NETs were shown to directly trigger inflammasome activation in platelets in vitro.14 Indeed, platelets represent more than half of caspase-1–positive cells within venous thrombi, and activated caspase-1 colocalizes with NETs at the thrombus sites enriched with platelets. This may suggest that inflammasome activation in neutrophils promotes NETosis, which in turn sustains platelet inflammasome activation as well as platelet recruitment and aggregation, thus favoring thrombus growth.14 Accordingly, mice treated with a caspase-1 inhibitor exhibit reduced NETosis and thrombus size.14 In line with these observations, venous thrombus growth in NLRP3-deficient mice was arrested sooner than in wild-type mice, and accompanied by reduced thrombus size at later timepoints.11
One important mechanism regulating venous thrombogenesis under flow-restricted and static blood flow conditions entails ectonucleoside tri(di)phosphohydrolase-1 (ENTPD1, also known as CD39). CD39 is a vascular enzyme that dissipates extracellular nucleotides including ATP and ADP released from damaged cells, thus preventing the activation of the P2X7R-mediated second signal for inflammasome assembly, and suppressing platelet activation by P2Y12R.15,91 Genetic deletion of CD39 aggravates venous thrombosis in parallel with enhanced NF-kB phosphorylation and exaggerated expression of NLRP3, caspase-1, and IL-1β within thrombi, and increased plasma IL-1β.15 Treatment of CD39-deficient mice with an IL-1β–neutralizing antibody or anakinra, a recombinant human IL-1RA approved for clinical use, markedly reduces venous thrombogenesis as shown by significantly fewer and smaller thrombi, without prolonging tail bleeding time.15 Although awaiting confirmation in clinical studies, these observations may support the therapeutic potential of inflammasome pathway blockade in VTE, and suggest that this therapeutic strategy may reduce venous thrombus formation and propagation while preserving hemostasis.15 Concurrently, they may raise concern about the potential thrombotic risk associated with the use of inhibitors to immune checkpoints such as CD39 in patients with cancer.15 Recent preliminary data suggest that CRP increases after initiation of immune checkpoint inhibitor therapy is associated with an increased risk of VTE.92 In mice, administration of pembrolizumab or ipilimumab induced myocardial expression of NLRP3, myeloid differentiation factor 88, and proinflammatory cytokines including IL-1β and IL-6, with enhanced NF-kB expression detected in the vascular endothelium and increased cytokine levels in the plasma.93 Additional studies are required to assess the potential contribution of inflammasome pathway activation to vascular toxicity associated with the use of immune checkpoint inhibitors.
Colchicine blocks microtubule polymerization and interferes with neutrophil diapedesis and degranulation.94 Colchicine appears to also exert nonspecific inhibitory activity on the NLRP3 inflammasome.95,96 In transgenic mice expressing human α-defensin-1, an antimicrobial protein released from activated neutrophils, colchicine administration 1 h after venous thrombosis and every 12 h thereafter significantly decreased clot size.97 Coadministration with heparin reduced by 20-fold the heparin dose required to prevent thrombosis, without increasing tail vein bleeding time.97 Interestingly, coadministration of subtherapeutic doses of colchicine and heparin associated with a 60% reduction in thrombus size with no impact on hemostasis.97 In another mouse study, colchicine, given 24 h after venous thrombosis, alleviated thrombus-induced vein wall scarring at days 8 and 14.98 This was associated with significant reductions in macrophage infiltration and fibroblast activation, with attenuated expression of profibrotic and proinflammatory markers, suggesting that colchicine may potentially represent a clinically viable option to reduce PTS after DVT.98 Additional studies are, however, warranted to validate these findings, and to clarify the precise molecular mechanisms underlying the potential antithrombotic and antiscarring effects of colchicine in the setting of venous thrombosis.
A role for IL-1α and IL-18 in VTE might be hypothesized also, although limited evidence is available to date.16,84,87 Increased IL-18 levels have been found in rodents and patients with DVT.84,87 IL-18 overexpression by means of lentiviral vectors resulted in larger venous thrombi compared with control rats, whereas IL-18 down-regulation curtailed thrombosis.84
Targeting Inflammation in VTE
Therapeutic efforts in VTE have mostly focused on addressing the coagulation cascade, with the introduction of vitamin K antagonists (VKAs), heparins, and direct oral anticoagulants (DOACs).17 Although these agents effectively target the coagulation cascade, they are associated with a risk of bleeding, and cannot completely prevent venous thrombosis and its complications including PTS and recurrent VTE,17 potentially unveiling a therapeutic gap due to a yet partially addressed pathogenic mechanism, namely thromboinflammation.16,99 As multiple thromboinflammatory pathways including the NLRP3 inflammasome are emerging contributors to venous thrombosis, pharmacologic agents targeting these pathways may potentially reduce VTE and its sequelae.
Anticoagulant agents reportedly have anti-inflammatory effects, which, however, remain difficult to appraise in humans. Heparins reduce neutrophil extravasation, dismantle NETs, and promote vein re-endothelization in animal models.16,99 DOACs targeting factor IIa or factor Xa, which activate proteinase-activated receptors (PARs), can reduce PAR-mediated inflammation.16,99 Inhibition of factor Xa, but not of factor IIa, reduced inflammasome activation resulting in attenuated fibrosis after murine myocardial ischemia-reperfusion.100 Rivaroxaban was found to reduce IL-6 and CRP in patients with atrial fibrillation,101 and a putative anti-inflammatory effect of rivaroxaban in slowing atherosclerotic vascular disease is advocated by the COMPASS (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary and Peripheral Artery Disease) and VOYAGER PAD (Efficacy and Safety of Rivaroxaban in Reducing the Risk of Major Thrombotic Vascular Events in Subjects with Symptomatic Peripheral Artery Disease Undergoing Peripheral Revascularization Procedures of the Lower Extremities) trials.99 Limited, mostly indirect, evidence exists on the potential anti-inflammatory effects of anticoagulants in the setting of VTE. Some studies suggested that, compared with VKA, heparins and DOACs could improve vein recanalization and reduce PTS, possibly due to profibrinolytic signaling.99 In a small observational study, rivaroxaban was associated with reduced fibrinogen and lower PTS when compared with VKA.102 Nevertheless, no significant reductions in PTS were found with rivaroxaban compared with enoxaparin or VKA in the EINSTEIN DVT (Oral Direct Factor Xa Inhibitor Rivaroxaban in Patients with Acute Symptomatic Deep Vein Thrombosis) study.103 Although further evaluation of the potential anti-inflammatory activity of current anticoagulants in VTE is needed, the persistence despite anticoagulant therapy of residual inflammation, either clinical or subclinical, in subgroups of patients with VTE may indicate that current anticoagulants alone cannot completely address inflammation associated with venous thrombosis.
Statins have established anti-inflammatory activity including reduction of leukocyte infiltration, NETosis, and inflammatory biomarkers, potentially improving thrombus resolution and vein scarring after DVT.99 A randomized trial enrolling 234 patients with acute DVT reported that the addition of rosuvastatin to low–molecular weight heparins reduced CRP and the 3-month incidence of PTS, highlighting the potential of anti-inflammatory strategies for PTS prevention.104 A phase 3 trial evaluating rosuvastatin to decrease the risk of recurrent VTE is currently ongoing (NCT04319627). Statins were also evaluated for the primary prevention of VTE. In the phase 3 JUPITER (Justification for the Use of statins in Primary prevention: an Intervention Trial Evaluating Rosuvastatin) trial enrolling normo-lipidemic subjects with high-sensitivity CRP ≥2 mg/L, rosuvastatin significantly reduced symptomatic VTE.105 A pooled analysis of the JUPITER and HOPE-3 (HOPE-3 (Heart Outcomes Prevention Evaluation-3) trials showed that rosuvastatin was associated with a 47% reduction in VTE incidence, regardless of the presence of VTE-related risk factors.106
Inflammasome Signaling Blockade in Atherothrombosis
To date, no trial has been conducted to evaluate the effects of selective targeting of the NLRP3 inflammasome or IL-1 for the prevention or treatment of VTE. However, rapidly accumulating proof-of-concept evidence from preclinical DVT models, data from patients with VTE, and indirect evidence derived from atherothrombosis trials may suggest that inflammasome signaling blockade could potentially represent a promising therapeutic strategy to prevent or manage VTE without increasing the risk of bleeding.
Selective NLRP3 inflammasome inhibitors have been developed and successfully tested in multiple preclinical models of disease, and are under clinical investigation for different indications.55,107,108 Colchicine may be regarded to as a nonselective NLRP3 inhibitor.94 In the LoDoCo (Low-Dose Colchicine)-2 trial, low-dose colchicine (0.5 mg once daily) was associated with a 31% reduction in the primary composite endpoint of cardiovascular death, ischemic stroke, myocardial infarction, or revascularization in patients with chronic coronary disease.109,110 A proteomic substudy found that low-dose colchicine suppressed inflammasome activation, as reflected by reduced IL-18, IL-1RA, and IL-6 levels, but also neutrophil degranulation, suggesting that the antithrombotic effects of colchicine may extend beyond inflammasome blockade.111 In COLCOT (Colchicine Cardiovascular Outcomes Trial), low-dose colchicine reduced coronary and cerebral thrombotic events in patients with recent acute myocardial infarction.112
IL-1 inhibitors are available for clinical use.7,55 Anakinra, a recombinant IL-1RA inhibiting both IL-1α and IL-1β, and canakinumab, an anti–IL-1β monoclonal antibody, are indicated for the treatment of numerous autoinflammatory and rheumatologic diseases.7,55 Anakinra and rilonacept, a soluble IL-1 receptor chimeric fusion protein neutralizing IL-1α and IL-1β, are standard of care for the treatment of recurrent pericarditis.55 Multiple trials have tested anakinra in patients with acute myocardial infarction (Table 2).113,114 The CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcomes Study) pilot trial provided preliminary evidence showing that canakinumab reduces IL-6, CRP, and fibrinogen independent from lipid- and glucose-lowering effects.115 In the CANTOS trial, in which 10,061 patients with prior myocardial infarction and high-sensitivity CRP ≥2 mg/L were randomized to canakinumab or placebo, canakinumab significantly reduced systemic inflammation and the occurrence of the composite of cardiovascular death, nonfatal acute myocardial infarction, or nonfatal stroke.116 This landmark study, together with the already-mentioned colchicine trials, contributed to validate the inflammatory hypothesis of arterial thrombosis and established the antithrombotic efficacy of inflammasome pathway inhibition, which remains, however, unexplored in the setting of venous thrombosis.16 These studies also suggested that targeting this pathway may preserve hemostasis, thus potentially circumventing the risk of bleeding associated with conventional antithrombotic agents.16 Yet, given that agents targeting the NLRP3/IL-1β axis are not devoid of potential side effects, including infection and gastrointestinal toxicity, both the safety and efficacy of these therapies should be carefully assessed in patients with or at risk for VTE.
Table 2.
Clinical Trials Testing Inflammasome Signaling Blockade in Atherothrombosis
| Trial (Ref. #) | Population (N) | Intervention | Duration | Main Outcomes |
|---|---|---|---|---|
| LoDoCo109 | Stable CAD (532) |
Colchicine 0.5 mg daily |
3 y (median) | ↓Cardiovascular events (CRP not reported) |
| LoDoCo2110 | Chronic, stable CAD (5,522) |
Colchicine 0.5 mg daily |
2.4 y (median) | ↓Ischemic events (CRP not reported) |
| COLCOT112 | Recent AMI (>30 d) (4,754) |
Colchicine 0.5 mg daily |
1.9 y (median) | ↓Cardiovascular ischemic events ↑Pneumonia (no effect on CRP beyond reduction seen in placebo; no effect on HF events) |
| MRC-ILA114 | Acute NSTEMI (<48 h) (182) |
Anakinra 100 mg daily | 2 wk treatment (1 y follow-up) |
↓CRP at 7 and 14 d (no effect on ischemic events at 30 d and 3 mo, but ↑at 1 y) |
| VCUART/ VCUART2108 |
Acute STEMI (<12 h) (40) |
Anakinra 100 mg daily | 2 wk treatment (3 mo follow-up) |
↓CRP, ↓ HF incidence (no effect on ischemic events) |
| VCUART3108 | Acute STEMI (<12 h) (99) |
Anakinra 100 mg once or twice daily | 2 wk treatment (1 y follow-up) |
↓CRP, ↓HF incidence, ↓HF hospitalization (no effect on ischemic events) |
| CANTOS Pilot115 | Well-controlled T2DM, high cardiovascular risk (556) |
Canakinumab 5/15/50/150 mg monthly | 4 mo | ↓CRP, ↓IL-6, ↓fibrinogen (no effect on glucose, lipids, and blood pressure) |
| CANTOS116 | Prior AMI (>30 d), CRP ≥2 mg/L (10,061) |
Canakinumab 50/150/300 mg every 3 mo | 3.7 y (median) | ↓CRP, ↓ischemic events, ↓HF hospitalization, ↑infection-related deaths, ↓cancer-related deaths, ↓rheumatologic diseases, ↓lung cancer |
AMI = acute myocardial infarction; CAD = coronary artery disease; CANTOS = Canakinumab Anti-inflammatory Thrombosis Outcomes Study; COLCOT = Colchicine Cardiovascular Outcome Trial; CRP = C-reactive protein; HF = heart failure; COVERT-MI = Colchicine for Left Ventricular Infarct Size Treatment in Acute Myocardial Infarction; LoDoCo = Low-Dose Colchicine; MI = myocardial infarction; MRC-ILA = Medical Royal Council InterLeukin-1 Antagonist; NSTEMI = non–ST-segment–elevation myocardial infarction; STEMI = ST-segment–elevation myocardial infarction; T2DM = type 2 diabetes mellitus; VUCART = Virginia Commonwealth University Anakinra Remodeling/Response Trial; other abbreviations as in Table 1.
Concluding Remarks and Future Perspectives
NLRP3 inflammasome pathway activation and heightened levels of IL-1β and IL-18 are found in patients with VTE. Recent preclinical studies have provided solid proof-of-concept evidence indicating that genetic deletion or pharmacologic inhibition of either NLRP3, caspase-1, GSDMD, IL-1β, or IL-18 markedly reduce venous thrombus formation and propagation. Addressing unopposed inflammation may also favor thrombus clearance and reduce vein scarring and dysfunction after VTE, thus potentially protecting from PTS and recurrent thromboembolism. This large body of rapidly accumulating evidence may suggest that targeting the NLRP3 inflammasome pathway may represent an attractive therapeutic strategy to prevent and manage VTE, eventually maximizing the benefits of anticoagulation without increasing the risk of bleeding. Adequately designed clinical trials to evaluate these hypotheses are needed. Additional in vivo studies are also necessary to elucidate the exact contribution of distinct IL-1 cytokines and pyroptosis to venous thrombosis and vein wall pathology, and to determine the effects, with corresponding therapeutic windows, of distinct agents blocking inflammasome signaling. Finally, as multiple inflammasome- and IL-1–based therapeutics are on the horizon for the treatment of a wide range of inflammatory disorders that carry an increased thrombotic risk, assessing the clinical impact of such interventions on VTE is of utmost importance.
Funding Support and Author Disclosures
Dr Potere has received a training fellowship from the International Society on Thrombosis and Haemostasis, and research grant funding from the International Network of VENous Thromboembolism Clinical Research Networks (INVENT). Dr Abbate has received research grant funding and has served as a paid scientific advisor to Implicit Biosciences, Kiniksa, Lilly, Merck, Novartis, Novo Nordisk, Olatec, R-Pharm, Serpin Pharma, and Swedish Orphan Biovitrum. Dr Kanthi has received research grant funding from the Intramural Research Program of the National Heart, Lung and Blood Institute, National Institutes of Health, and the Lasker Foundation, and is an inventor on a patent application by the University of Michigan on the use of biogases in vascular disease (US20180369278A1). Dr Carrier has received research funding from Leo Pharma, Bristol Myers Squibb, and Pfizer, and honoraria from Bayer, Bristol Myers Squibb, Sanofi, Servier, Pfizer, and Leo Pharma. Dr Toldo and Dr Abbate are supported by a National Institutes of Health grant (R01HL150115). Dr Toldo has served as a paid scientific advisor to Cardiol Therapeutics and Novo Nordisk, and received research grant funding from Kiniksa, Olatec, Serpin Pharma, and Cardiol Therapeutics. Dr Di Nisio has received personal fees from Bayer, Daiichi Sankyo, Sanofi, Bristol Myers Squibb, Pfizer, Leo Pharma, and Viatris. Dr Porreca has reported that he has no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an interactive version of the Central Illustration please see the online version of this paper.
Appendix
References
- 1.Foley J.H., Conway E.M. Cross talk pathways between coagulation and inflammation. Circ Res. 2016;118(9):1392–1408. doi: 10.1161/CIRCRESAHA.116.306853. [DOI] [PubMed] [Google Scholar]
- 2.Engelmann B., Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 3.Bonaventura A., Vecchié A., Dagna L., et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21:319–329. doi: 10.1038/S41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Potere N., Del Buono M.G., Caricchio R., et al. Interleukin-1 and the NLRP3 inflammasome in COVID-19: pathogenetic and therapeutic implications. EBioMedicine. 2022;85 doi: 10.1016/J.EBIOM.2022.104299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swanson K.V., Deng M., Ting J.P.Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toldo S., Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. 2018;15:203–214. doi: 10.1038/nrcardio.2017.161. [DOI] [PubMed] [Google Scholar]
- 7.Abbate A., Toldo S., Marchetti C., Kron J., Van Tassell B.W., Dinarello C.A. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res. 2020:1260–1280. doi: 10.1161/CIRCRESAHA.120.315937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi M. Cell-specific roles of NLRP3 inflammasome in myocardial infarction. J Cardiovasc Pharmacol. 2019;74:188–193. doi: 10.1097/FJC.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 9.Wu C., Lu W., Zhang Y., et al. Inflammasome activation triggers blood clotting and host death through pyroptosis. Immunity. 2019;50:1401–1411.e4. doi: 10.1016/J.IMMUNI.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahlenberg J.M., Carmona-Rivera C., Smith C.K., Kaplan M.J. Neutrophil extracellular trap–associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol. 2013;190:1217–1226. doi: 10.4049/jimmunol.1202388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Münzer P., Negro R., Fukui S., et al. NLRP3 inflammasome assembly in neutrophils is supported by PAD4 and promotes NETosis under sterile conditions. Front Immunol. 2021;12:1–16. doi: 10.3389/fimmu.2021.683803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta N., Sahu A., Prabhakar A., et al. Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc Natl Acad Sci U S A. 2017;114:4763–4768. doi: 10.1073/pnas.1620458114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y., Cui J., Zhang G., et al. Inflammasome activation promotes venous thrombosis through pyroptosis. Blood Adv. 2021;5:2619–2623. doi: 10.1182/bloodadvances.2020003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campos J., Ponomaryov T., de Prendergast A., et al. Neutrophil extracellular traps and inflammasomes cooperatively promote venous thrombosis in mice. Blood Adv. 2021;5:2319–2324. doi: 10.1182/bloodadvances.2020003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav V., Pinsky D.J., Kanthi Y., et al. ENTPD-1 disrupts inflammasome IL-1β – driven venous thrombosis. J Clin Invest. 2019;129:2872–2877. doi: 10.1172/JCI124804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stark K., Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol. 2021;18:666–682. doi: 10.1038/s41569-021-00552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Nisio M., van Es N., Büller H.R. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388:3060–3073. doi: 10.1016/S0140-6736(16)30514-1. [DOI] [PubMed] [Google Scholar]
- 18.Heit J.A. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12:464–474. doi: 10.1038/nrcardio.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan F., Rahman A., Carrier M., et al. Long term risk of symptomatic recurrent venous thromboembolism after discontinuation of anticoagulant treatment for first unprovoked venous thromboembolism event: systematic review and meta-analysis. BMJ. 2019;366:l4363. doi: 10.1136/BMJ.L4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahn S.R., Comerota A.J., Cushman M., et al. The postthrombotic syndrome: evidence-based prevention, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2014;130:1636–1661. doi: 10.1161/CIR.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 21.Saghazadeh A., Rezaei N. Inflammation as a cause of venous thromboembolism. Crit Rev Oncol Hematol. 2016;99:272–285. doi: 10.1016/j.critrevonc.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Colling M.E., Tourdot B.E., Kanthi Y. Inflammation, infection and venous thromboembolism. Circ Res. 2021:2017–2036. doi: 10.1161/CIRCRESAHA.121.318225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White R.H., Zhou H., Romano P.S. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost. 2003;90:446–455. doi: 10.1160/TH03-03-0152. [DOI] [PubMed] [Google Scholar]
- 24.Grimnes G., Isaksen T., Tichelaar Y.I.G.V., Brækkan S.K., Hansen J.B. Acute infection as a trigger for incident venous thromboembolism: results from a population-based case-crossover study. Res Pract Thromb Haemost. 2017;2:85–92. doi: 10.1002/rth2.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smeeth L., Cook C., Thomas S., Hall A.J., Hubbard R., Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet (London, England) 2006;367:1075–1079. doi: 10.1016/S0140-6736(06)68474-2. [DOI] [PubMed] [Google Scholar]
- 26.Kröger K., Weiland D., Ose C., et al. Risk factors for venous thromboembolic events in cancer patients. Ann Oncol Off J Eur Soc Med Oncol. 2006;17:297–303. doi: 10.1093/annonc/mdj068. [DOI] [PubMed] [Google Scholar]
- 27.Horvei L.D., Grimnes G., Hindberg K., et al. C-reactive protein, obesity, and the risk of arterial and venous thrombosis. J Thromb Haemost. 2016;14:1561–1571. doi: 10.1111/jth.13369. [DOI] [PubMed] [Google Scholar]
- 28.Fanola C.L., Norby F.L., Shah A.M., et al. Incident heart failure and long-term risk for venous thromboembolism. J Am Coll Cardiol. 2020;75:148–158. doi: 10.1016/j.jacc.2019.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wattanakit K., Cushman M., Stehman-Breen C., Heckbert S.R., Folsom A.R. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol. 2008;19:135–140. doi: 10.1681/ASN.2007030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zöller B., Li X., Sundquist J., Sundquist K. Risk of pulmonary embolism in patients with autoimmune disorders: a nationwide follow-up study from Sweden. Lancet. 2012;379:244–249. doi: 10.1016/S0140-6736(11)61306-8. [DOI] [PubMed] [Google Scholar]
- 31.Ridker P.M. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res. 2016;118:145–156. doi: 10.1161/CIRCRESAHA.115.306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smilowitz N.R., Kunichoff D., Garshick M., et al. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J. 2021;42:2270–2279. doi: 10.1093/eurheartj/ehaa1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li P., Zhao W., Kaatz S., Latack K., Schultz L., Poisson L. Factors associated with risk of postdischarge thrombosis in patients with COVID-19. JAMA Netw Open. 2021;4(11):e2135397 doi: 10.1001/JAMANETWORKOPEN.2021.35397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimnes G., Isaksen T., Tichelaar Y.I.G.V., Brox J., Brækkan S.K., Hansen J.B. C-reactive protein and risk of venous thromboembolism: results from a population-based case-crossover study. Haematologica. 2018;103:1245–1250. doi: 10.3324/haematol.2017.186957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zacho J., Tybjærg-Hansen A., Nordestgaard B.G. C-reactive protein and risk of venous thromboembolism in the general population. Arterioscler Thromb Vasc Biol. 2010;30:1672–1678. doi: 10.1161/ATVBAHA.109.198473. [DOI] [PubMed] [Google Scholar]
- 36.Kunutsor S.K., Seidu S., Blom A.W., Khunti K., Laukkanen J.A. Serum C-reactive protein increases the risk of venous thromboembolism: a prospective study and meta-analysis of published prospective evidence. Eur J Epidemiol. 2017;32:657–667. doi: 10.1007/s10654-017-0277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roumen-Klappe E.M., Den Heijer M., Van Uum S.H.M., Van Der Ven-Jongekrijg J., Van Der Graaf F.D., Wollersheim H. Inflammatory response in the acute phase of deep vein thrombosis. J Vasc Surg. 2002;35:701–706. doi: 10.1067/mva.2002.121746. [DOI] [PubMed] [Google Scholar]
- 38.Bakirci E.M., Topcu S., Kalkan K., et al. The role of the nonspecific inflammatory markers in determining the anatomic extent of venous thromboembolism. Clin Appl Thromb. 2015;21:181–185. doi: 10.1177/1076029613494469. [DOI] [PubMed] [Google Scholar]
- 39.Rabinovich A., Cohen J.M., Cushman M., et al. Association between inflammation biomarkers, anatomic extent of deep venous thrombosis, and venous symptoms after deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2015;3:347–353.e1. doi: 10.1016/j.jvsv.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Roumen-Klappe E.M., Janssen M.C.H., Van Rossum J., et al. Inflammation in deep vein thrombosis and the development of post-thrombotic syndrome: a prospective study. J Thromb Haemost. 2009;7:582–587. doi: 10.1111/j.1538-7836.2009.03286.x. [DOI] [PubMed] [Google Scholar]
- 41.Engeseth M., Enden T., Sandset P.M., Wik H.S. Predictors of long-term post-thrombotic syndrome following high proximal deep vein thrombosis: a cross-sectional study. Thromb J. 2021;19:1–7. doi: 10.1186/s12959-020-00253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reitsma P.H., Rosendaal F.R. Activation of innate immunity in patients with venous thrombosis: the Leiden Thrombophilia Study. J Thromb Haemost. 2004;2:619–622. doi: 10.1111/j.1538-7836.2004.00689.x. [DOI] [PubMed] [Google Scholar]
- 43.Krieger E., Van Der Loo B., Amann-Vesti B.R., Rousson V., Koppensteiner R. C-reactive protein and red cell aggregation correlate with late venous function after acute deep venous thrombosis. J Vasc Surg. 2004;40:644–649. doi: 10.1016/j.jvs.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Bittar L.F., da Silva L.Q., de Andrade Orsi F.L., et al. Increased inflammation and endothelial markers in patients with late severe post-thrombotic syndrome. PLoS One. 2020;15 doi: 10.1371/JOURNAL.PONE.0227150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shbaklo H., Holcroft C.A., Kahn S.R. Levels of inflammatory markers and the development of the post-thrombotic syndrome. Thromb Haemost. 2009;101:505–512. [PubMed] [Google Scholar]
- 46.Rabinovich A., Cohen J.M., Cushman M., et al. Inflammation markers and their trajectories after deep vein thrombosis in relation to risk of post-thrombotic syndrome. J Thromb Haemost. 2015;13:398–408. doi: 10.1111/jth.12814. [DOI] [PubMed] [Google Scholar]
- 47.Bouman A.C., Smits J.J.M., Ten Cate H., Ten Cate-Hoek A.J. Markers of coagulation, fibrinolysis and inflammation in relation to post-thrombotic syndrome. J Thromb Haemost. 2012;10:1532–1538. doi: 10.1111/j.1538-7836.2012.04798.x. [DOI] [PubMed] [Google Scholar]
- 48.Van Aken B.E., Den Heijer M., Bos G.M., Van Deventer S.J.H., Reitsma P.H. Recurrent venous thrombosis and markers of inflammation. Thromb Haemost. 2000;83:536–539. [PubMed] [Google Scholar]
- 49.Jara-Palomares L., Solier-Lopez A., Elias-Hernandez T., et al. D-dimer and high-sensitivity C-reactive protein levels to predict venous thromboembolism recurrence after discontinuation of anticoagulation for cancer-associated thrombosis. Br J Cancer. 2018;119:915–921. doi: 10.1038/s41416-018-0269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bontekoe E., Brailovsky Y., Hoppensteadt D., et al. Upregulation of inflammatory cytokines in pulmonary embolism using biochip-array profiling. Clin Appl Thromb. Published online May 10, 2021 doi: 10.1177/10760296211013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abul Y., Karakurt S., Ozben B., Toprak A., Celikel T. C-reactive protein in acute pulmonary embolism. J Investig Med. 2011;59:8–14. doi: 10.2310/jim.0b013e31820017f2. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi M. NLRP3 inflammasome as a key driver of vascular disease. Cardiovasc Res. 2022;118:372–385. doi: 10.1093/CVR/CVAB010. [DOI] [PubMed] [Google Scholar]
- 53.Mogensen T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Broz P., Dixit V.M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 55.Toldo S., Mezzaroma E., Buckley L.F., et al. Targeting the NLRP3 inflammasome in cardiovascular diseases. Pharmacol Ther. 2021;236:108053. doi: 10.1016/J.PHARMTHERA.2021.108053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schulz C., Engelmann B., Massberg S. Crossroads of coagulation and innate immunity: the case of deep vein thrombosis. J Thromb Haemost. 2013;11(suppl 1):233–241. doi: 10.1111/jth.12261. [DOI] [PubMed] [Google Scholar]
- 57.Giesen P.L.A., Rauch U., Bohrmann B., et al. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci U S A. 1999;96:2311–2315. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Müller I., Klocke A., Alex M., et al. Intravascular tissue factor initiates coagulation via circulating microvesicles and platelets. FASEB J. 2003;17:476–478. doi: 10.1096/fj.02-0574fje. [DOI] [PubMed] [Google Scholar]
- 59.von Brühl M.L., Stark K., Steinhart A., et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rothmeier A.S., Marchese P., Petrich B.G., et al. Caspase-1–mediated pathway promotes generation of thromboinflammatory microparticles. J Clin Invest. 2015;125:1471–1484. doi: 10.1172/JCI79329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Geddings J.E., Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122:1873–1880. doi: 10.1182/blood-2013-04-460139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rider P., Carmi Y., Guttman O., et al. IL-1α and IL-1β recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187:4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- 63.Darbousset R., Thomas G.M., Mezouar S., et al. Tissue factor–positive neutrophils bind to injured endothelial wall and initiate thrombus formation. Blood. 2012;120:2133–2143. doi: 10.1182/blood-2012-06-437772. [DOI] [PubMed] [Google Scholar]
- 64.Thålin C., Hisada Y., Lundström S., Mackman N., Wallén H. Neutrophil extracellular traps: villains and targets in arterial, venous, and cancer-associated thrombosis. Arterioscler Thromb Vasc Biol. 2019;39:1724–1738. doi: 10.1161/ATVBAHA.119.312463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murthy P., Durco F., Miller-Ocuin J.L., et al. The NLRP3 inflammasome and bruton’s tyrosine kinase in platelets co-regulate platelet activation, aggregation, and in vitro thrombus formation. Biochem Biophys Res Commun. 2017;483:230–236. doi: 10.1016/j.bbrc.2016.12.161. [DOI] [PubMed] [Google Scholar]
- 66.Allam O., Samarani S., Jenabian M.A., et al. Differential synthesis and release of IL-18 and IL-18 binding protein from human platelets and their implications for HIV infection. Cytokine. 2017;90:144–154. doi: 10.1016/j.cyto.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 67.Thornton P., McColl B.W., Greenhalgh A., Denes A., Allan S.M., Rothwell N.J. Platelet interleukin-1α drives cerebrovascular inflammation. Blood. 2010;115:3632–3639. doi: 10.1182/blood-2009-11-252643. [DOI] [PubMed] [Google Scholar]
- 68.Vogel S., Murthy P., Cui X., Lotze M.T., Zeh H.J., Sachdev U. TLR4-dependent upregulation of the platelet NLRP3 inflammasome promotes platelet aggregation in a murine model of hindlimb ischemia. Biochem Biophys Res Commun. 2019;508:614–619. doi: 10.1016/j.bbrc.2018.11.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pennings G.J., Reddel C.J., Traini M., et al. Rapid release of interleukin-1β from human platelets is independent of NLRP3 and caspase. Thromb Haemost. 2022;122(4):517–528. doi: 10.1055/s-0041-1731288. [DOI] [PubMed] [Google Scholar]
- 70.Beaulieu L.M., Lin E., Mick E., et al. Interleukin 1 receptor 1 and interleukin 1β regulate megakaryocyte maturation, platelet activation, and transcript profile during inflammation in mice and humans. Arterioscler Thromb Vasc Biol. 2014;34:552–564. doi: 10.1161/ATVBAHA.113.302700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qiao J., Wu X., Luo Q., et al. NLRP3 regulates platelet integrin αIIbβ3 outside-in signaling, hemostasis and arterial thrombosis. Haematologica. 2018;103:1568–1576. doi: 10.3324/haematol.2018.191700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rolfes V., Ribeiro L.S., Hawwari I., et al. Platelets fuel the inflammasome activation of innate immune cells. Cell Rep. 2020;31(6) doi: 10.1016/J.CELREP.2020.107615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hottz E.D., Lopes J.F., Freitas C., et al. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood. 2013;122:3405–3414. doi: 10.1182/blood-2013-05-504449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bai B., Yang Y., Wang Q., et al. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020;11(9):776. doi: 10.1038/S41419-020-02985-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ito H., Kimura H., Karasawa T., et al. NLRP3 inflammasome activation in lung vascular endothelial cells contributes to intestinal ischemia/reperfusion-induced acute lung injury. J Immunol. 2020;205:1393–1405. doi: 10.4049/jimmunol.2000217. [DOI] [PubMed] [Google Scholar]
- 76.Bevilacqua M.P., Pober J.S., Majeau G.R., Cotran R.S., Gimbrone M.A. Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984;160:618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salmeron K., Aihara T., Redondo-Castro E., Pinteaux E., Bix G. IL-1alpha induces angiogenesis in brain endothelial cells in vitro: implications for brain angiogenesis after acute injury. J Neurochem. 2016;136:573–580. doi: 10.1111/jnc.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nawroth P.P., Handley D.A., Esmon C.T., Stern D.M. Interleukin 1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc Natl Acad Sci. 1986;83:3460–3464. doi: 10.1073/pnas.83.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Folco E.J., Mawson T.L., Vromman A., et al. Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1α and cathepsin G. Arterioscler Thromb Vasc Biol. 2018;38:1901–1912. doi: 10.1161/ATVBAHA.118.311150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Puhlmann M., Weinreich D.M., Farma J.M., Carroll N.M., Turner E.M., Alexander H.R. Interleukin-1beta induced vascular permeability is dependent on induction of endothelial tissue factor (TF) activity. J Transl Med. 2005;3:37. doi: 10.1186/1479-5876-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ching S., Zhang H., Belevych N., et al. Endothelial-specific knockdown of interleukin-1 (IL-1) type 1 receptor differentially alters CNS responses to IL-1 depending on its route of administration. J Neurosci. 2007;27:10476–10486. doi: 10.1523/JNEUROSCI.3357-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Merton R.E., Hockley D., Gray E., Poole S., Thomas D.P. The effect of interleukin-1 on venous endothelium--an ultrastructural study. Thromb Haemost. 1991;66:725–729. [PubMed] [Google Scholar]
- 83.Gerdes N., Sukhova G.K., Libby P., Reynolds R.S., Young J.L., Schönbeck U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages implications for atherogenesis. J Exp Med. 2002;195:245–257. doi: 10.1084/jem.20011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li G., Zhou R., Liu R., Ye C., Zhao X. Correlation between the expression of IL-18 and deep venous thrombosis. Int J Mol Med. 2018;42:883–896. doi: 10.3892/ijmm.2018.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Minkelen R., De Visser M.C.H., Houwing-Duistermaat J.J., Vos H.L., Bertina R.M., Rosendaal F.R. Haplotypes of IL1B, IL1RN, IL1R1, and IL1R2 and the risk of venous thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1486–1491. doi: 10.1161/ATVBAHA.107.140384. [DOI] [PubMed] [Google Scholar]
- 86.Abuduhalike R., Sun J., Zhao L., Mahemuti A. Correlation study of venous thromboembolism with SAA, IL-1, and TNF-a levels and gene polymorphisms in Chinese population. J Thorac Dis. 2019;11:5527–5534. doi: 10.21037/jtd.2019.11.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Y.L., Shou L.H., Zhang Z.X. Association of interleukin-18 gene polymorphism and its protein expression with the lower extremity deep venous thrombosis in the Chinese han population: a case-control study. J Clin Lab Anal. 2018;32:e22345. doi: 10.1002/jcla.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jesus A.A., Osman M., Silva C.A., et al. A novel mutation of IL1RN in the deficiency of interleukin-1 receptor antagonist syndrome: description of two unrelated cases from Brazil. Arthritis Rheum. 2011;63:4007–4017. doi: 10.1002/art.30588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garg M., de Jesus A.A., Chapelle D., et al. Rilonacept maintains long-term inflammatory remission in patients with deficiency of the IL-1 receptor antagonist. JCI Insight. 2017;2:e94838. doi: 10.1172/JCI.INSIGHT.94838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mala Ponte G., Signorelli S.S., Bevilacqua V., et al. Increased levels of NF-dB-dependent markers in cancer-associated deep venous thrombosis. Plops One. 2015;10:e0132496. doi: 10.1371/journal.pone.0132496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anyanwu A.C., Kanthi Y., Ukase K., et al. Tuning the thromboinflammatory response to venous flow interruption by the ectonucleotidase CD39. Arterioscler Thromb Vasc Biol. 2019;39:e118–e129. doi: 10.1161/ATVBAHA.119.312407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moik F., Riedl J., Barth D., et al. Early dynamics of C-reactive protein predict risk of venous thromboembolism in patients with cancer treated with immune checkpoint inhibitors. Blood. 2022;140(suppl 1):1250–1251. [Google Scholar]
- 93.Quagliariello V., Passariello M., Di Mauro A., et al. Immune checkpoint inhibitor therapy increases systemic SDF-1, cardiac DAMPs Fibronectin-EDA, S100/Calgranulin, galectine-3, and NLRP3-MyD88-chemokine pathways. Front Cardiovasc Med. 2022;9:930797. doi: 10.3389/FCVM.2022.930797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leung Y.Y., Yao Hui L.L., Kraus V.B. Colchicine-Update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015;45:341–350. doi: 10.1016/j.semarthrit.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Misawa T., Takahama M., Kozaki T., et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol. 2013;14:454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 96.Taskiran E.Z., Cetinkaya A., Balci-Peynircioglu B., Akkaya Y.Z., Yilmaz E. The effect of colchicine on pyrin and pyrin interacting proteins. J Cell Biochem. 2012;113:3536–3546. doi: 10.1002/jcb.24231. [DOI] [PubMed] [Google Scholar]
- 97.Abu-Fanne R., Stepanova V., Litvinov R.I., et al. Neutrophil α-defensins promote thrombosis in vivo by altering fibrin formation, structure, and stability. Blood. 2019;133:481–493. doi: 10.1182/blood-2018-07-861237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qi G., Kessinger C.W., Mauskapf A., Jhajj H.S., Henke P., Jaffer F.A. Abstract 127: colchicine attenuates vein wall scarring in murine venous thrombosis: implications for limiting the post-thrombotic syndrome. Arterioscler Thromb Vasc Biol. 2017;37(suppl 1):A127. doi: 10.1161/ATVB.37.SUPPL_1.127. [DOI] [Google Scholar]
- 99.Borgel D., Bianchini E., Lasne D., Pascreau T., Saller F. Inflammation in deep vein thrombosis: a therapeutic target? Hematol (United Kingdom) 2019;24:742–750. doi: 10.1080/16078454.2019.1687144. [DOI] [PubMed] [Google Scholar]
- 100.Gadi I., Fatima S., Elwakiel A., et al. Different DOACs control inflammation in cardiac ischemia-reperfusion differently. Circ Res. 2021;128:513–529. doi: 10.1161/CIRCRESAHA.120.317219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kirchhof P., Ezekowitz M.D., Purmah Y., et al. Effects of rivaroxaban on biomarkers of coagulation and inflammation: a post hoc analysis of the X-VeRT trial. TH Open. 2020;04:e20–e32. doi: 10.1055/s-0040-1701206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jeraj L., Jezovnik M.K., Poredos P. Rivaroxaban versus warfarin in the prevention of post-thrombotic syndrome. Thromb Res. 2017;157:46–48. doi: 10.1016/j.thromres.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 103.Cheung Y.W., Middeldorp S., Prins M.H., et al. Post-thrombotic syndrome in patients treated with rivaroxaban or enoxaparin/vitamin K antagonists for acute deep-vein thrombosis: a post-hoc analysis. Thromb Haemost. 2016;116:733–738. doi: 10.1160/TH16-01-0041. [DOI] [PubMed] [Google Scholar]
- 104.San Norberto E.M., Gastambide M.V., Taylor J.H., García-Saiz I., Vaquero C. Effects of rosuvastatin as an adjuvant treatment for deep vein thrombosis. 2016;45:133–140. doi: 10.1024/0301-1526/a000507. [DOI] [PubMed] [Google Scholar]
- 105.Glynn R.J., Danielson E., Fonseca F.A.H., et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–1861. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Joseph P., Glynn R., Lonn E., et al. Rosuvastatin for the prevention of venous thromboembolism: a pooled analysis of the HOPE-3 and JUPITER randomized controlled trials. Cardiovasc Res. 2022;18(3):897–903. doi: 10.1093/cvr/cvab078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wohlford G.F., Van Tassell B.W., Billingsley H.E., et al. Phase 1B, randomized, double-blinded, dose escalation, single-center, repeat dose safety and pharmacodynamics study of the oral NLRP3 inhibitor dapansutrile in subjects with NYHA II-III systolic heart failure. J Cardiovasc Pharmacol. 2020;77:49–60. doi: 10.1097/FJC.0000000000000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Klück V., Jansen T.L.T.A., Janssen M., et al. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol. 2020;2:e270–e280. doi: 10.1016/s2665-9913(20)30065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nidorf S.M., Eikelboom J.W., Budgeon C.A., Thompson P.L. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404–410. doi: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 110.Nidorf S.M., Fiolet A.T.L., Mosterd A., et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838–1847. doi: 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 111.Opstal T.S.J., Hoogeveen R.M., Fiolet A.T.L., et al. Colchicine attenuates inflammation beyond the inflammasome in chronic coronary artery disease: a LoDoCo2 proteomic substudy. Circulation. 2020;142:1996–1998. doi: 10.1161/CIRCULATIONAHA.120.050560. [DOI] [PubMed] [Google Scholar]
- 112.Tardif J.-C., Kouz S., Waters D.D., et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 113.Morton A.C., Rothman A.M.K., Greenwood J.P., et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur Heart J. 2015;36:377–384. doi: 10.1093/eurheartj/ehu272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abbate A., Wohlford G.F., Del Buono M.G., et al. Interleukin-1 blockade with anakinra and heart failure following ST-segment elevation myocardial infarction: results from a pooled analysis of the VCUART clinical trials. Eur Hear J - Cardiovasc Pharmacother. 2022;8(5):503–510. doi: 10.1093/ehjcvp/pvab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ridker P.M., Howard C.P., Walter V., et al. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–2748. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 116.Ridker P.M., Everett B.M., Thuren T., et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.