Abstract
Background.
Despite intensified use of monovalent oral poliovirus type 1 vaccine and improved coverage of immunization campaigns, wild poliovirus type 1 persisted in Indian states of Uttar Pradesh and Bihar during 2006 to 2009.
Methods.
A serosurvey was conducted among cases of acute flaccid paralysis in the 25 high-polio-incidence districts of western Uttar Pradesh. Children were recruited by age group (6–11 months, 12–24 months, and 25–69 months) from among cases reported through the acute flaccid paralysis surveillance system between November 2008 and August 2009.
Results.
Seroprevalence for type 1 wild poliovirus was >96.4% for each age group. The seroprevalence of wild poliovirus types 2 and 3 increased with age, from 36.7% to 73.4% for type 2 and from 39.0% to 74.1% for type 3. In addition to the number of type-specific vaccine doses, father’s level of education, being from a Muslim family, height for age, and female sex were the socioeconomic risk factors associated with seronegativity to poliovirus.
Conclusions.
The seroprevalence and risk factors identified in this study were consistent with the epidemiology of polio, and the findings were instrumental in optimizing vaccination strategy in western Uttar Pradesh with respect to the choice of OPV types, the frequency of supplementary immunization campaigns, and the urgency to improve routine immunization services.
Keywords: seroprevalence, wild poliovirus, vaccination strategy, oral polio vaccine, immunization campaigns
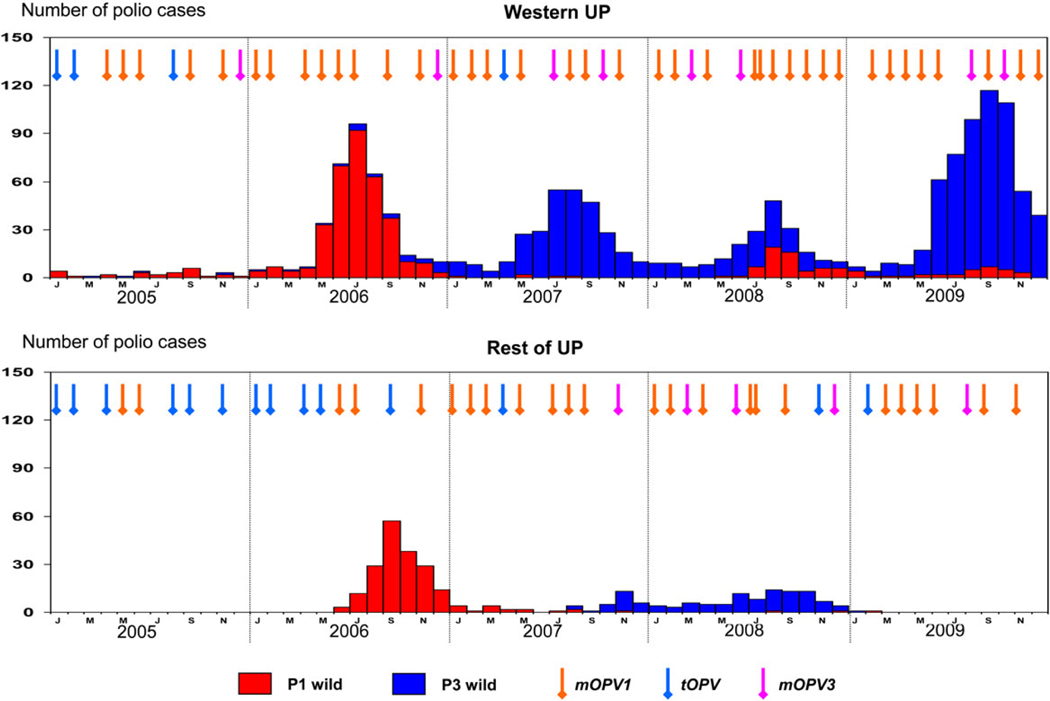
In 1988, the World Health Assembly resolved to eradicate polio worldwide. India launched its polio eradication program in 1995. By the end of 1999, wild poliovirus type 2 was eradicated from India. By 2001, all states and Union Territories, except the states of Uttar Pradesh (UP) and Bihar, had eliminated their indigenous strains of wild poliovirus types 1 and 3. Poliovirus genomic sequence data indicated that polio cases detected in other parts of India or viruses exported to other countries after 2001 were related to strains that were circulating in UP or Bihar. Within UP, intense circulation of poliovirus and the highest incidence of poliomyelitis occurred predominantly in the western districts, compared with the districts in the eastern and central regions of the state. During 2005 through 2009, the number of polio cases was 1504 in the 25 western districts of UP, compared with 321 in the 38 districts in the central and eastern regions of the state (Figure 1).
Figure 1.
Comparison of polio cases in western Uttar Pradesh (UP) and the rest of UP in relation to the supplementary immunization activity campaigns. Abbreviations: mOPV1, monovalent oral polio vaccine type 1; mOPV3, monovalent oral polio vaccine type 3; tOPV, trivalent oral polio vaccine.
Case-control studies demonstrated a lower per-dose efficacy of trivalent oral polio vaccine (tOPV) in Uttar Pradesh, compared with other parts of India, and confirmed that the per-dose efficacy of monovalent oral polio vaccine type 1 (mOPV1) was higher against type 1 poliovirus, compared with that of tOPV [1]. To interrupt poliovirus transmission in western UP, almost monthly, supplementary immunization activities (SIAs) were conducted from 2005 through 2009 [2, 3]. These SIAs preferentially used mOPV1. Despite an increase in the frequency of SIAs, improvements in the quality of campaigns, and more-frequent use of mOPV1, cases due to poliovirus type 1 reemerged in western UP in 2008 after a gap of 8 months. During this period, multiple outbreaks of poliovirus type 3 infection also occurred, mainly affecting western UP. Surveillance data from 2006 to 2008 also showed that the incidence of polio cases was higher in the younger age groups, peaking among infants 12–23 months of age. Yet, a large majority of the polio cases had received ≥4 doses of OPV. A clinic-based serosurvey in late 2007 in a district of western UP (Moradabad) showed seroprevalence of 88%, 70%, and 75% to poliovirus types 1, 2, and 3, respectively, among children 6–12 months of age [4].
The best way to monitor the population immunity against poliovirus is by determining the type-specific prevalence of antibodies against the virus. Ideally, surveys should be done on a population basis, using a randomly selected sample of children. Doing this, however, is logistically difficult, so previous serosurveys have been conducted by enrolling children who have been brought to a local clinic for care. This leads to a biased sample more heavily weighted toward families that seek out health care. Also, the sample contains only children who are living within the area served by the participating clinics. We used a novel approach to survey a group of children that is more representative of the population in western UP. We recruited children who were identified through the acute flaccid paralysis (AFP) surveillance system, which is the basic program strategy to detect poliovirus in the population. Since cases of non–polio-associated AFP occur almost randomly in the community without any bias toward geographic, economic, or other population groups, patients identified by the AFP surveillance system who do not have polio as the cause of their paralysis are more representative of the population than those identified in a clinic-based study, even though they still are not a random sample of the population.
To better understand the factors associated with a higher risk of polio and to optimize the vaccination strategy for interrupting poliovirus transmission, a study to assess seroprevalence and identify risk factors for polio was conducted in 25 districts of western UP. The specific objectives of the study were to (1) directly evaluate immunity to the 3 poliovirus serotypes among children in different age groups, (2) assess the effectiveness of OPV and the supplementary immunization strategy, and (3) identify risk factors associated with susceptibility to polio in western UP. This report presents the results of a serosurvey based on AFP surveillance that was conducted in 25 districts of western UP from November 2008 through August 2009.
METHODS
Participants in this study were selected from among the children identified through the AFP surveillance system in western UP. This system seeks to detect circulating poliovirus by identifying and testing cases of AFP among children ≤15 years of age or among persons of any age suspected by a physician of having polio. This is done through both immediate and weekly reporting of AFP cases by identified health facilities that have been enrolled as reporting sites and an active review of records in these sites by the surveillance medical officers on a periodic basis. Once identified, the AFP case is investigated by a district immunization officer/surveillance medical officer (SMO) who collects demographic and clinical information and arranges for the collection of 2 stool samples from the child. The SMOs belong to the National Polio Surveillance Project of World Health Organization Country Office for India. They are positioned in the districts to provide technical assistance for AFP surveillance and polio immunization activities targeted for polio eradication in India.
For this study, AFP cases were recruited from 3 age groups: 6–11 months, 12–24 months, and 25–59 months. A sample size of 369 was targeted for each of the 2 older age groups, to give us a 90% probability of obtaining an estimated prevalence between 55% and 65% if the population prevalence is 60%. Because the incidence of non–polio-associated AFP is much lower in the youngest age group, we chose a sample size of 133, which would give us a 90% probability of obtaining an estimate between 53% and 67%, again with the population prevalence of 60%. During visits to the family of the child with AFP in one of the target age groups, the SMO would explain the seroprevalence study to the parent and obtain informed consent for participation of the patient in the study. If the parent consented, the SMO would collect an approximately 2-mL venous blood sample from the child in a vacuum tube with serum separator. The SMO would also collect additional information about the child that was specific to this study. Routine immunization history was determined by immunization card, physician’s prescription, or parental recall. Vaccination through supplemental immunization activities (SIAs) was ascertained by asking the parent which of the SIAs conducted in the district during the preceding 12 months were missed by the child. An events calendar was used to aid in recall.
Within 3 hours of collection, the blood sample was taken in a cold carrier to a local laboratory and centrifuged to separate the serum. The sample was then transported to the National Polio Surveillance Unit in Delhi, where it was stored while awaiting transport to the Enterovirus Research Center in Mumbai. After arrival at the Enterovirus Research Center, the specimen was tested for the presence of poliovirus-neutralizing antibody, using a previously described modified microneutralization assay [5,6]. A portion of one of the stool specimens collected from the child for poliovirus testing as part of AFP surveillance was sent to a Delhi laboratory for testing for the presence of ova and parasites.
In addition to the biological samples collected from each child, anthropometric data were also collected. Based on WHO standards [7], each child’s height for age (stunting) and weight for height (wasting) were expressed as z scores. The child was classified as having moderate stunting or wasting, if the corresponding z score was −2 or less but greater than −3, and as having severe stunting or wasting, if the score was −3 or less.
For analysis, recurrent diarrhea was defined as having ≥4 episodes of diarrhea within the previous 3 months.
Ninety-five percent confidence intervals for percentages were calculated using the Wilson score method [8]. Analyses for individual risk factors were done using the Mantel–Haenszel χ2 test, stratified by age group. For the ordered risk factors father’s education level, height for age, and weight for height, the Cochran–Mantel–Haenszel χ2 test for trend was used. Potential risk factors with associated P values of ≤.10 were then analyzed jointly, using logistic regression. The analyses were performed using SAS, version 9.2 [9], and R, version 2.12.1 [10].
Samples from study subjects were collected until the third week of August 2009, and analyzed information from the study was presented to the India Expert Advisory Group on polio eradication during its meeting in early November 2009.
RESULTS
Participant Characteristics
A total of 1157 children aged 6–59 months with AFP were enrolled in the study. Of these, 37 AFP cases were virologically confirmed as polio cases and therefore were omitted from the analyses. An additional 170 had inadequate blood samples (167 were severely hemolyzed, and 3 were of insufficient quantity), leaving 950 children available for analysis: 169 (17.8%) in the 6–11-month-old group, 383 (40.3%) in the 12–23-monthold group, and 398 (41.9%) in the 24–59-month-old group. Characteristics of the study participants are summarized in Table 1. The father’s education level was skewed toward less schooling, with the largest percentage in each age group being illiterate. Severe wasting decreased with age (from 24% in the 6–11-month-old group to 11.6% in the 24–59-month-old group), and severe stunting increased with age (from 12.6% in the 6–11-month-old group to 47.4% in the 24–59-month-old group). The most common parasite detected in stool specimens was Giardia lamblia, which was found in 8.3% of the children aged 6–11 months, 21.9% of those aged 12–23 month, and 22.6% of those aged 24–59 months.
Table 1.
Characteristics of Study Participants, by Age Group
| Age Group | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| 6–11 mo |
12–23 mo |
24–59 mo |
||||
| Characteristic | Proportion With Characteristic | Percentage (95% CI) | Proportion With Characteristic | Percentage (95% CI) | Proportion With Characteristic | Percentage (95% CI) |
|
| ||||||
| Male sex | 100/169 | 59.2 (51.6–66.3) | 242/383 | 63.2 (58.2–67.9) | 210/398 | 52.8 (47.9–57.6) |
| Father’s education level | ||||||
| Illiterate | 55/169 | 32.5 (25.9–40.0) | 123/383 | 32.1 (27.6–36.9) | 144/398 | 36.2 (31.6–41.0) |
| Primary school | 34/169 | 20.1 (14.8–26.8) | 64/383 | 16.7 (13.3–20.8) | 62/398 | 15.6 (12.3–19.5) |
| Middle school | 45/169 | 26.6 (20.5–33.8) | 103/383 | 26.9 (22.7–31.5) | 90/398 | 22.6 (18.8–27.0) |
| Tenth grade | 16/169 | 9.5 (5.9–14.8) | 53/383 | 13.8 (10.7–17.7) | 52/398 | 13.1 (10.1–16.7) |
| Twelfth grade | 13/169 | 7.7 (4.6–12.7) | 22/383 | 5.7 (3.8–8.5) | 28/398 | 7.0 (4.9–10.0) |
| High school graduate | 5/169 | 3.0 (1.3–6.7) | 14/383 | 3.7 (2.2–6.0) | 13/398 | 3.3 (1.9–5.5) |
| Postgraduate | 1/169 | 0.6 (.03–3.3) | 4/383 | 1.0 (.4–2.6) | 9/398 | 2.3 (1.2–4.2) |
| Muslim religion | 76/169 | 45.0 (37.7–52.5) | 155/383 | 40.5 (35.7–45.4) | 146/398 | 36.7 (32.1–41.5) |
| Rural setting | 135/169 | 79.9 (73.2–85.2) | 314/383 | 82.0 (77.8–85.5) | 319/398 | 80.2 (76.0–83.8) |
| 0 routine doses | 55/155 | 35.5 (28.4–43.3) | 82/337 | 24.3 (20.1–29.2) | 154/359 | 42.9 (37.9–48.1) |
| Persistent diarrhea | 33/168 | 19.6 (14.3–26.3) | 63/383 | 16.4 (13.1–20.5) | 46/398 | 11.6 (8.8–15.1) |
| Weight for height | ||||||
| Moderate wasting | 39/167 | 23.4 (17.6–30.3) | 65/382 | 17.0 (13.6–21.1) | 64/396 | 16.1 (12.8–20.1) |
| Severe wasting | 40/167 | 24.0 (18.1–31.0) | 51/382 | 13.4 (10.3–17.1) | 46/396 | 11.6 (8.8–15.1) |
| Height for age | ||||||
| Moderate stunting | 26/167 | 15.6 (10.8–21.8) | 94/382 | 24.6 (20.6–29.2) | 98/396 | 24.7 (20.7–29.2) |
| Severe stunting | 21/167 | 12.6 (8.4–18.5) | 127/382 | 33.2 (18.7–38.1) | 188/396 | 47.4 (42.5–52.3) |
| Giardia lamblia cyst | 14/169 | 8.3 (5.0–13.4) | 84/383 | 21.9 (18.1–26.3) | 90/398 | 22.6 (18.8–27.0) |
| Ascaris lumbricoides | 0/169 | 0.0 (.0–2.2) | 3/383 | 0.8 (.3–2.3) | 11/398 | 2.8 (1.6–4.9) |
| Entamoeba coli cyst | 0/169 | 0.0 (.0–2.2) | 1/383 | 0.3 (.01–1.5) | 9/398 | 2.3 (1.2–4.2) |
| Hymenolepis nana | 0/169 | 0.0 (.0–2.2) | 0/383 | 0.0 (.0–1.0) | 5/398 | 1.3 (.5–2.9) |
Abbreviation: CI, confidence interval.
The median number of tOPV doses received through the routine immunization program varied little with age: 1 dose (range, 0–7 doses) in the 6–11-month-old group, 2 doses (range, 0–5 doses) in the 12–23-month-old group, and 1 dose (range, 0–5 doses) in the 24–59-month-old group. The median numbers of doses received through SIAs in the previous 12 months were 7 (range, 1–12 doses) in the 6–11-month-old group, 11 (range, 5–16 doses) in the 12–23-month-old group, and 11 (range, 7–13 doses) in the 24–59-month-old group.
The median numbers of total (routine immunization plus SIA) OPV doses received in the 6–11-month-old group in the previous 12 months were 8 (range, 2–13 doses) for type 1 (tOPV or mOPV1), 1 (range, 0–7 doses) for type 2 (tOPV), and 2 (range, 0–7 doses; tOPV or mOPV3) for type 3.
Seroprevalence
The estimated seroprevalence for type 1 was high in each age group, the lowest estimate being 96.4% in the 6–11–month-old group. Even in the youngest age group, the estimated seroprevalence to type 1 was uniformly high across the 24 districts, ranging from 92.3% to 100%. Seroprevalence of types 2 and 3 increased with age (Table 2), ranging from 36.7% to 73.4% for type 2 and from 39.0% to 74.1% for type 3.
Table 2.
Seroprevalence, by Age and Poliovirus Serotype
| Type 1 |
Type 2 |
Type 3 |
|||||
|---|---|---|---|---|---|---|---|
| Age, mo | Patients, No. | No. Positive | Percentage Positive (95% CI) | No. Positive | Percentage Positive (95% CI) | No. Positive | Percentage Positive (95% CI) |
|
| |||||||
| 6–11 | 169 | 165 | 96.4 (92.5–98.4) | 62 | 36.7 (29.8–44.2) | 66 | 39.0 (32.0–46.6) |
| 12–23 | 383 | 374 | 97.6 (95.6–98.8) | 213 | 55.6 (50.8–60.5) | 236 | 61.6 (56.7–66.4) |
| 24–59 | 398 | 391 | 98.2 (96.4–99.1) | 292 | 73.4(68.8–77.5) | 295 | 74.1 (69.6–78.2) |
Abbreviation: CI, confidence interval.
Risk Factors for Seronegativity
Because seroprevalence for type 1 was so high, we investigated risk factors for seronegativity only for types 2 and 3. Also, because the total number of SIA doses received was only ascertained accurately for the preceding 12 months, analyses of the relationship between number of OPV doses and seronegativity were performed separately on children ≤12 months of age. Individual Mantel–Haenszel analyses for potential risk factors, stratified by age group, are presented in Table 3. For the analysis of all age groups, the number of OPV doses received through SIAs earlier than the preceding 12 months was only available from the AFP surveillance case investigation form. Because these data are not serotype specific and the majority of the SIAs during the relevant periods used mOPV1, we only used tOPV doses for these analyses. Significant associations were found with sex, religion, father’s education level, recurrent diarrhea (type 2 only), height for age (ie, stunting), and number of tOPV doses received. Among children ≤12 months of age, the total number of type-specific doses of OPV was significantly associated with seronegativity for each serotype (Table 4). Other significant associations with type 2 seronegativity found among this younger age group were with religion (P < .001), stunting (P = .001), and father’s education level (P = .005). Additional significant associations with type 3 seronegativity were found with religion (P = .003) and father’s education level (P = .005).
Table 3.
Risk Factors for Poliovirus Seronegativity, by Poliovirus Type
| Type 2 |
Type 3 |
||||||
|---|---|---|---|---|---|---|---|
| Characteristic, Age | Patients, No. | No. Negative | Percentage Negative (95% CI) | P Value | No. Negative | Percentage Negative (95% CI) | P Value |
|
| |||||||
| Sex | .04 | .03 | |||||
| 6–11 mo | |||||||
| Female | 65 | 47 | 72.3 (60.4–81.7) | 44 | 67.6 (55.6–77.8) | ||
| Male | 94 | 55 | 58.5 (48.4–67.9) | 56 | 59.6 (49.5–68.9) | ||
| 12–23 mo | |||||||
| Female | 144 | 72 | 50.0 (41.9–58.1) | 63 | 43.8 (35.9–51.9) | ||
| Male | 244 | 102 | 41.8 (35.8–48.1) | 87 | 35.7 (29.9–41.8) | ||
| 24–59 mo | |||||||
| Female | 189 | 52 | 26.5 (21.6–34.3) | 53 | 28.0 (22.1–34.8) | ||
| Male | 214 | 55 | 25.7 (20.3–32.0) | 50 | 23.4 (18.2–29.5) | ||
| Religion | <.001 | .001 | |||||
| 6–11 mo | |||||||
| Hindu | 87 | 45 | 51.7 (41.4–61.9) | 49 | 56.3 (45.8–66.3) | ||
| Muslim | 72 | 57 | 79.2 (68.4–87.0) | 51 | 70.8 (59.5–80.1) | ||
| 12–23 mo | |||||||
| Hindu | 229 | 90 | 39.3 (33.2–45.8) | 76 | 33.2 (27.5–39.5) | ||
| Muslim | 159 | 84 | 52.8 (45.1–60.5) | 74 | 46.5 (39.0–54.3) | ||
| 24–59 mo | |||||||
| Hindu | 257 | 61 | 23.7 (18.9–29.3) | 61 | 23.7 (18.9–29.3) | ||
| Muslim | 146 | 46 | 31.5 (24.5–39.4) | 42 | 29.8 (22.0–36.6) | ||
| Father’s education level | <.001 | <.001 | |||||
| 6–11 mo | |||||||
| Illiterate | 51 | 37 | 72.6 (59.0–82.9) | 37 | 72.6 (59.0–82.9) | ||
| Primary school | 30 | 21 | 70.0 (52.1–83.4) | 20 | 66.7 (48.8–80.8) | ||
| Middle school | 44 | 29 | 65.9 (51.1–78.1) | 31 | 70.4 (55.8–81.8) | ||
| Tenth grade | 15 | 8 | 53.3 (30.1–75.2) | 6 | 40.0 (19.8–64.2) | ||
| Twelfth grade | 13 | 6 | 46.2 (23.2–70.9) | 5 | 38.5 (17.7–64.5) | ||
| High school graduate/postgraduate | 6 | 1 | 16.7 (.9–56.4) | 1 | 16.7 (.9–56.4) | ||
| 12–23 mo | |||||||
| Illiterate | 126 | 70 | 55.6 (46.8–63.9) | 55 | 43.6 (35.3–52.4) | ||
| Primary school | 68 | 35 | 51.5 (39.8–63.0) | 36 | 52.9 (41.2–64.3) | ||
| Middle school | 103 | 40 | 38.8 (30.0–48.5) | 39 | 36.9 (28.2–46.5) | ||
| Tenth grade | 54 | 22 | 40.7 (28.7–54.0) | 11 | 20.4 (11.8–32.9) | ||
| Twelfth grade | 20 | 3 | 15.0 (5.2–36.0) | 5 | 25.0 (11.2–46.9) | ||
| High school graduate/ postgraduate | 17 | 4 | 23.5 (9.6–47.3) | 5 | 29.4 (13.3–53.1) | ||
| 24–59 mo | |||||||
| Illiterate | 145 | 49 | 33.8 (26.6–41.8) | 43 | 29.7 (22.8–37.5) | ||
| Primary school | 62 | 22 | 35.5 (24.7–47.9) | 18 | 29.0 (19.2–41.3) | ||
| Middle school | 91 | 20 | 22.0 (14.7–31.5) | 23 | 25.3 (17.5–35.1) | ||
| Tenth grade | 52 | 11 | 21.2 (12.2–34.0) | 13 | 25.0 (15.2–38.2) | ||
| Twelfth grade | 30 | 4 | 13.3 (5.3–29.7) | 5 | 16.7 (7.3–33.6) | ||
| High school graduate/postgraduate | 23 | 1 | 4.4 (.8–21.0) | 1 | 4.4 (.8–21.0) | ||
| Setting | .63 | .10 | |||||
| 6–11 mo | |||||||
| Rural | 128 | 81 | 63.3 (54.7–71.1) | 80 | 62.5 (53.9–70.4) | ||
| Urban | 31 | 21 | 67.7 (50.1–81.4) | 20 | 64.5 (47.0–78.9) | ||
| 12–23 mo | |||||||
| Rural | 318 | 175 | 45.0 (39.6–50.5) | 128 | 40.2 (35.0–45.7) | ||
| Urban | 70 | 31 | 44.3 (33.2–55.9) | 22 | 31.4 (21.8–43.0) | ||
| 24–59 mo | |||||||
| Rural | 322 | 89 | 27.6 (23.0–32.8) | 87 | 27.0 (22.5–32.1) | ||
| Urban | 81 | 18 | 22.2 (14.5–32.4) | 16 | 19.8 (12.5–29.7) | ||
| Routine OPV doses, no. | <.001 | .001 | |||||
| 6–11 mo | |||||||
| 0 | 53 | 49 | 92.4 (82.1–97.0) | 41 | 77.4 (64.5–86.6) | ||
| ≥1 | 92 | 40 | 44.6 (34.8–54.7) | 47 | 51.1 (41.0–61.0) | ||
| 12–23 mo | |||||||
| 0 | 83 | 57 | 68.7 (58.1–77.6) | 40 | 48.2 (37.8–58.8) | ||
| ≥1 | 260 | 89 | 34.2 (28.7–40.2) | 90 | 34.6 (29.1–40.6) | ||
| 24–59 mo | |||||||
| 0 | 155 | 49 | 31.6 (24.8–39.3) | 45 | 29.0 (22.5–36.6) | ||
| ≥1 | 208 | 47 | 22.6 (17.4–28.8) | 52 | 25.0 (19.6–31.3) | ||
| Recurrent diarrhea | .03 | .99 | |||||
| 6–11 mo | |||||||
| Yes | 30 | 23 | 76.7 (59.1–88.2) | 21 | 70.0 (52.1–83.3) | ||
| No | 128 | 78 | 60.9 (52.3–69.0) | 78 | 60.9 (52.3–69.0) | ||
| 12–23 mo | |||||||
| Yes | 65 | 35 | 53.8 (41.8–65.4) | 23 | 35.4 (24.9–47.5) | ||
| No | 323 | 139 | 43.0 (37.8–48.5) | 127 | 39.3 (34.2–44.7) | ||
| 24–59 mo | |||||||
| Yes | 47 | 14 | 29.8 (18.6–44.0) | 12 | 25.5 (15.2–39.5) | ||
| No | 356 | 93 | 26.1 (21.8–30.9) | 91 | 25.6 (21.3–30.3) | ||
| Height for age | .001 | <.001 | |||||
| 6–11 mo | |||||||
| Severe stunting | 21 | 15 | 71.4 (50.0–86.2) | 14 | 66.7 (45.4–82.8) | ||
| Moderate stunting | 26 | 24 | 92.3 (75.9–97.9) | 16 | 61.5 (42.5–77.6) | ||
| No stunting | 110 | 63 | 57.3 (47.9–66.1) | 69 | 62.7 (53.4–71.2) | ||
| 12–23 mo | |||||||
| Severe stunting | 126 | 60 | 47.6 (39.1–56.3) | 54 | 42.9 (34.6–51.6) | ||
| Moderate stunting | 94 | 43 | 45.7 (36.0–55.8) | 43 | 45.7 (36.0–55.8) | ||
| No stunting | 168 | 71 | 42.3 (35.0–49.8) | 53 | 31.6 (25.0–38.9) | ||
| 24–59 mo | |||||||
| Severe stunting | 190 | 64 | 33.7 (27.4–40.7) | 63 | 33.2 (26.9–40.1) | ||
| Moderate stunting | 98 | 22 | 22.4 (15.3–31.7) | 24 | 24.5 (17.0–33.9) | ||
| No stunting | 115 | 21 | 18.3 (12.3–26.3) | 16 | 13.9 (8.8–21.4) | ||
| Weight for height | .30 | .96 | |||||
| 6–11 mo | |||||||
| Severe wasting | 39 | 25 | 64.1 (48.4–77.3) | 28 | 71.8 (56.2–83.5) | ||
| Moderate wasting | 35 | 23 | 65.6 (49.2–79.2) | 22 | 62.9 (46.3–76.8) | ||
| No wasting | 83 | 54 | 65.1 (54.3–74.4) | 49 | 59.0 (48.3–69.0) | ||
| 12–23 mo | |||||||
| Severe wasting | 52 | 20 | 38.5 (26.5–52.0) | 16 | 30.8 (19.9–44.3) | ||
| Moderate wasting | 68 | 34 | 50.0 (38.4–61.6) | 26 | 38.2 (27.6–50.1) | ||
| No wasting | 267 | 119 | 44.6 (38.7–50.6) | 108 | 40.4 (34.7–46.4) | ||
| 24–59 mo | |||||||
| Severe wasting | 46 | 13 | 28.3 (17.3–42.6) | 14 | 30.4 (19.1–44.8) | ||
| Moderate wasting | 65 | 22 | 33.8 (23.5–46.0) | 17 | 26.2 (17.0–38.0) | ||
| No wasting | 290 | 72 | 24.8 (20.2–30.1) | 72 | 24.8 (20.2–30.1) | ||
| Giardia lamblia | .44 | .09 | |||||
| 6–11 mo | |||||||
| Positive | 14 | 10 | 71.4 (45.5–88.3) | 9 | 64.3 (38.8–83.7) | ||
| Negative | 145 | 92 | 63.4 (55.4–70.8) | 91 | 62.8 (54.7–70.2) | ||
| 12–23 mo | |||||||
| Positive | 84 | 45 | 53.6 (43.0–63.8) | 35 | 41.7 (31.7–52.4) | ||
| Negative | 304 | 129 | 42.4 (37.0–48.0) | 115 | 37.8 (32.6–43.4) | ||
| 24–59 mo | |||||||
| Positive | 90 | 20 | 22.2 (14.9–31.8) | 30 | 33.3 (24.4–43.6) | ||
| Negative | 313 | 87 | 27.8 (23.1–33.0) | 73 | 23.3 (19.0–28.3) | ||
Abbreviations: CI, confidence interval; OPV, oral polio vaccine.
Table 4.
Poliovirus Seronegativity Among Children Aged 6–12 Months, by Number of Type-Specific Doses
| Vaccine, No. of Doses | Patients, No. | No. Negative | Percentage Negative (95% CI) | P Valuea |
|---|---|---|---|---|
|
| ||||
| tOPV | <.001 | |||
| 0 | 58 | 54 | 93.1 (83.6–97.3) | |
| 1 | 29 | 19 | 65.5 (47.3–80.1) | |
| 2 | 29 | 16 | 55.2 (37.5–71.6) | |
| ≥3 | 53 | 13 | 24.5 (14.9–37.6) | |
| OPV3 | <.001 | |||
| 0 | 21 | 18 | 85.7 (65.4–95.0) | |
| 1 | 15 | 9 | 60.0 (35.7–80.2) | |
| 2 | 37 | 28 | 75.7 (59.9–86.6) | |
| 3 | 38 | 22 | 57.9 (42.2–72.1) | |
| 4 | 24 | 11 | 45.8 (27.9–64.9) | |
| ≥5 | 35 | 11 | 31.4 (18.6–48.0) | |
Abbreviations: CI, confidence interval; OPV3, oral polio vaccine type 3; tOPV, trivalent oral polio vaccine.
By the Cochran-Armitage trend test.
As a final analysis, risk factors with P values of ≤.10 in the individual variable analyses were examined in a logistic regression model. For these analyses, the “moderate stunting” and “severe stunting” categories were combined. Also, the ordered categories for father’s education level were numbered from 1 to 7 and treated as a continuous variable in the model. The number of routine tOPV doses was also treated as a continuous variable. The results of these analyses are summarized in Table 5. For the analyses of all children, age, number of routine tOPV doses, sex, religion, stunting, and father’s education level were found to be significantly associated with type 2 seronegativity. Age, number of routine tOPV doses, sex rural setting, stunting, and father’s education level were found to be significantly associated with type 3 seronegativity. For the 6–12-month-old children analyzed for number of doses, Muslim religion and number of tOPV doses were found to be associated with seronegativity for type 2, and only the numbers of tOPV and mOPV3 SIA doses were found to be significantly associated with type 3 seronegativity. In this age group, number of mOPV3 doses did not differ by religion (median for Hindus, 1.2; median for Muslims, 1.0; P = .115, by the Wilcoxon test), nor was number of mOPV3 SIA doses associated with father’s education level (Spearman r = 0.02; P = .824). However, the number of routine doses was significantly associated with father’s education level (Spearman r = 0.24; P = .001) and was lower among Muslims (median for Hindus, 1.30; median for Muslims, 0.6; P = .036, by the Wilcoxon test).
Table 5.
Logistic Regression Analysis of Risk Factors for Poliovirus Seronegativity
| Risk Factor | OR (95% CI) | P Value |
|---|---|---|
|
| ||
| All age groups | ||
| Type 2 poliovirus | ||
| Age | <.001 | |
| 6–11 mo | Reference | |
| 12–23 mo | 0.46 (.30–.73) | |
| 24–59 mo | 0.14 (.09–.24) | |
| Routine OPV doses | 0.62 (.55–.70) | <.001 |
| Muslim religion | 1.49 (1.07–2.05) | .017 |
| Female sex | 1.45 (1.06–1.99) | .022 |
| Moderate/severe stunting | 1.43 (1.01–2.01) | .042 |
| Recurrent diarrhea | 1.23 (.80–1.89) | .344 |
| Father’s education level | 0.84 (.75–.94) | .003 |
| Type 3 poliovirus | ||
| Age | <.001 | |
| 6–11 mo | Reference | |
| 12–23 mo | 0.34 (.22–.52) | |
| 24–59 mo | 0.16 (.10–.25) | |
| Routine OPV doses | 0.82 (.73–.92) | <.001 |
| Rural setting | 1.51 (1.00–2.26) | .049 |
| Muslim religion | 1.34 (.97–1.84) | .076 |
| Female sex | 1.40 (1.03–1.90) | .032 |
| Moderate/severe stunting | 1.76 (1.26–2.46) | .001 |
| Giardia lamblia | 1.33 (.92–1.92) | .131 |
| Father’s education level | 0.87 (.78–.97) | .015 |
| Age 6–12 mo | ||
| Type 2 poliovirus | ||
| Muslim religion | 2.46 (1.11–5.46) | .027 |
| Stunting | 2.69 (1.08–6.68) | .034 |
| Recurrent diarrhea | 2.00 (.74–5.41) | .174 |
| Father’s education level | 0.82 (.61–1.09) | .164 |
| Total no. of tOPV doses (RI) | 0.44 (.32–.61) | <.001 |
| Type 3 poliovirus | ||
| Muslim religion | 1.43 (.71–2.90) | .318 |
| Father’s education level | 0.80 (.63–1.03) | .083 |
| Total no. of mOPV3 doses (SIA) | 0.53 (.41–.90) | .004 |
| Total no. of tOPV doses (RI) | 0.65 (.35–.82) | .006 |
Abbreviations: CI, confidence interval; mOPV3, monovalent oral polio vaccine type 3; OPV, oral polio vaccine; OR, odds ratio; RI, routine immunization; SIA, supplementary immunization activity; tOPV, trivalent oral polio vaccine.
DISCUSSION
The high seroprevalence rates for type 1 indicate that the ongoing mOPV1 SIAs prior to this study were effective in raising the population immunity to levels that were approaching the threshold required to interrupt transmission of wild poliovirus type 1 in western UP. Supported by these findings, the program further intensified the use of mOPV1, which proved to be an effective strategy. The last case of type 1 polio in UP was detected in November 2009 in Moradabad district, in the western part of the state, which remained free of polio due to type 1 through the end of all poliovirus transmission across India.
As was expected, immunity gaps were identified for poliovirus types 2 and 3, especially in the 6–11-month-old age group, consistent with the low median number of tOPV and mOPV3 doses received by study children through routine immunization and SIAs. The gap in type 3 immunity helps explain the higher case incidence attributable to type 3 poliovirus during 2009 [2].
Findings from the study guided the program in India on the type of vaccine to be used during the subsequent SIAs and also highlighted the need for initiating actions to improve routine immunization, with a focus on areas with the underserved Muslim populations that had lower type 2 immunity because of receipt of fewer tOPV doses.
The availability of bivalent OPV (bOPV types 1 and 3) and its aggressive use from beginning of 2010 led to rapid control and ultimately cessation of polio due to type 3 in UP and across India in 2010. Use of bOPV, which was more immunogenic against serotypes 1 and 3 in the absence of interference by type 2 Sabin, enabled the program to simultaneously increase the level of immunity to type 3 poliovirus and maintain the high level of protection against type 1. India reported its last case of polio in January 2011.
The gap in type 2 immunity helps explain the emergence of circulating vaccine-derived poliovirus (VDPV) type 2 in western UP during 2009–2010. Targeted SIAs using tOPV rapidly stopped the circulation of type 2 cVDPV. It is important to note that the finding of low seroprevalence and subsequent emergence of cVDPV created further urgency to improve routine immunization coverage in UP.
This study included evaluation of sociodemographic and biological risk factors for seronegativity to poliovirus. Belonging to a Muslim family and having a father who is illiterate or with a low education status were factors that were significantly associated with seronegativity to both type 2 and type 3 poliovirus. Being a female was associated with type 3 seronegativity. Moderate-to-severe stunting was also associated with seronegativity to both serotypes. These risk factors are most likely surrogates for lack of access to health and nutritional services for children from the lower socioeconomic strata. In children aged 6–12 months, being a Muslim and the presence of stunting were found to be risk factors for being seronegative to type 2. The lack of type-specific doses was found to be highly associated with seronegativity for both serotypes, which explains the higher risk among Muslims for being seronegative for both type 2 and type 3 poliovirus. The number of routine tOPV doses received was lower among Muslim children. These findings again highlighted the urgent need for prioritizing improvements in routine immunization and broader health services and awareness among the most disadvantaged populations.
In addition to wasting and stunting, we evaluated the presence of intestinal parasites and diarrhea as potential biological risk factors for poliovirus seronegativity. Intestinal infection and alterations in the local microbiome have been hypothesized to interfere with the immunogenicity of the live attenuated oral polio vaccine. Despite high prevalence rates of diarrhea and giardiasis, this study did not demonstrate any significant association between these factors and seronegativity.
Although the children tested in this study do not represent a random sample of the population, risk factors for non–polio-associated AFP are unrelated to those for poliovirus seronegativity. Since occurrence of non–polio-associated AFP is random and is related to the population size, children in this study are highly likely to be representative of the population in western UP. One limitation of the study relates to the age distribution of individuals with non–polio-associated AFP; therefore, the 6–11-month-old children were relatively underrepresented in our sample. Other limitations include a recall bias for the number of OPV doses by age, with the bias being greater in the older age group than in the younger age groups.
Financial support.
This work was supported by the Ministry of Health and Family Welfare, Government of India, and by the World Health Organization.
Footnotes
Supplement sponsorship. This article is part of a supplement entitled “The Final Phase of Polio Eradication and Endgame Strategies for the Post-Eradication Era,” which was sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Grassly NC, Fraser C, Wenger J, et al. New strategies for the elimination of polio from India. Science 2006; 314:1150. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication–India, January 2006-September 2007. MMWR Morb Mortal Wkly Rep 2007; 56:1187–91. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication–India, January 2007-May 2009. MMWR Morb Mortal Wkly Rep 2009; 58:719–23. [PubMed] [Google Scholar]
- 4.Deshpande JM, Bahl S, Sarkar BK, et al. Assessing population immunity in a persistently high-risk area for wild poliovirus transmission in India: a serological study in Moradabad, Western Uttar Pradesh. J Infect Dis 2014; 210(suppl 1):S225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Expanded Programme on Immunization. Report of a WHO Informal consultation on polio neutralizing antibody assays, Nashville, 5–6 December 1991. WHO/EPI/RD/91.3 Rev 1. Geneva: World Health Organization, 1991. [Google Scholar]
- 6.WHO Collaborative Study Group on Oral and Inactivated Poliovirus Vaccines. Combined immunization of infants with oral and inactivated poliovirus vaccines: results of a randomized trial in The Gambia, Oman, and Thailand. J Infect Dis 1997; 175(suppl 1): S215–227. [DOI] [PubMed] [Google Scholar]
- 7.WHO Multicentre Growth Reference Study Group. WHO child growth standards: length/ height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization, 2006. http://www.who.int/childgrowth/standards/technical_report/en/index.html. Accessed 3 December 2010. [Google Scholar]
- 8.Agresti A, Coul BA. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat 1998; 52:119–26. [Google Scholar]
- 9.Institute SAS. SAS 9.2 help and documentation. Cary, NC: SAS Institute, 2002–2011. [Google Scholar]
- 10.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2010. http://www.R-project.org/. Accessed 30 October 2012. [Google Scholar]