Abstract
Objective
Studies have reported inconsistent results regarding the extent to which neurocognitive recovery occurs in abstinent patients with alcohol use disorder (AUD). In addition to abstinence, other factors may have influenced this process and contributed to the inconsistencies. This review examines the factors investigated in this regard and describes the possible influence of each factor based on the evidence collected.
Methodology
PubMed was systematically searched for articles published between January 2000 and July 2023. Longitudinal humane studies investigating neurocognitive recovery in abstinent adult AUD patients were included. Studies with a cross-sectional design were excluded, as were studies that did not classify AUD according to the DSM-IV or 5 criteria, only examined binge use, did not report neuropsychological outcomes or duration of abstinence, or where neurological disorders were present.
Results
Sixteen categories of factors were distinguished from 31 full-text articles. Consistent patterns were found, indicating an association between neurocognitive recovery and the “smoking” and ‘brain volume” factors. Consistent patterns were also found indicating that there is no relationship with “quantities of alcohol used” and “education level.” A similar consistent pattern was also found for “polysubstance use”, “gender” and “verbal reading”, but the number of studies is considered limited. The association with “age” is studied frequently but with inconsistent findings. The remaining eight factors were regarded as understudied.
Conclusion
The clearest patterns emerging from the evidence are a predominantly negative influence of smoking on neurocognitive recovery, associations between changes in brain area volume and neurocognitive recovery, and no association between neurocognitive recovery and the amount of alcohol consumed, as measured by self-report, nor with educational attainment. Future research on the understudied factors and factors with inconsistent evidence is needed, preferably through longitudinal designs with multiple assessment periods starting after at least two weeks of abstinence.
Keywords: substance use disorder, alcohol, neurocognitive recovery, contextual neuropsychology, abstinence
Introduction
Impaired neurocognitive function is common in treatment seeking patients with an alcohol use disorder (AUD).1 Alcohol intoxication is associated with loss of control and an increased probability of potentially harmful behaviors, complicating the maintenance of sobriety.2,3 Moreover, chronic and excessive alcohol consumption may result in long-lasting cognitive impairments.4–7 Since commonly used therapies such as cognitive behavioral therapy or motivational interviewing require intact cognitive function,8 cognitive impairments impede treatment success9 and are related to higher drop-out rates.10 Abstinence from alcohol can lead to (partial) recovery of neurocognitive function. However, studies have reported inconsistent results regarding the extent of neurocognitive recovery.4,11–14 This could be explained by two major problems.
First, most studies on neurocognitive recovery in abstinent AUD patients used a cross-sectional design.4 While these studies allow insight into neurocognitive recovery,15 they only provide a snapshot of functions over time16 and, as a result, are unable to reliably detect within-subject changes over a period of abstinence.17 Therefore, longitudinal studies are needed to improve our understanding of neurocognitive recovery.4,18,19 However, it is challenging to follow patients over time and collect the data needed to test these hypotheses because participants with AUD often show lower adherence10,15 and limited abstinence rates.20–22
A second problem that may underlie these inconsistent findings is the lack of knowledge about factors other than abstinence that influence the course of neurocognitive recovery.16,18 Frequently mentioned are the number of previous medical detoxifications and biological factors such as liver complications and thiamine deficiencies, polydrug use, age, smoking, family history of alcohol dependence, and individual historical aspects of alcohol use (eg, total amount of alcohol consumed over a lifetime, recent alcohol consumption, and duration of AUD).9,13,19,23 While these factors are often discussed as potentially contributing to the variability seen in neurocognitive recovery,13,24 they have received insufficient attention in observational research.12 In addition, studies do not always provide suitable data or use validated methods to measure these factors. This was illustrated by two meta-analyses on the recovery of neurocognitive function in abstinent AUD patients. Insufficient detail of moderating factors4 or unreliable data14 limits the analysis of such variables leading to inconsistent findings where the latter author concluded that cognitive dysfunction abates after one year of sobriety, while the results from Crowe et al4 suggested pervasive cognitive impairments on several timeframes of abstinence duration, even after one year.
In summary, longitudinal research is necessary to increase our understanding of the recovery of neurocognitive function in abstinent AUD patients. Insight into the factors that influence this process can aid in realizing an effective longitudinal study design and in lowering the threshold for conducting this challenging longitudinal research.
In the present review, we aimed to answer the following questions: (a) Which factors are investigated in research on the recovery of neurocognitive function in abstinent AUD patients and (b) what is the influence of these factors on the recovery of neurocognitive function during abstinence from alcohol? In addition, the clinical value and applicability of the current knowledge of these factors will be discussed and considered in terms of their putative relevance for future research on the recovery of neurocognitive function in patients with abstinent AUD.
Method
This scoping review aims to answer the aforementioned questions using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines.25 Our primary outcome measure was the change in neurocognitive function, as measured with neuropsychological instruments.
Search Strategy
In order to identify articles, a literature search in PubMed was performed using the following search terms: ((Alcohol AND (recov* OR abstin*) AND (execut* OR memor* OR visu* OR attent* OR social OR longit* OR cogn* OR neuropsy* OR MRI OR DTI OR VBM OR MRS OR CT OR PET OR SPECT OR EEG OR ERP OR spectroscopy OR neuroimaging OR neurophysiology))) AND (cogn*[Title/Abstract] OR neuropsy*[Title/Abstract] OR memory[Title/Abstract] OR attention[Title/Abstract] OR execut*[Title/Abstract] OR visu*[Title/Abstract]), filters: Humans.
Article Selection
During the initial screening, articles were selected by two reviewers (JS and TK) based on their titles or abstracts. Only English-language manuscripts published in peer-reviewed journals were considered for inclusion, with the aim of including well-established methodologically sound studies. Humane, longitudinal studies investigating neuropsychological or neurocognitive function in adult abstinent patients (≥18 years) with alcohol dependency according to DSM-IV (TR) or alcohol use disorder according to DSM-5 (Diagnostic and Statistical Manual of Mental Disorders) criteria were included. The included reviews were systematically screened for relevant references to additional papers. Studies on abuse (DSM-IV), binge or heavy episodic drinking only, were excluded, as were cross-sectional studies, as this review aimed to clarify the factors associated with cognitive recovery over time in abstinent AUD patients. Finally, studies were excluded if a classification of alcohol use disorder for participants included in the study was missing, if there were no neuropsychological instruments used, when the duration of abstinence was not described, or when patients were diagnosed with an alcohol-related neurological disease (eg, Korsakoff, Marchiafava-Bignami, Alcohol Dementia, epilepsy, dementia).
The search encompassed articles published between January 2000 and July 2023, following the suggestion by Fernández-Serrano et al26 to include studies that were published after the surge of contemporary neuroscientific models of addiction and similar to other reviews on the effects of alcohol on neuropsychological function in AUD patients.13,27
The full article was retrieved and discussed by the two reviewers when there was uncertainty regarding the inclusion of a study. A consensus was reached for all discussed articles. The remaining articles were full-text articles and read to be used for data extraction.
Data Extraction
For each study, all relevant data from the results and discussion sections were selected using a coding form (available via the corresponding author) with fields containing information about the author, year of publication, study design, number of participants/controls, neuropsychological measure(s), and (non-)significant predictors of neuropsychological outcomes.
In order to quantify the design quality, validity, and utility for patient care, the level of evidence (1 (very strong) to 5 (weak)) of each included article was rated by two independent reviewers (JS and TK) using the Oxford Classification for Evidence-Based Medicine (OCEBM Levels of Evidence Working Group) (Howick et al, 2011). Evidence strength was rated per study following the recommendations of the OCEBM. The studies included for the purpose of this review were considered as prognostic.
The effects on neuropsychological outcome measures were placed under the most appropriate neuropsychological domain after a consensus meeting with both the reviewers. The classification of neuropsychological domains was largely adopted from the study by Fernández-Serrano et al26 Regarding this classification, it is important to point out the overlap between various neuropsychological domains and the fact that neurocognitive tasks used are not specific indicators of one neuropsychological function.
All predictors from the full-text articles included for this review were sorted into categories using a coding form. During the collection process, reviewers (JS and TK) identified 16 categories. The extracted variables were placed by the reviewer under the most appropriate category at their own discretion. In case of doubt, the other reviewer was consulted, and consensus was reached. The categories were regrouped into five domains, again with the agreement of both the reviewers. The following five domains were distinguished: drinking-related factors, demographic factors, psychiatric factors, premorbid neurocognitive function, and medical factors.
The effects and associations found for a specific factor and their direction, are narratively synthesized and discussed. Findings are considered significant at alpha level ≤.05.
Results
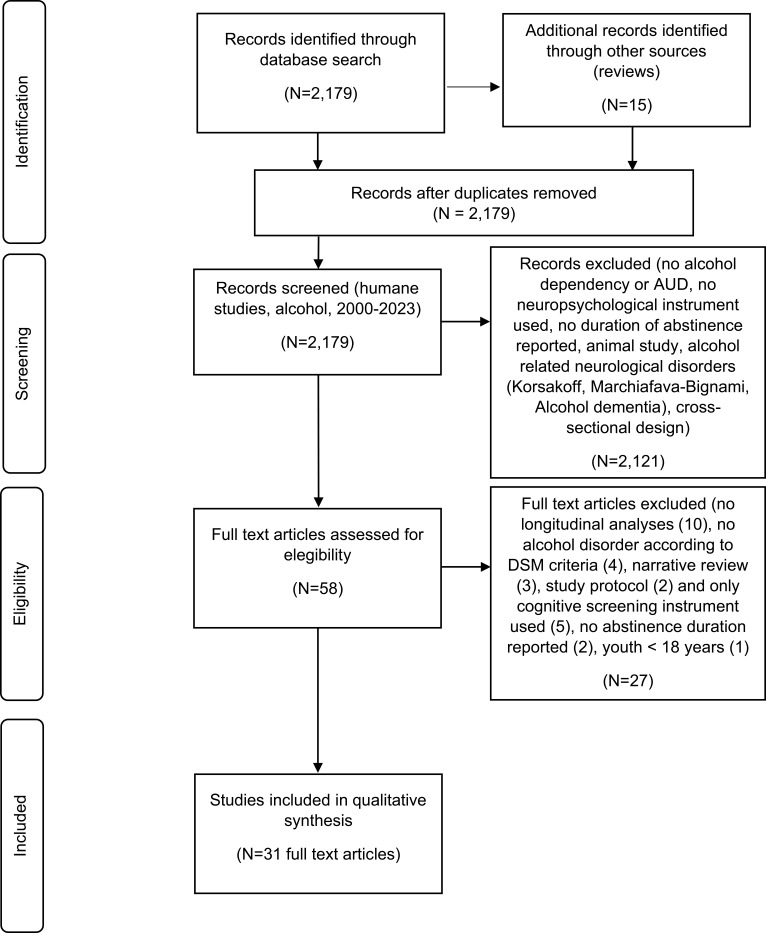
During the screening, 2179 articles, including 15 reviews, were retrieved based on the search criteria (Figure 1). A total of 58 articles remained for inclusion and inspected by two reviewers. Within this stage, the primary aim was to include studies that explicitly considered the association between abstinence duration, recovery of neurocognitive function, and other relevant factors. During this final process, we excluded several studies (no longitudinal analyses (10), no alcohol disorder according to DSM-IV or DSM-5 criteria (4), narrative review (3), study protocol (2), only a cognitive screening instrument used (5), no abstinence duration reported (2), and age <18 years (1)), resulting in 31 articles for which data could be extracted and used for the purpose of this review.
Figure 1.
Prisma flow diagram detailing the screening process.
Summary of Factors
Regarding the 31 studies included for analysis, most (25/31) started cognitive assessments within two weeks after admission, using neuropsychological tasks covering domains of executive function (21/31), memory and learning (22/31), attention (9/31), processing speed (13/31), spatial processing (8/31), intelligence (11/31), and social cognition (2/31). Abstinence from alcohol was verified by means of biomarkers (and in some studies also self-report) (13/31 studies), by means of self-report only (5/31 studies), or abstinence control measures were not mentioned (13/31 studies).
Table 1 summarizes the results per factor based on the number of studies that found an association or effect with neurocognitive recovery and the number that did not. Found associations and effects for each factor pointed in one direction. Per study, the rated OCEBM level is marked in superscript. Studies in this review have been rated from 2 to 4.
Table 1.
Neurocognitive Recovery in Abstinent AUD Patients: Factors and Evidence
| Domain | Factors | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drinking related | Smoking | A | 9b | 11b | 20b | 21b | 26c | 28a | |||||
| NA | 22b | 30b | |||||||||||
| Quantities used | A | 29b | 15c | ||||||||||
| NA | 9b | 21b | 30b | 6b | 2a | 10c | 3b | 20b | 13c | 28a | 31a | ||
| Polysubstance | A | 26c | |||||||||||
| NA | 4b | 21b | 13c | ||||||||||
| Detoxifications | A | 17b | |||||||||||
| NA | 6b | 31a | |||||||||||
| Positive family history | A | 2a | 4b | 5b | |||||||||
| NA | 8b | 31a | |||||||||||
| Demographic | Age | A | 2a | 15c | 21b | 20b | |||||||
| NA | 4b | 9b | 22b | 31a | 8b | 30b | |||||||
| Gender | A | – | |||||||||||
| NA | 2a | 7 c | 31a | ||||||||||
| Education | A | 4b | |||||||||||
| NA | 2a | 15c | 9b | 21b | 28a | ||||||||
| Psychiatric | Depression | A | 2a | 20b | |||||||||
| NA | 3b | 21b | 28a | 8b | 31a | ||||||||
| ASPD* | A | 2a | 4b | ||||||||||
| NA | – | ||||||||||||
| Premorbid cognitive functioning | Verbal reading | A | 20b | ||||||||||
| NA | 9b | 21b | 22b | 3b | |||||||||
| Neurocognitive task | A | 2a | |||||||||||
| NA | – | ||||||||||||
| Medical | Blood values | A | 7c | 1c | 2a | ||||||||
| NA | – | ||||||||||||
| Medical conditions | A | 4b | |||||||||||
| NA | 21b | 28a | |||||||||||
| Brain volumes | A | 12b | 23b | 11b | 22b | 10c | |||||||
| NA | 10c | ||||||||||||
| Perfusion | A | – | |||||||||||
| NA | 14b |
Notes: Numbers refers to the study that did, or did not, found an association or effect (<.05) of the specific factor on neurocognitive recovery: 149, 239, 315, 443 545, 637, 747, 846, 934, 1038, 1132, 1252, 1340, 1453, 1517, 1694, 1744, 1896, (no factors), 1948, 2028, 2131, 2235, 2351, 2495, (no factors), 2512, 2633, 2797, 2829, 2942, 3036, 3141. Indication of level of evidence according to Oxford Classification for Evidence Based Medicine (OCEBM), varying from 1 (very strong) to 5 (very weak): aLevel 2, bLevel 3, cLevel 4. *Antisocial Personality Disorder.
Abbreviations: A, associated; NA, not associated.
Drinking Related Factors
Smoking
Eight studies examined the association between tobacco smoking and neurocognitive recovery in abstinent AUD patients. Six studies found significant effects of chronic smoking on neurocognitive recovery, with abstinence duration from alcohol varying from five weeks28 to 9 months.29,30 Smoking adversely affects neurocognitive recovery during short- and long-term abstinence, in terms of processing speed,28–31 learning and memory,18,30,32,33 spatial processing,30,32 executive function18,34 and fine motor skills.33
Additionally, greater current smoking severity was related to less neurocognitive improvement29,30 and former smoking status (never smoking or former smoker) modulated neurocognition, with never-smoking AUD patients showing the greatest recovery.18,28,29 Active smokers showed less recovery than former- or never-smoking AUD patients. Former smoking AUD patients showed less neurocognitive recovery than never smoking AUD patients.18,29 In addition, Yeh et al32 found lower performance on domains of learning and memory in smoking AUD patients compared to never smoking AUD patients after 7 months of abstinence. Moreover, the results indicated that smoking status interacted with both abstinence duration and age, with older age leading to poorer neurocognitive performance.
Two studies did not find an association between chronic smoking and recovery of neurocognitive function. In a study on the effect of brain-derived neurotrophic factor (BDNF) genotype within subgroups of AUD patients classified by smoking status, Hoefer et al35 found changes in learning and memory to correlate with hippocampal volume depending on BDNF genotype. They concluded that BDNF genotype, but not smoking status or measures of drinking severity, regulates functionally relevant hippocampal volume recovery in abstinent AUD patients. In another study, smoking status was not associated with neurocognitive recovery on domains of executive function, memory and speed.36
In summary, the results show a mostly consistent pattern of a negative association between tobacco use and recovery on several neurocognitive domains during varying periods of abstinence.
Quantities of Alcohol Used
Thirteen studies analyzed the association between quantified alcohol use and recovery of neurocognitive function. Variables included among other things “number of average drinks per month at 1, 3 and 8 years of drinking”, “lifetime years of drinking”, “lifetime drinks per month”, “months of heavy drinking”, “age of onset of heavy drinking”,30 ‘lifetime drinks’ and ‘1 year average drinks’,18,29 “alcohol dose” or “duration of dependence”,37 “lifetime alcohol use”.38 Eleven studies did not find an association between variables quantifying alcohol use and recovery on domains of executive function, learning and memory, attention, processing speed15,18,28–30,36–40 and recovery of social cognitive function.41 The abstinence duration periods ranged from 1 day39 up to 48 months,15 with most studies using abstinence periods within one year (8/11 studies).18,28,34,36,38,40,41 Other studies have investigated neurocognitive recovery during abstinence periods up to 48 months.15,37,39
Two studies found an association between quantified alcohol use (length of alcohol use and abuse, duration of dependency and non-dependency, average intake per day, last intake) and recovery on domains of learning and memory, intelligence, spatial processing, and executive function, using abstinence periods of three42 and six months.17
In summary, the evidence shows a largely consistent pattern in which no association between quantities of previous alcohol use and recovery on several neurocognitive domains during varying periods of abstinence could be found.
Polysubstance Use
Four studies examined the association between polysubstance use and recovery of neurocognitive function. One study investigating neurocognitive recovery in patients with polysubstance use disorder (including AUD) showed significant improvements on domains of general intelligence, executive function, working memory, and global cognition after four months of abstinence. No significant improvements were observed on domains of visuospatial skills and processing speed. No longitudinal comparison with AUD-only patients was made.33 Three studies did not find an association between single or polysubstance use next to alcohol use and recovery on domains of executive function, learning and memory, and processing speed, with abstinence duration periods ranging from one month to eight months.18,40,43
In summary, the number of studies is limited but shows a pattern in which no association could be found between polysubstance use and recovery on several neurocognitive domains, during varying periods of abstinence.
Number of Previous Detoxifications
Three studies analyzed the association between the number of previous detoxifications and the recovery of neurocognitive function. In a study of patients with AUD, a distinction was made between groups with few medical detoxifications (≤1) and many medical detoxifications (≥2). The former group performed better on tasks of executive function, as well as learning and memory after three to six months of abstinence. No differences were found in the first three months of abstinence.44 One study did not find the number of inpatient detoxifications to be a predictor in similar domains after 24 months of abstinence.37 Another study did not find a significant correlation between the number of previous detoxifications and recovery of social cognitive function after eight weeks of abstinence.41 In summary, evidence for an association between previous detoxification and recovery of neurocognitive function is considered limited and inconsistent.
Positive Family History
Five studies examined the association between a positive family history (PFH) and recovery of neurocognitive function. Three studies found a negative association between PFH status and recovery on domains of executive function after six to seven weeks of abstinence43,45 or 15 months of abstinence.39 Two studies did not find an association between PFH and social cognitive function after two months of abstinence.41,46 In summary, the evidence is considered limited and suggests a negative association between PFH and executive function and no association between PFH and social cognitive function.
Demographic Factors
Age
Four out of ten studies found a negative association between increasing age and recovery of executive function, learning and memory, and speed after 6 to 15 months of abstinence.17,18,28,39 Six studies did not find an association between age and recovery of executive function, learning and memory, attention, speed, and social cognitive function after an abstinence duration varying from three weeks to nine months of abstinence.30,35,36,41,43,46 In summary, a larger number of studies have shown inconsistent results regarding the association between age and recovery of neurocognitive function.
Gender
Three studies examined the association between gender and neurocognitive recovery and did not find any effects on domains of executive function after 15 months of abstinence,39 nor on attention after one and a half months of abstinence,47 or social cognitive function after eight weeks of abstinence.41 Evidence is considered limited, but consistently shows no association between gender and recovery of neurocognitive function.
Education
Five of the six studies did not find an association between educational level and neurocognitive recovery. After controlling for education level in their analyses, no association was found with the performance on domains of executive function, learning and memory, attention, processing speed and visuospatial processing, between subgroups, after abstinence duration periods of 1.5 months till 15 months.17,18,29,30,39 One study found that higher education predicted a better recovery of verbal abilities after an abstinence period of 1.5 months.43 Taken together, the evidence largely shows a consistent pattern showing that educational attainment is not associated with the recovery of neurocognitive function.
Psychiatric Factors
Depression
Seven studies examined the association between depressive symptoms and recovery of neurocognitive function, of which three studies did not find an association between depressive symptoms and recovery on domains of executive function, learning and memory, or processing speed after abstinence periods varying from eight weeks to 48 months of abstinence.15,18,29 Two studies did not find an association between depressive symptoms and social cognitive function after two months of abstinence.41,46
Two studies found a negative association between depressive symptoms and recovery of executive function at five weeks of abstinence28 and after 15 months of abstinence.39 All studies examined depressive symptoms but did not classify depression using the DSM criteria.
One study analyzed the association between AUD and Bipolar Disorder (BD) and found that patients with co-occurring BD and AUD may suffer from more severe cognitive dysfunction and less favorable recovery of cognitive deficits than patients without AUD over the course of remission from a mood episode.48
In summary, evidence of an association between depressive symptoms and recovery of neurocognitive function is considered insufficient. The results show an inconsistent pattern of an association between depressive symptoms and recovery of executive function, limited evidence of an association with recovery on domains of learning and memory and processing speed, and limited evidence showing no association between depressive symptoms and social cognitive function.
Antisocial Personality Disorder
Two studies examined the association between antisocial personality disorder (ASPD) and neurocognitive recovery.39,43 One study did not clearly state whether ASPD coincided with a lack of abstinence,43 and in the other, ASPD was only associated with executive function at baseline and not with changes in neurocognitive function.39 The evidence found is considered to be limited.
Premorbid Neurocognitive Function
Verbal Reading Task
Four out of five studies found no association between performance on a verbal reading task and the recovery of executive function, learning and memory, processing speed, and spatial processing after six till 48 months of abstinence.15,18,30,35 In addition, Rosenbloom et al15 argued that overall test results showed higher scores on most tests by patients completing their trajectory compared to patients who dropped out of the study in a later phase, suggesting the possibility that improvements in sobriety were due to higher initial function.
In one study, the results of a verbal reading task explained part of the found improvement in executive function, processing speed, and spatial processing after an abstinence period of five weeks.28
In summary, a mostly consistent pattern of limited studies shows no association between performance on a verbal reading task and the recovery of neurocognitive function, after six to 48 months of abstinence. One study found an association after five weeks of abstinence, indicating a possible relationship with short-term abstinence.
Neurocognitive Performance at Baseline
One study showed that executive function at baseline predicted executive function after an abstinence duration of 15 months.39
Medical
Blood Values
Three studies examined the association between blood values and recovery of neurocognitive function. Two studies, using assessments at admission and at one and a half month after admission, found improvement in domains of learning and memory, and attention to be associated with NAA (N-acetylaspartate) values.47,49
A third study found that baseline medical test results (only specified as “abnormal results from blood and urine tests used to detect signs of liver, blood, kidney, and connective tissue disease”) directly predicted less recovery on measures of executive function after 15 months of abstinence.39
In summary, evidence of an association between blood values and recovery of neurocognitive function is considered limited.
Medical Conditions
Three studies examined the association between medical conditions and recovery of neurocognitive function, two of which found no association between hypertension and hepatitis C (measured at baseline) in domains of executive function, learning and memory, processing speed, attention, and visuospatial processing after abstinence periods of 4 and 8 months.18,29
Bates et al43 did find fewer medical conditions at baseline (not specified and measured with the Life Experiences and Social Resources Inventory50) to contribute to the recovery of verbal ability assessed after 1.5 months of abstinence.
In summary, the evidence for an association between medical conditions and recovery of neurocognitive function is considered inconsistent, and the number of studies is limited.
Brain Volumes
Five of the six studies found an association between brain volumes and recovery of (visuospatial) memory, visuospatial learning, and processing speed using abstinence periods varying from one week of abstinence32 to eight months of abstinence.51
In non-smoking AUD patients, increasing hippocampal volumes correlated with visuospatial memory improvements after one month of abstinence.52 A moderate-to-strong correlation was found between hippocampal volume change and improvements in the domain of learning and memory after one month of abstinence, depending on the brain-derived neurotrophic factor (BDNF) genotype (Val homozygotes only, not in MET carriers).35 Between one month and seven months, however, hippocampal volume changes did not correlate significantly with changes in neurocognitive function.
Increasing volumes in all gray matter and white matter regions (with the exception of the thalamus) were associated with improved processing speed over nearly eight months of abstinence, in non-smoking AUD patients, only.51 Yeh et al32 found significant correlations between total brain volume changes and recovery of visuospatial learning between one week and one month of abstinence from alcohol for smoking AUD but not for non-smoking AUD. However, the greater rate of brain volume increase in the first period of abstinence (one week to one month) in smoking AUD patients was inversely related to the improvement of visuospatial memory during the second period of abstinence (one month to seven months). Despite numerically faster brain volume gains and a trend toward faster ventricular shrinkage in smoking AUD patients, the recovery of visuospatial learning and memory of smoking AUD patients was below that of non-smoking AUD patients in the second period.
Changes in lateral ventricular volume were negatively correlated with overall memory performance after abstinence periods varying from three weeks to two years.38 Changes in the fourth ventricle were not associated with changes in memory performance after one month of abstinence.38
In summary, most studies have shown a consistent pattern of association between changes in brain volume during abstinence and recovery of neurocognitive function. This process can be influenced by smoking.
Perfusion
One study found no correlation between changes in frontal and parietal gray matter perfusion and changes in neurocognitive performance in executive function, learning and memory, processing speed, spatial processing, and intelligence after one month of abstinence.53
Discussion
This scoping review describes the scientific evidence for factors associated with the recovery of neurocognitive function in abstinent AUD patients (see Table 2 for a comprehensive overview of the strength of evidence found per factor). In this section, we discuss the most consistent patterns found, relate them to evidence in the relevant literature, and identify research gaps.
Table 2.
Overview of the Strength of Evidence per Factor
| Factor | # Studies | Strength of Evidence for Association with Neurocognitive Recovery |
|---|---|---|
| Smoking | 8 | Sufficient & associated |
| Quantities used | 13 | Sufficient & not associated |
| Polysubstance | 4 | Limited & not associated |
| Detoxifications | 3 | Limited |
| Positive family history | 5 | Limited |
| Age | 10 | Sufficient & inconsistent |
| Gender | 3 | Limited & not associated |
| Education | 6 | Sufficient & not associated |
| Depression | 7 | Limited |
| ASPD | 2 | Limited |
| Verbal reading | 5 | Limited & not associated |
| Blood values | 3 | Limited |
| Neurocognitive task | 1 | Limited |
| Medical conditions | 3 | Limited |
| Brain volumes | 6 | Sufficient & associated |
| Perfusion | 1 | Limited |
Notes: Sufficient & associated = enough studies, consistent pattern of associations with recovery of neurocognitive functions; Sufficient & not associated = enough studies, consistent pattern of not being associated with recovery of neurocognitive function; limited and not associated = not enough studies, most studies show no associations with recovery of neurocognitive functions; Limited = not enough studies; Sufficient & inconsistent = enough studies with mixed results.
First, the accumulated evidence suggests a largely consistent pattern of a negative association between smoking and neurocognitive recovery in the domains of processing speed, learning and memory, spatial processing, executive function, and fine motor skills, up to nine months of abstinence from alcohol. The interaction of nicotine and alcohol on neurocognitive function is of interest because it is highly prevalent in AUD patients,54–56 creating synergy and multiplying health risks.57–59 Future studies of neurocognitive recovery in AUD patients should incorporate smoking status and differentiate between active smoking, former smoking, and never smoking status.28
Second, increasing brain volumes during abstinence from alcohol within one year is associated with improvements in (visuospatial) memory and learning, and speed. Some evidence has also been found suggesting an association between decreasing lateral ventricular volume and memory improvement. Due to the process of sustained abstinence, ventricular volumes decrease and brain structures such as the temporal, insular, anterior cingulate cortices, amygdala, thalamus, hippocampus, brainstem, and cerebellar cortex increase.60 Identifying the reversibility of the structural and functional changes caused by alcohol use is important.6 The results of this review further support the association between the recovery of neurocognitive function with changes in brain volumes. In addition, findings from the included studies suggest that other factors may influence this relationship, such as smoking status31,32 or BDNF genotype.35
Two other well-studied factors, amounts of alcohol used and education level, show consistent evidence that they are predominantly not associated with the recovery of neurocognitive function. First, factors reflecting alcohol quantities were not, or sparsely, associated with neurocognitive recovery in various domains (executive function, learning and memory, attention and processing speed, and social cognition) after varying abstinence periods of up to 4 years. This finding has been noted before,49 and is somewhat counterintuitive. Excessive alcohol use is related to structural and functional abnormalities in the brain,6 which, in turn, are associated with neuropsychological impairments.61,62 There may be a methodological problem underlying this finding. Former alcohol use was retrospectively assessed in these studies by relying on self-report measures and the ability to adequately retrieve information from the past. AUD, however, is associated with episodic memory deficits, varying from learning impairments to spatiotemporal coding deficiencies (ie, binding spatial and temporal information), and deficiencies in autonoetic awareness (ie, recalling situations from the past by introspectively reliving these situations). These memory impairments may compromise the validity of the results obtained using the self-report measures.17,19 In addition, it can be considered that other factors associated with excessive alcohol use may cause severe neurocognitive impairments, such as thiamine deficiency.63
Second, accumulated evidence suggests that educational attainment is not associated with the recovery of neurocognitive function in multiple domains. This finding seems to contradict the robust association between education level and cognitive performance.64,65 Indeed, the level of education is believed to influence the recovery of neurocognitive function4,66 and to reflect cognitive reserve.65 However, these results were derived from cross-sectional studies, which showed a correlation at one point in time. Recovery of function, as reflected by changes in cognitive function with multiple assessments, was not measured. In line with our findings, other studies on cognitive reserve have shown no substantial relationship between educational attainment and changes in cognitive performance67 or cognitive reserve.68
The evidence for an association between the factors “polysubstance use”, “premorbid neurocognitive function” or “gender” with the recovery of neurocognitive function is considered insufficient. In most cases, the patterns consistently show no evidence of an association. Firstly, polysubstance use has been studied to a limited extent. Only four studies were included, of which two studies had lower levels of evidence ratings (small sample sizes:33,40 and no control group40). Moreover, two studies that did not find an effect did not include smoking status in their analyses. This excludes that part of the effects can be ascribed to smoking status, given the evidence for the influence of smoking status on neurocognitive recovery in AUD patients. The field of research on polysubstance use has long been overlooked,69,70 and the use of other substances was largely seen as a nuisance before 2010.29 Given the few longitudinal studies available, the assumed impact of smoking status, and specific neurobiological alterations coinciding with polysubstance use, more studies are needed.
Second, premorbid neurocognitive function, assessed by means of a verbal reading task, could not be associated with the recovery of neurocognitive function. Regarding the results, the following considerations are made to enhance future research. Investigations of alcohol-related neurocognitive impairments often use a verbal reading task to establish premorbid intelligence, such as the NART (National Adult Reading Task71). Previous research on the use of the NART provided evidence for justified use in estimating premorbid intelligence in patients with frontal lobe damage, Korsakoff syndrome, and mild or moderate stages of Alzheimer’s disease.72 Indeed, studies indicate that reading tests index prior intellectual ability73 and provide the most reliable and precise estimates of the WAIS-IV full scale IQ.74 However, in our review, most studies used these measures to investigate the effect of premorbid intelligence on performance or recovery on other neurocognitive instruments. To our knowledge, studies on the relationship between verbal reading tasks and neurocognitive measures other than intelligence tasks are scarce, with one study indicating a moderate correlation between performance on the NART and performance on measures of memory.75 Therefore, it seems appropriate to be careful when using a verbal reading task to predict specific neurocognitive function or recovery of neurocognitive function. In addition, tests assessing premorbid intelligence are likely to provide the most reliable premorbid estimates of full-scale intelligence in the mean range while overestimating intelligence in those with very low scores and underestimating those with very high scores.76 Finally, it is considered that a lower verbal ability can precede heavy alcohol use77 or can be the result of both lower premorbid intelligence and more intellectual decline.78
A third factor, gender, shows no association with the recovery of neurocognitive function but has been studied to a limited extent. The strength and generalizability of the evidence is limited for several reasons. Most studies included in this review consisted largely of men, which may have biased the results. Research within AUD populations largely consists of men, which complicates research on gender effects.79 Furthermore, the included studies had a small sample size47 or focused on only one domain of recovery (social cognitive function).41 Literature on the effect of gender differences on cognitive function states that men and women are generally evenly capable of cognitive capacities.80–82 It remains unclear whether this also applies to the recovery of neurocognitive function in AUD patients, since only a few studies have been performed on this matter, to our knowledge.83 Neurobiological studies suggest gender-specific neural recovery effects84 and greater neurotoxic effects of alcohol in binge drinking women than in men.85 Findings from a recent study showed a significant association between no recovery of cognitive function and the female gender in recovering AUD patients.86
One factor that has been analyzed in multiple studies on the recovery of neurocognitive function is age. However, the results showed an inconsistent pattern of studies that found a negative association and studies that found no association. These findings may partially cohere with the relatively low and equally distributed age of patients included in the studies (between 40 and 50 years, with, roughly estimated, most patients predominantly aged between 30 and 60 years). Moreover, the interaction of age with alcohol is complex, since the recovery of neurocognitive function can be modulated by age at exposure, aging following alcohol toxicity or thiamine deficiency, and aging during chronic alcohol use.87
Finally, the findings of this review indicate that most of these factors are understudied. In this regard, studies on the influence of polysubstance use, the number of previous detoxifications, positive family history, sex, depression, ASPD, verbal reading, blood values, baseline neurocognitive function, medical conditions, and perfusion on recovery of neurocognitive function during abstinence are limited and in need of further research. In addition, it is noted that alcohol use disorder is highly comorbid with other psychiatric disorders, such as depression or personality disorder,88 as was also found within some of the studies included. This, so-called “dual disorder”,89,90 is associated with poorer prognosis (eg, greater psychopathological severity, higher frequency of psychiatric admissions) and may be accompanied by other, distinct, neuropsychological characteristics.91
Recommendations for Future (Longitudinal) Research
Based on the findings of this review, a number of recommendations can be made that may lower the threshold for clinically relevant scientific research and further aid in modelling the recovery of neuropsychological function with sustained abstinence.
The recovery of neurocognitive function was negatively associated with smoking. Smoking status (never smoking, former smoking, and smoking) is associated with differential effects on the recovery of neurocognitive function and should be incorporated in future research.
There is sufficient evidence for the lack of an association between “quantities used” (by means of self-report) and recovery of neurocognitive function during abstinence. It should be noted that episodic memory deficits might obscure the self-reported findings of AUD patients regarding alcohol use in the past. It is therefore recommended to consider carefully the use of self-report tools to measure prior alcohol consumption, and their purpose in neurocognitive recovery research.
Most studies (25/31) included in this review started the first assessments within two weeks of abstinence. Given the possible negative effects of withdrawal on neurocognitive function, it is recommended that neurocognitive function be assessed after at least two weeks of abstinence.92 Another study recommends assessment after six weeks of abstinence.93
Findings from this review indicate that most factors are understudied, while scarce evidence is indicative of follow-up research. In this regard, associations of neurocognitive recovery with age, gender, polysubstance use, the number of previous detoxifications, blood values, medical conditions, depression and ASPD (also studied as dual disorders89,91) should be studied in well-controlled cross-sectional, or preferably longitudinal, designs that account for abstinence duration.
Social cognition, or the recovery of social cognitive function, is an understudied neurocognitive domain that requires further research.
Limitations
This review is not without limitations, four of which we will mention here. First, methodological issues must be considered when interpreting our findings. Determining the predictive value of a factor, or its association, is always done in the context of other factors, and is valid, within the model used. Therefore, not finding an association or an effect means that there is no effect within the model handled. Studies within this review show considerable differences in the number of variables included in their analyses. In this regard, we notice that well-controlled, cross-sectional studies, including multiple relevant variables associated with neurocognitive functioning in abstinent AUD, also contribute to our understanding of neurocognitive recovery (see for example90) but have not been included in this review. Second, we did not pool data to meta-analyze the effects of individual factors since not all data were available from the included studies. Third, the instruments used to measure neurocognitive function in these studies are diverse and differ in terms of validity and reliability. In addition, there is diversity in the extent to which studies take into account possible learning effects associated with the instruments used, ranging from merely stating a limitation of the study to using parallel forms or calculating a reliable change index. Finally, there is a risk of publication bias by including only published studies in this review. We consider the risk, however, to be limited, as the factors studied were often not the primary focus of the included study, resulting, for example, in multiple null findings (eg, multiple studies showing no association between quantities of alcohol used with neurocognitive recovery).
Conclusion
Notwithstanding the aforementioned limitations, to our knowledge, this is the first review to systematically map the factors that may influence the recovery of neurocognitive function in abstinent AUD patients. The clearest patterns emerging from the evidence collected are a predominantly negative influence of smoking on the recovery of neurocognitive function, and associations between changes in volumes of various brain areas (hippocampal, ventricular, and total brain volume), and recovery of neurocognitive function. There is sufficient evidence for the lack of an association between neurocognitive recovery and the amount of alcohol consumed, as measured by self-report, or between neurocognitive recovery and educational attainment.
Future research on several factors is indicated because these have been sparsely investigated using a longitudinal design (polysubstance use, the number of previous detoxifications, positive family history, gender, depression, ASPD, verbal reading, blood values, baseline neurocognitive function, medical conditions, and perfusion) and/or show conflicting findings (age). Longitudinal designs should preferably be used, with multiple assessment periods, starting at least after two weeks of abstinence, given the possibility of withdrawal symptoms interfering with neurocognitive performance.
Findings from these studies may help resolve the conflicting results found so far on the recovery of neurocognitive function and may expand our understanding of this process and the extent to which it occurs in the context of AUD. Ultimately, this will aid in creating a clinical profile that can be used to identify patients at risk of slower or limited recovery and to improve personalized care.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bernardin F, Maheut-Bosser A, Paille F. Cognitive impairments in alcohol-dependent subjects. Front Psychiatry. 2014;5. doi: 10.3389/fpsyt.2014.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perry CJ. Cognitive decline and recovery in alcohol abuse. J Mol Neurosci. 2016;60(3):383–389. doi: 10.1007/s12031-016-0798-4 [DOI] [PubMed] [Google Scholar]
- 3.Bailey K, Bartholow BD, Saults JS, Lust SA. Give me just a little more time: effects of alcohol on the failure and recovery of cognitive control. J Abnorm Psychol. 2014. doi: 10.1037/a0035662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowe SF, Cammisuli DM, Stranks EK. Widespread cognitive deficits in alcoholism persistent following prolonged abstinence: an updated meta-analysis of studies that used standardised neuropsychological assessment tools. Arch Clin Neuropsychol. 2019;35(1):31–45. doi: 10.1093/arclin/acy106 [DOI] [PubMed] [Google Scholar]
- 5.Crews FT, Buckley T, Dodd PR, et al. Alcoholic neurobiology: changes in dependence and recovery. Alcohol Clin Exp Res. 2005;29(8):1504–1513. doi: 10.1097/01.alc.0000175013.50644.61 [DOI] [PubMed] [Google Scholar]
- 6.Harper C. The neuropathology of alcohol-related brain damage. Alcohol Alcohol. 2009;44(2):136–140. doi: 10.1093/alcalc/agn102 [DOI] [PubMed] [Google Scholar]
- 7.Oscar-Berman M, Maleki N. Alcohol dementia, Wernicke’s encephalopathy, and Korsakoff’s syndrome. In: The Oxford Handbook of Adult Cognitive Disorders. Oxford Academic; 2019:742–758. [Google Scholar]
- 8.Domínguez-Salas S, Díaz-Batanero C, Lozano-Rojas OM, Verdejo-García A. Impact of general cognition and executive function deficits on addiction treatment outcomes: systematic review and discussion of neurocognitive pathways. Neurosci Biobehav Rev. 2016. doi: 10.1016/j.neubiorev.2016.09.030 [DOI] [PubMed] [Google Scholar]
- 9.Bates ME, Buckman JF, Nguyen TT. A role for cognitive rehabilitation in increasing the effectiveness of treatment for alcohol use disorders. Neuropsychol Rev. 2013;23(1):27–47. doi: 10.1007/s11065-013-9228-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brorson HH, Ajo Arnevik E, Rand-Hendriksen K, Duckert F. Drop-out from addiction treatment: a systematic review of risk factors. Clin Psychol Rev. 2013;33(8):1010–1024. doi: 10.1016/j.cpr.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 11.Le Berre A-P, Fama R, Sullivan EV. Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: a critical review to inform future research. Alcohol Clin Exp Res. 2017;41(8):1432–1443. doi: 10.1111/acer.13431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petit G, Luminet O, Cordovil De Sousa Uva M, Zorbas A, Maurage P, De Timary P. Differential spontaneous recovery across cognitive abilities during detoxification period in alcohol-dependence. PLoS One. 2017;12(8):e0176638. doi: 10.1371/journal.pone.0176638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulte MHJ, Cousijn J, den Uyl TE, et al. Recovery of neurocognitive functions following sustained abstinence after substance dependence and implications for treatment. Clin Psychol Rev. 2014;34(7):531–550. doi: 10.1016/j.cpr.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 14.Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol. 2013;18(2):203–213. doi: 10.1111/j.1369-1600.2011.00418.x [DOI] [PubMed] [Google Scholar]
- 15.Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Recovery of short-term memory and psychomotor speed but not postural stability with long-term sobriety in alcoholic women. Neuropsychology. 2004;18(3):589–597. doi: 10.1037/0894-4105.18.3.589 [DOI] [PubMed] [Google Scholar]
- 16.Oscar-Berman M, Valmas MM, Sawyer KS, Ruiz SM, Luhar RB, Gravitz ZR. Profiles of impaired, spared, and recovered neuropsychologic processes in alcoholism. Handb Clin Neurol. 2014. doi: 10.1016/B978-0-444-62619-6.00012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitel AL, Rivier J, Beaunieux H, Vabret F, Desgranges B, Eustache F. Changes in the episodic memory and executive functions of abstinent and relapsed alcoholics over a 6-month period. Alcohol Clin Exp Res. 2009;33(3):490–498. doi: 10.1111/j.1530-0277.2008.00859.x [DOI] [PubMed] [Google Scholar]
- 18.Durazzo TC, Pennington DL, Schmidt TP, Meyerhoff DJ. Effects of cigarette smoking history on neurocognitive recovery over 8 months of abstinence in alcohol-dependent individuals. Alcohol Clin Exp Res. 2014;38(11):2816–2825. doi: 10.1111/acer.12552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Berre AP, Fama R, Sassoon SA, Pfefferbaum A, Sullivan EV, Zahr NM. Cognitive and motor impairment severity related to signs of subclinical wernicke’s encephalopathy in HIV infection. J Acquir Immune Defic Syndr. 2019;81(3):345–354. doi: 10.1097/QAI.0000000000002043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui C, Noronha A, Warren KR, et al. Brain pathways to recovery from alcohol dependence. Alcohol. 2015;49(5):435–452. doi: 10.1016/j.alcohol.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thygesen LC, Johansen C, Keiding N, Giovannucci E, Grønbæk M. Effects of sample attrition in a longitudinal study of the association between alcohol intake and all-cause mortality. Addiction. 2008;103(7):1149–1159. doi: 10.1111/j.1360-0443.2008.02241.x [DOI] [PubMed] [Google Scholar]
- 22.Torvik FA, Rognmo K, Tambs K. Alcohol use and mental distress as predictors of non-response in a general population health survey: the HUNT study. Soc Psychiatry Psychiatr Epidemiol. 2012;47(5):805–816. doi: 10.1007/s00127-011-0387-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan EV, Pfefferbaum A. Brain-behavior relations and effects of aging and common comorbidities in alcohol use disorder: a review. Neuropsychology. 2019;33(6):760. doi: 10.1037/NEU0000557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitel AL, Beaunieux H, Desgranges B, Sullivan EV, Eustache F. Memory disorders in alcohol use disorder without clinically-detectable neurological complication. Curated Ref Collect Neurosci Biobehav Psychol. 2016;2016:215–221. doi: 10.1016/B978-0-12-809324-5.00358-8 [DOI] [Google Scholar]
- 25.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 26.Fernández-Serrano MJ, Pérez-García M, Verdejo-García A. What are the specific vs. generalized effects of drugs of abuse on neuropsychological performance? Neurosci Biobehav Rev. 2011;35(3):377–406. doi: 10.1016/j.neubiorev.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 27.Stevens L, Verdejo-García A, Goudriaan AE, Roeyers H, Dom G, Vanderplasschen W. Impulsivity as a vulnerability factor for poor addiction treatment outcomes: a review of neurocognitive findings among individuals with substance use disorders. J Subst Abuse Treat. 2014. doi: 10.1016/j.jsat.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 28.Pennington DL, Durazzo TC, Schmidt TP, Mon A, Abé C, Meyerhoff DJ. The effects of chronic cigarette smoking on cognitive recovery during early abstinence from alcohol. Alcohol Clin Exp Res. 2013;37(7):1220–1227. doi: 10.1111/acer.12089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durazzo TC, Meyerhoff DJ. Cigarette smoking history is associated with poorer recovery in multiple neurocognitive domains following treatment for an alcohol use disorder. Alcohol. 2020;85:135–143. doi: 10.1016/j.alcohol.2019.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durazzo TC, Rothlind JC, Gazdzinski S, Banys P, Meyerhoff DJ. Chronic smoking is associated with differential neurocognitive recovery in abstinent alcoholic patients: a preliminary investigation. Alcohol Clin Exp Res. 2007;31(7):1114–1127. doi: 10.1111/j.1530-0277.2007.00398.x [DOI] [PubMed] [Google Scholar]
- 31.Durazzo TC, Mon A, Pennington D, Abé C, Gazdzinski S, Meyerhoff DJ. Interactive effects of chronic cigarette smoking and age on brain volumes in controls and alcohol-dependent individuals in early abstinence. Addict Biol. 2014;19(1):132–143. doi: 10.1111/j.1369-1600.2012.00492.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh P-H, Gazdzinski S, Durazzo TC, Sjöstrand K, Meyerhoff DJ. Hierarchical linear modeling (HLM) of longitudinal brain structural and cognitive changes in alcohol-dependent individuals during sobriety. Drug Alcohol Depend. 2007;91(2–3):195–204. doi: 10.1016/j.drugalcdep.2007.05.027 [DOI] [PubMed] [Google Scholar]
- 33.Schmidt TP, Pennington DL, Cardoos SL, Durazzo TC, Meyerhoff DJ. Neurocognition and inhibitory control in polysubstance use disorders: comparison with alcohol use disorders and changes with abstinence. J Clin Exp Neuropsychol. 2017;39(1):22–34. doi: 10.1080/13803395.2016.1196165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durazzo TC, Meyerhoff DJ. Neurobiological and neurocognitive effects of chronic cigarette smoking and alcoholism. Front Biosci. 2007;12:4079–4100. [DOI] [PubMed] [Google Scholar]
- 35.Hoefer ME, Pennington DL, Durazzo TC, et al. Genetic and behavioral determinants of hippocampal volume recovery during abstinence from alcohol. Alcohol. 2014;48(7):631–638. doi: 10.1016/j.alcohol.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maillard A, Poussier H, Boudehent C, et al. Short-term neuropsychological recovery in alcohol use disorder: a retrospective clinical study. Addict Behav. 2020:105. doi: 10.1016/j.addbeh.2020.106350 [DOI] [PubMed] [Google Scholar]
- 37.Bartels C, Kunert H-J, Stawicki S, Kroner-Herwig B, Ehrenreich H, Krampe H. Recovery of hippocampus-related functions in chronic alcoholics during monitored long-term abstinence. Alcohol Alcohol. 2006;42(2):92–102. doi: 10.1093/alcalc/agl104 [DOI] [PubMed] [Google Scholar]
- 38.Rosenbloom MJ, Rohlfing T, O’Reilly AW, Sassoon SA, Pfefferbaum A, Sullivan EV. Improvement in memory and static balance with abstinence in alcoholic men and women: selective relations with change in brain structure. Psychiatry Res Neuroimaging. 2007;155(2):91–102. doi: 10.1016/j.pscychresns.2006.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bates ME, Barry D, Labouvie EW, Fals-Stewart W, Voelbel G, Buckman JF. Risk factors and neuropsychological recovery in clients with alcohol use disorders who were exposed to different treatments. J Consult Clin Psychol. 2004;72(6):1073–1080. doi: 10.1037/0022-006X.72.6.1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manning V, Wanigaratne S, Best D, et al. Changes in neuropsychological functioning during alcohol detoxification. Eur Addict Res. 2008;14(4):226–233. doi: 10.1159/000156479 [DOI] [PubMed] [Google Scholar]
- 41.Rupp CI, Junker D, Kemmler G, Mangweth-Matzek B, Derntl B. Do social cognition deficits recover with abstinence in alcohol-dependent patients? Alcohol Clin Exp Res. 2021;45(2):470–479. doi: 10.1111/acer.14537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaur P, Sidana A, Malhotra N, Gupta A. Effects of abstinence of alcohol on neurocognitive functioning in patients with alcohol dependence syndrome. Asian J Psychiatr. 2020;50. doi: 10.1016/j.ajp.2020.101997 [DOI] [PubMed] [Google Scholar]
- 43.Bates ME, Voelbel GT, Buckman JF, Labouvie EW, Barry D. Short-term neuropsychological recovery in clients with substance use disorders. Alcohol Clin Exp Res. 2005;29(3):367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loeber S, Duka T, Márquez HW, et al. Effects of repeated withdrawal from alcohol on recovery of cognitive impairment under abstinence and rate of relapse. Alcohol Alcohol. 2010. doi: 10.1093/alcalc/agq065 [DOI] [PubMed] [Google Scholar]
- 45.Moriyama Y, Murumatsu T, Kato M, Mimura M, Kashima H. Family history of alcoholism and cognitive recovery in subacute withdrawal. Psychiatry Clin Neurosci. 2006;60(1):85–89. doi: 10.1111/j.1440-1819.2006.01464.x [DOI] [PubMed] [Google Scholar]
- 46.Foisy ML, Kornreich C, Petiau C, et al. Impaired emotional facial expression recognition in alcoholics: are these deficits specific to emotional cues? Psychiatry Res. 2007;150(1):33–41. doi: 10.1016/j.psychres.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 47.Bartsch AJ, Homola G, Biller A, et al. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130(1):36–47. doi: 10.1093/brain/awl303 [DOI] [PubMed] [Google Scholar]
- 48.Levy B, Manove E, Weiss RD. Recovery of cognitive functioning in patients with co-occurring bipolar disorder and alcohol dependence during early remission from an acute mood episode. Ann Clin Psychiatry. 2012;24(2):143–154. [PMC free article] [PubMed] [Google Scholar]
- 49.Bendszus M, Weijers HG, Wiesbeck G, et al. Sequential MR imaging and proton MR spectroscopy in patients who underwent recent detoxification for chronic alcoholism: correlation with clinical and neuropsychological data. Am J Neuroradiol. 2001;22(10):1926–1932. [PMC free article] [PubMed] [Google Scholar]
- 50.Moos RH. Life stressors and social resources inventory; 2011. Available from: https://www.parinc.com/Products/Pkey/218. Accessed September 13, 2023.
- 51.Durazzo TC, Mon A, Gazdzinski S, Yeh P-H, Meyerhoff DJ. Serial longitudinal magnetic resonance imaging data indicate non-linear regional gray matter volume recovery in abstinent alcohol-dependent individuals. Addict Biol. 2015;20(5):956–967. doi: 10.1111/adb.12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gazdzinski S, Durazzo TC, Yeh PH, Hardin D, Banys P, Meyerhoff DJ. Chronic cigarette smoking modulates injury and short-term recovery of the medial temporal lobe in alcoholics. Psychiatry Res. 2008;162(2):133–145. doi: 10.1016/j.pscychresns.2007.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mon A, Durazzo TC, Gazdzinski S, Meyerhoff DJ. The impact of chronic cigarette smoking on recovery from cortical gray matter perfusion deficits in alcohol dependence: longitudinal arterial spin labeling MRI. Alcohol Clin Exp Res. 2009;33(8):1314–1321. doi: 10.1111/j.1530-0277.2009.00960.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller NS, Gold MS. Comorbid cigarette and alcohol addiction: epidemiology and treatment. J Addict Dis. 1998;17(1):55–66. doi: 10.1300/J069v17n01_06 [DOI] [PubMed] [Google Scholar]
- 55.Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107–1115. doi: 10.1001/archpsyc.61.11.1107 [DOI] [PubMed] [Google Scholar]
- 56.Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14(2):106–123. doi: 10.1080/10550490590924728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hurt RD, Offord KP, Croghan IT, et al. Mortality following inpatient addictions treatment: role of tobacco use in a community-based cohort. J Am Med Assoc. 1996;275(14):1097–1103. doi: 10.1001/jama.275.14.1097 [DOI] [PubMed] [Google Scholar]
- 58.Castellsagué X, Munoz N, De Stefani E, et al. Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. Int J Cancer. 1999;82(5):657–664. doi: [DOI] [PubMed] [Google Scholar]
- 59.Pelucchi C, Gallus S, Garavello W, Bosetti C, La Vecchia C. Cancer risk associated with alcohol and tobacco use: focus on upper aero-digestive tract and liver. Alcohol Res Heal. 2006;29(3):193–198. [PMC free article] [PubMed] [Google Scholar]
- 60.Zahr NM, Pfefferbaum A. Alcohol’s effects on the brain: neuroimaging results in humans and animal models. Alcohol Res. 2017;38(2):183–206. [PMC free article] [PubMed] [Google Scholar]
- 61.Chanraud S, Martelli C, Delain F, et al. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32(2):429–438. doi: 10.1038/sj.npp.1301219 [DOI] [PubMed] [Google Scholar]
- 62.Chanraud S, Reynaud M, Wessa M, et al. Diffusion tensor tractography in mesencephalic bundles: relation to mental flexibility in detoxified alcohol-dependent subjects. Neuropsychopharmacology. 2009;34(5):1223–1232. doi: 10.1038/npp.2008.101 [DOI] [PubMed] [Google Scholar]
- 63.Arts NJM, Walvoort SJW, Kessels RPC. Korsakoff’s syndrome: a critical review. Neuropsychiatr Dis Treat. 2017. doi: 10.2147/NDT.S130078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caneva S, Ottonello M, Torselli E, Pistarini C, Spigno P, Fiabane E. Cognitive impairments in early-detoxified alcohol-dependent inpatients and their associations with socio-demographic, clinical and psychological factors: an exploratory study. Neuropsychiatr Dis Treat. 2020;16:1705–1716. doi: 10.2147/NDT.S254369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Requena-Ocaña N, Araos P, Flores M, et al. Evaluation of neurotrophic factors and education level as predictors of cognitive decline in alcohol use disorder. Sci Rep. 2021;11(1). doi: 10.1038/S41598-021-95131-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oscar-Berman M, Marinković K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17(3):239–257. doi: 10.1007/s11065-007-9038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seblova D, Berggren R, Lövdén M. Education and age-related decline in cognitive performance: systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2020;58. doi: 10.1016/j.arr.2019.101005 [DOI] [PubMed] [Google Scholar]
- 68.Wilson RS, Yu L, Lamar M, Schneider JA, Boyle PA, Bennett DA. Education and cognitive reserve in old age. Neurology. 2019;92(10):E1041–E1050. doi: 10.1212/WNL.0000000000007036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Skike CE, Maggio SE, Reynolds AR, et al. Critical needs in drug discovery for cessation of alcohol and nicotine polysubstance abuse. Prog Neuro Psychopharmacol Biol Psychiatry. 2016;65:269–287. doi: 10.1016/j.pnpbp.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCabe SE, West BT, Jutkiewicz EM, Boyd CJ. Multiple DSM-5 substance use disorders: a national study of US adults. Hum Psychopharmacol Clin Exp. 2017;32(5):e2625. doi: 10.1002/hup.2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nelson HE. National Adult Reading Test (NART): for the assessment of premorbid intelligence in patients with dementia; 1982.
- 72.Bright P, Jaldow E, Kopelman MD. The national adult reading test as a measure of premorbid intelligence: a comparison with estimates derived from demographic variables. J Int Neuropsychol Soc. 2002;8(6):847–854. doi: 10.1017/S1355617702860131 [DOI] [PubMed] [Google Scholar]
- 73.Crawford JR, Deary IJ, Starr J, Whalley LJ. The NART as an index of prior intellectual functioning: a retrospective validity study covering a 66-year interval. Psychol Med. 2001;31(3):451–458. doi: 10.1017/s0033291701003634 [DOI] [PubMed] [Google Scholar]
- 74.Bright P, Van der linde I. Comparison of methods for estimating premorbid intelligence. Neuropsychol Rehabil. 2020;30(1):1–14. doi: 10.1080/09602011.2018.1445650 [DOI] [PubMed] [Google Scholar]
- 75.Frick A, Wahlin TBR, Pachana NA, Byrne GJ. Relationships between the national adult reading test and memory. Neuropsychology. 2011;25(3):397–403. doi: 10.1037/a0021988 [DOI] [PubMed] [Google Scholar]
- 76.Bright P, Hale E, Gooch VJ, Myhill T, van der Linde I. The national adult reading test: restandardisation against the Wechsler adult intelligence scale-fourth edition. Neuropsychol Rehabil. 2018;28(6):1019–1027. doi: 10.1080/09602011.2016.1231121 [DOI] [PubMed] [Google Scholar]
- 77.Schottenbauer MA, Momenan R, Kerick M, Hommer DW. Relationships among aging, IQ, and intracranial volume in alcoholics and control subjects. Neuropsychology. 2007;21(3):337–345. doi: 10.1037/0894-4105.21.3.337 [DOI] [PubMed] [Google Scholar]
- 78.Grønkjaer M, Flensborg-Madsen T, Osler M, Sørensen HJ, Becker U, Mortensen EL. Adult-life alcohol consumption and age-related cognitive decline from early adulthood to late midlife. Alcohol Alcohol. 2019;54(4):446–454. doi: 10.1093/alcalc/agz038 [DOI] [PubMed] [Google Scholar]
- 79.Walter H, Dvorak A, Gutierrez K, Zitterl W, Lesch OM. Gender differences: does alcohol affect females more than males? Neuropsychopharmacol Hung. 2005;7(2):78–82. [PubMed] [Google Scholar]
- 80.Hyde JS. Sex and cognition: gender and cognitive functions. Curr Opin Neurobiol. 2016;38:53–56. doi: 10.1016/j.conb.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 81.Jäncke L. Sex/gender differences in cognition, neurophysiology, and neuroanatomy [version 1; referees: 3 approved]. F1000Research. 2018;7. doi: 10.12688/f1000research.13917.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van den Berg JF, Dogge B, Kist N, Kok RM, Van der Hiele K. Gender differences in cognitive functioning in older alcohol-dependent patients. Subst Use Misuse. 2017;52(5):574–580. doi: 10.1080/10826084.2016.1245341 [DOI] [PubMed] [Google Scholar]
- 83.Fama R, Le Berre AP, Sullivan EV. Alcohol’s unique effects on cognition in women: a 2020 (Re)view to envision future research and treatment. Alcohol Res. 2020;40(2):1–17. doi: 10.35946/ARCR.V40.2.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Charlet K, Rosenthal A, Lohoff FW, Heinz A, Beck A. Imaging resilience and recovery in alcohol dependence. Addiction. 2018;113(10):1933–1950. doi: 10.1111/add.14259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vinader-Caerols C, Talk A, Montañés A, Duque A, Monleón S. Differential effects of alcohol on memory performance in adolescent men and women with a binge drinking history. Alcohol Alcohol. 2017;52(5):610–616. doi: 10.1093/alcalc/agx040 [DOI] [PubMed] [Google Scholar]
- 86.Luquiens A, Rolland B, Pelletier S, et al. Role of patient sex in early recovery from alcohol-related cognitive impairment: women penalized. J Clin Med. 2019;8(6). doi: 10.3390/jcm8060790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nunes PT, Kipp BT, Reitz NL, Savage LM. Aging with alcohol-related brain damage: critical brain circuits associated with cognitive dysfunction. In: International Review of Neurobiology. Vol. 148. Academic Press Inc.; 2019:101–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Howe LK, Fisher LR, Atkinson EA, Finn PR. Symptoms of anxiety, depression, and borderline personality in alcohol use disorder with and without comorbid substance use disorder. Alcohol. 2021;90:19–25. doi: 10.1016/J.ALCOHOL.2020.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Adan A, Torrens M. Special issue: diagnosis and management of addiction and other mental disorders (dual disorders). J Clin Med. 2021;10(6):1307. doi: 10.3390/JCM10061307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Torrens M, Adan A. Recent advances in dual disorders (addiction and other mental disorders). J Clin Med. 2023;12(9):12. doi: 10.3390/JCM12093315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beerten-Duijkers JCLM, Vissers CTWM, Rinck M, Egger JIM. Inhibitory control and craving in dual disorders and recurrent substance use. Preliminary findings. Front Psychiatry. 2021;12:569817. doi: 10.3389/FPSYT.2021.569817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Laniepce A, Cabe N, Andre C, et al. The effect of alcohol withdrawal syndrome severity on sleep, brain and cognition. Brain Commun. 2020;2(2). doi: 10.1093/BRAINCOMMS/FCAA123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walvoort SJW, Wester AJ, Egger JIM. Neuropsychologische diagnostiek en cognitieve functies bij alcoholabstinentie. Tijdschr Psychiatr. 2013;55(2):101–111. [PubMed] [Google Scholar]
- 94.Cordovil De Sousa Uva M, Luminet O, Cortesi M, Constant E, Derely M, De Timary P. Distinct effects of protracted withdrawal on affect, craving, selective attention and executive functions among alcohol-dependent patients. Alcohol Alcohol. 2010;45(3):241–246. doi: 10.1093/alcalc/agq012 [DOI] [PubMed] [Google Scholar]
- 95.Czapla M, Simon JJ, Richter B, et al. The impact of cognitive impairment and impulsivity on relapse of alcohol-dependent patients: implications for psychotherapeutic treatment. Addict Biol. 2016. doi: 10.1111/adb.12229 [DOI] [PubMed] [Google Scholar]
- 96.Alhassoon OM, Sorg SF, Taylor MJ, et al. Callosal white matter microstructural recovery in abstinent alcoholics: a longitudinal diffusion tensor imaging study. Alcohol Clin Exp Res. 2012;36(11):1922–1931. doi: 10.1111/j.1530-0277.2012.01808.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mulhauser K, Weinstock J, Ruppert P, Benware J. Changes in neuropsychological status during the initial phase of abstinence in alcohol use disorder: neurocognitive impairment and implications for clinical care. Subst Use Misuse. 2018;53(6):881–890. doi: 10.1080/10826084.2017.1408328 [DOI] [PubMed] [Google Scholar]