Abstract
Objective
A relevance of fear and concerns about vaccine development and its side effects are suggested to explain COVID-19 vaccine hesitancy. However, evidence supporting the phobic origin hypothesis of hesitancy for COVID-19 and other vaccinations remains indirect and elusive.
Method
We addressed this issue by investigating the existence of a relationship between fear conditioning, extinction, and the respective vaccination hesitancy and anxiety scores in a group of 25 individuals.
Results
Overall, we show that the general mechanism of fear extinction learning is impaired in individuals with high vaccine hesitancy. State and trait anxiety scores do not account for this result.
Conclusions
These findings suggest that attitudes against vaccination could be linked to an altered inhibitory learning process.
Keywords: vaccine hesitancy, pavlovian fear conditioning, fear extinction learning, anxiety, inhibitory learning process
Introduction
The public health risk and concerns of the COVID-19 infection are far from over. In December 2022, an impressive rise of COVID-19 infections took place in China (Akriti, 2022). Furthermore, a recent model from the Institute for Health Metrics and Evaluation (covid19.healthdata.org) predicted that the number of COVID-19 infections could be destined to rise due to the absence of lockdowns, mask mandates and other prevention measures. This requires the strengthening and consolidation of available strategies to diminish COVID-19 infections. An important tool is vaccination, as well as diminishing vaccination hesitancy, which is estimated to involve approximately a quarter of the general population (Murphy et al., 2021). That can be achieved through vaccination campaigns, whose success is dependent on the ability to overcome attitudes of hesitation/resistance towards vaccination in general.
Mounting evidence stresses the role of sociodemographic and economic variables, as well as misinformation (Larson et al., 2021), to explain hesitancy/resistance to COVID-19 vaccination. Some reports suggest the relevance of emotions, such as fear of needles (e.g., Freeman et al., 2021), and concerns about vaccine development and its side effects (Rosenbaum, 2021). Moreover, “fear appeals” are considered relevant to explain the negative influence of misinformation on attitudes towards vaccines (van der Linden, 2022), as some individuals may be more sensitive to threatening messages that could undermine scientifically valid information campaigns. Nevertheless, evidence supporting the phobic origin hypothesis of hesitancy for vaccination remains indirect and elusive.
We addressed this issue by investigating whether vaccine hesitancy, as measured by the adult vaccine hesitancy scale (aVHS), can predict the neurovegetative response (i.e., skin conductance response – SCR) associated with fear conditioning/extinction learning. In line with evidence suggesting that generalized anxiety is associated with unwillingness or indecision regarding being vaccinated against COVID-19 (Sekizawa et al., 2022), we also investigated individual differences in anxiety using the state-trait anxiety inventory (STAI-2). In this way we explored the predictive role of anxiety to explain the psychophysiological (SCR) activity of vaccine-hesitant individuals during fear conditioning, or extinction.
The exploration of SCR, which reflects the activation of the sympathetic nervous system (Dawson et al., 1990), in combination with the Pavlovian paradigm of fear conditioning, has proved to be particularly effective in the investigation of the psychophysiological correlates of fear learning and extinction in healthy subjects (Haaker et al., 2019; Vicario et al., 2020), and in clinical populations affected by anxiety and post-traumatic stress disorder (See Marković et al., 2021; Rabinak et al., 2017).
According to the phobic origin hypothesis of vaccine hesitancy we predicted higher fear acquisition and/or reduced fear extinction learning in individuals with high aVHS scores, as compared to individuals with low aVHS scores. Moreover, in line with evidence for a relation between vaccine hesitancy and anxiety (Bendau et al., 2021), we sought to clarify whether any difference in fear conditioning between the two groups could reflect interindividual differences in trait or state anxiety.
Methods
Participants
25 participants (10 males, 16 right-handed) with a mean age of 22.6 ± 3.20 years were recruited by means of online advertisements. Informed consent was obtained from all participants before inclusion, and the protocol was approved by the local ethics committee of the Department. The experimental procedures were conducted according to the Declaration of Helsinki principles (Rickham, 1964), and subsequent updated versions (WMA, 2013).
Instruments
2.2.1 Adult Vaccine Hesitancy Scale
The Italian version of the adult Vaccine Hesitancy Scale (aVHS) is a valid and reliable self-report instrument for evaluating attitudes towards vaccination against respective preventable diseases (Ledda et al., 2022). Akel et al. (2021) developed the original version of this instrument and assessed its reliability and validity in cross-sectional internet surveys conducted in the United States and China, with a focus on the influence and acceptance of COVID-19 vaccination.The 10 items of the adult Vaccine Hesitancy Scale (aVHS) are answered by the participants on a five-point Likert-type rating scale ranging from least hesitant (1) to most hesitant (5). An example item of the Italian version is: “I vaccini sono importanti per la mia salute” (Vaccines are important for my health). The internal consistency (IC) of the Italian version of the aVHS, as assessed by the Cronbach alpha coefficient is 0.94, demonstrating an excellent IC reliability. The scale content of validity index (S-CVI) calculated for the total scale was 0.97 (Ledda et al., 2022). The cutoff score to establish whether participants are classified as highly hesitant to vaccination is > 25, according to Akel et al. (2021).
2.2.2 State-Trait Anxiety Disorder Inventory
We used both, the state (STAI-Y1) and the trait (STAI-Y2) anxiety subscales of the State-Trait Anxiety Disorder Inventory (STAI; Spielberger et al., 1970) to investigate relationships between anxiety and fear acquisition and extinction. For our study we referred to the Italian version created by Pedrabissi & Santinello (1989). The scale includes 20 items for assessing state anxiety and 20 for trait anxiety. For each item, participants had to provide a rating ranging from 1 “Almost Never” to 4 “Almost Always”. The total score of each scale ranges from 20 to 80 points. An example item in the Italian version is: “mi sento bene” (I feel well). Good internal consistency is reported for both the state and trait anxiety subscales of the STAI, with Cronbach's alpha coefficients ranging from 0.91-0.95 and 0.85-0.90, respectively.
2.2.3 Virtual reality Pavlovian fear conditioning/ extinction task
To study fear conditioning and extinction, we created) a 3D environment in virtual reality by using the software unity engine 3D, and the Oculus Rift device. Our experimental setup included a helmet for virtual reality presentation, the Oculus Rift, equipped with two Pentile OLED displays, with 1080 × 1200 resolution per eye, a 90 Hz refreshing rate and a 110° field of view. The device includes also features for rotation, position tracking and integrated headphones that provide a 3D sound effect (Daher et al., 2021; Grasso et al., 2019; Grasso et al., 2020; Lucifora et al., 2020). A graphics workstation, equipped with a NVIDIA Titan X graphics card, was used to run the simulation, ensuring a uniform high-resolution rendering of the virtual environment, which was projected to the VR headset. Our VR paradigm, successfully used in a previous study of our group (Lucifora et al., 2022) consisted of three sessions: habituation, acquisition, and extinction. Stimuli of the protocol consisted of two doors of different colours. During the habituation session (duration about 7 min), the participants watched the two different doors (blue and red) for a total of 8 trials (i.e., 4 times the blue door, 4 times the red door) presented in randomized order. During presentation, doors were kept closed for 3 s and opened for 9 s. The inter-trial interval was between 6 and 20 s. After a short break of 60 s, participants were prompted to start the fear acquisition stage (duration about 10 min), when they were ready for it. In the acquisition session, the blue door served as conditioning stimulus (CS+). It was paired (in 80% of all cases (8 of 10 trials)) with a threatening stimulus (i.e., a monster - serving as the unconditioned stimulus – US) jumping in the direction of the participant and screaming (80 dB) to induce a fear response. As in the habituation phase, the duration, and timing of appearance of the US were 3 s (closed door) plus 9 s (opened door). The red-coloured door was not paired with the US (CS−: the safety signal). The acquisition phase involved presentation of 10 CS+ trials and 10 CS− trials. The inter-trial interval was between 6 and 20 s. The conditioned fear response was extinguished in the extinction stage, which took place 5 min after the acquisition phase. During the break, participants stayed out of the virtual reality environment. For extinction learning, the blue door (CS+) was no longer accompanied by the jumping and screaming monster. This session consisted of two blocks, early and late extinction (duration about 10 min), involving presentation of a total of 20 trials (10 times the red door and 10 times the blue door), presented in randomized order, as in the acquisition phase.
Skin conductance
Skin conductance response (SCR) was measured via eSense (Mindfield Biosystems, Inc. Berlin. Germany) on a MEIZU M5C M710H, with electrodes attached to the middle and index fingers by means of Velcro straps. The electrodes were connected to the device using the audio connection input. eSense acquired data at a sampling rate of 5 Hz and the data were exported via email in csv files. The SCR to the CS+ and CS− was calculated in microSiemens (μS) by subtracting the mean SCL during the 2 s prior to stimulus onset from the maximum SCL during the 12 s stimulus presentation duration (3 s closed door plus 9 s opened door).
Procedure
After the participants had given informed consent, they completed the STAI-Y1, the STAI-Y2 and the aVHS questionnaires. Next, participants were connected to the GSR Amp (eSense), and two ring-shaped skin conductance electrodes were placed over the middle and index fingers of the right hand. Then, the virtual reality helmet (Oculus Rift) was placed on the head, and the fear conditioning/extinction task was conducted. Finally, at the end of the experiment participants were debriefed.
Data analysis
Data normality was assessed by visual inspection and using the Shapiro-Wilk test. Statistical comparisons including different variables such as age, gender, STAI, aVHS and SCR scores were conducted via nonparametric tests. We used the Mann-Whitney U test to investigate SCR differences between individuals with low and high aVHS scores for the different sessions of the fear conditioning task; the Wilcoxon Matched Pairs Test to investigate SCR differences associated with CS+ and CS- in each group of participants (see below) for the different sessions of the fear conditioning task; the Spearman correlation index to investigate the relationship between the different variables of interest; and the Chi-square to investigate the association between two categorical variables (e.g., gender). All these analyses were implemented to test the hypothesis of impaired fear conditioning in individuals with high aVHS scores, compared to individuals with low aVHS scores.
The SCR amplitude was determined off-line by subtracting the baseline of 2 s prior to CS presentation from the highest skin conductance level during each CS presentation. This was done for each individual value of each participant. We lost 2.6% of the trials due to technical failure during data collection. For SCR data analysis, a square root transformation was applied to the SCR data to reduce variability, in accordance with previous studies (e.g., Ney et al., 2021; Vicario et al., 2020). SCR scores that fell 3 Standard Deviations above or below each individual mean for each experimental condition were removed as outliers (1.3% of the total). A critical alpha level of α = 0.05 served as significance threshold for all tests. Statistical analysis was performed using STATISTICA (StatSoft. Inc., Tulsa, OK, USA) version 12.0.
Results
SCR data were not normally distributed (Shapiro-Wilk W = .901, p = .032). No significant difference was found for gender (X2= 0.243, p = .621), age (U = 54.00, p = .207), STAI-Y1 (U = 39.5, p = .204) and STAI-Y2 (U = 54.00, p = .763), when comparing individuals with low aVHS scores (≤ 25, N = 14), and high aVHS scores (> 25, N = 11 see table 1 for descriptive statistics).
Table 1.
Descriptive statistics of the variables of interest
| Age | Sex | aVHS | STAI-Y1 | STAI-Y2 | |
|---|---|---|---|---|---|
| Low aVHS | M=23.21 | 5 M, | M=18.07 | M=43.30 | M=46.07 |
| SD=3.09 | 9 F | SD=4.74 | SD=13.13 | SD=10.07 | |
| High aVHS | M=21.81 | 5 M, | M=29.90 | M=38.00 | M=47.00 |
| SD=3.31 | 6 F | SD=4.34 | SD=5.65 | SD=9.05 |
Note. aVHS= adult vaccine hesitancy scale; STAI-Y1= State Trait Anxiety Inventory, Form Y, Subscale 1; STAI-Y1= State Trait Anxiety Inventory, Form Y2, Subscale 2.
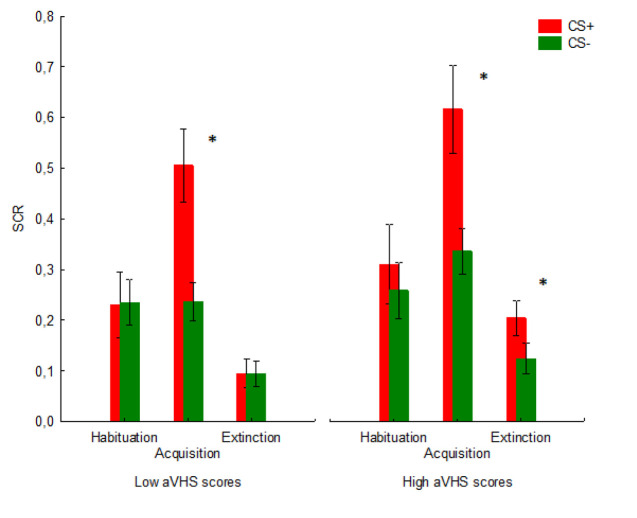
We used the Mann-Whitney U test to separately compare the mean differential [SC+ minus CS-] SCRs associated to the three sessions (i.e., habituation, acquisition, extinction) of individuals with low and high aVHS scores. The group with high aVHS showed a larger differential SCR (Median SCR = +137 μS) as compared to the low aVHS group of participants (Median SCR = +116 μS, U = 25.00, p = .025) in the extinction session. No significant results were found for habituation (U = 64.00, p = .63) and acquisition (U = 73.00, p = .826) sessions.
In a further analysis, we used the Wilcoxon Matched Pairs Test to separately compare the SCR to CS+ vs. CS- in the three sessions (figure 1). Individuals with low aVHS scores successfully extinguished fear to CS+, with no difference between CS+ and CS- in the extinction session (Z = .244, p = .806). In striking contrast, individuals with high aVHS scores failed to extinguish fear to CS+ as compared to CS- (Z = 2.445, p = .014). Moreover, as expected, we found a significant difference between CS+ and CS- for the acquisition session in individuals with both low and high aVHS scores (low aVHS group: Z = 2.793, p = .005; high aVHS group: Z = 2.510, p = .012). On the other hand, no significant between-groups difference was found for the habituation session (low aVHS group: Z = .596, p = .550; high aVHS group: Z= 1.658, p = .097, figure 1).
Figure 1.
Shown are the SCR mean patterns associated for CS+ and CS- in the three sessions (habituation session; Acquisition session; Extinction session). * Indicates a significant difference between CS- and CS+. Vertical bars denote ± standard error of means
We also investigated the pattern of correlations between aVHS, STAI, and SCR differences in the three sessions. The Spearman correlation index did not document significant relations between aVHS and SCR for the habituation (rho = -.009, p = .964), acquisition (rho = -.116, p = .580) or the extinction (rho = .392, p = .071) session, although the trend reported in the extinction session is in line with the results provided when comparing individuals with low and high aVHS scores. Moreover, no relationship was found between trait or state anxiety and SCR for the three conditions (STAI-Y2: habituation, rho = .348, p = .122; acquisition, rho = -.111, p = .619; extinction, rho = -.362, p = .127. STAI-Y1: habituation, rho = .412, p = .063; acquisition, rho = .115, p = .610; extinction, rho = -.126, p = .615). Finally, no correlation was found between STAI-Y2 (rho = .076, p = .735) or STAI-Y1 (rho = -.222, p = .319) anxiety, and aVHS scores.
Discussion
In this study we sought to investigate the role of abnormal fear processing to explain vaccine hesitancy during COVID-19 pandemics. Hence, we tested the physiological response in a Pavlovian fear conditioning-extinction paradigm, which is a valid and widely used translational model for the experimental investigation of mechanisms underlying pathological fear and anxiety (Battaglia et al., 2023; Milad & Quirk, 2012; Ney et al., 2021; Vicario et al., 2017; Vicario et al., 2019).
Overall, our findings provide the first psychophysiological evidence that the general process of fear extinction learning is impaired in highly vaccine hesitant individuals. On the other hand, no difference between individuals with low and high vaccine hesitancy scores was found for the fear acquisition session.
Fear acquisition reflects associative learning, where a neutral stimulus is conditioned as fearful (CS+) due to its association with an aversive event. Fear extinction reflects a re-learning process in which individuals arerepeatedly exposed to a CS+ without the associated aversive outcome (Lonsdorf et al., 2017). Consequently, individuals form a new memory, which suppresses the expression of the original fear memory through a relearning inhibitory mechanism (Myers & Davis, 2007). Evidence for a normal fear acquisition process together with evidence for impaired fear extinction suggests that the antivaccination attitude may be mainly linked to abnormalities of re-learning inhibitory mechanisms, which might indicate a difficulty to suppress fear memory traces. Failures to inhibit fear memory traces have been reported in PTSD and anxiety disorders (Rabinak et al., 2017), showing a SCR pattern like the one we found in participants with high aVHS scores. Additionally, failures in extinguishing fear have been associated with poor stress management (Maren & Holmes, 2016), which is also known to predict vaccination hesitancy (Zhang et al., 2022). Interestingly, the above-mentioned dissociation between fear acquisition and extinction could reflect the role of the ventromedial prefrontal cortex in mediating mechanisms of fear extinction learning, but not fear acquisition (Phelps et al., 2004; Milad et al., 2007). Future research is needed to directly evaluate this possibility.
Our results do not confirm the predictive role of state and trait anxiety on SCR associated to fear acquisition and extinction sessions. Importantly, no difference in state and trait anxiety was found between individuals with low and high aVHS scores. Finally, the correlation analysis did not confirm the relevance of both, state and trait anxiety to explain fear acquisition and extinction responses of our participants. This suggests that anxiety may be not relevant for the fear extinction learning deficit of our group of individuals with high vaccine hesitancy.
In our study we did not find evidence of a relationship between vaccine hesitancy and anxiety, as in previous works (Bendau et al., 2021). This might be due to the relatively low number of participants, or because the STAI does not allow to assess the relationship between vaccination and anxiety, in contrast to previous studies that have used more specific scales that focus on anxiety in response to viral pandemics (e.g., the COVID-19-Anxiety Questionnaire, Petzold et al., 2020).
Our study has some relevant limitations. First, sample size was relatively small. Therefore, further investigations involving larger participant samples are needed. A further limitation concerns the gender disbalance in our study, with a higher number of female participants. However, gender was comparable in the two groups of individuals with low and high aVHS scores. This allows to exclude a role of gender in accounting for the different physiological reactivity in the two groups. Further investigations are needed to study the predictive role of other variables that have been associated with antivaccination attitudes, such as conspiratorial thinking (Gioia et al., 2023), and sensitivity to misinformation (Larson et al., 2021). Other relevant variables of interest may also include disgust sensitivity, an important landmark of mental discomfort (Culicetto et al., In press). The link with such variables can be addressed by using self-report instruments such as the Misinformation Susceptibility Test (Maertens et al., 2021), the Generic Conspiracist Beliefs Scale (Drinkwater et al., 2020) and the Disgust Sensitivity Scale (Haidt et al., 1994). Finally, the addition of neurophysiological measures to elucidate underlying neural mechanisms would be intriguing and should be the subject of future research.
References
- Akel, K.B., Masters, N.B., Shih, S.F., Lu, Y., & Wagner, A.L. (2021). Modification of a vaccine hesitancy scale for use in adult vaccinations in the United States and China. Human Vaccines and Immunotherapy, 17(8), 2639-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia, S., Di Fazio, C., Vicario, C. M., & Avenanti, A. (2023). Neuropharmacological Modulation of N-methyl-D-aspartate, Noradrenaline and Endocannabinoid Receptors in Fear Extinction Learning: Synaptic Transmission and Plasticity. International Journal of Molecular Science, 24(6), 5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendau, A., Plag, J., Petzold, M. B., & Ströhle, A. (2021). COVID-19 vaccine hesitancy and related fears and anxiety. International Immunopharmacology, 97, 107724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, W. S., & Budenz, A. (2020). Considering Emotion in COVID-19 Vaccine Communication: Addressing Vaccine Hesitancy and Fostering Vaccine Confidence. Health Communication, 35, 1718-1722. [DOI] [PubMed] [Google Scholar]
- Culicetto, L., Ferraioli, F., Lucifora, C., Falzone, A., Martino, G., Craparo, G., Avenanti, A., & Vicario, C. M. (2023). Disgust as a transdiagnostic index for mental illness: A narrative review on clinical populations. Bulletin of the Menninger Clinic, 87(Supplement A), 2023. [DOI] [PubMed] [Google Scholar]
- Daher, K., Capallera, M., Lucifora, C., Casas, J., Meteier, Q., El Kamali, M., & Mugellini, E. (2021). Empathic interactions in automated vehicles. In Extended Abstracts of the 2021 CHI Conference on Human Factors in Computing Systems, 1–4. [Google Scholar]
- Dawson, M. E., Schell, A. M., & Filion, D. L. (1990). The electrodermal system. In Cacioppo J. T. & Tassinary L. G. (Eds.), Principles of psychophysiology: Physical, social, and inferential elements (pp. 295-324). Cambridge, England: Cambridge University Press. [Google Scholar]
- Drinkwater, K. G., Dagnall, N., Denovan, A., & Neave, N. (2020). Psychometric assessment of the Generic Conspiracist Beliefs Scale. PLoS One, 15(3), e0230365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, D., Lambe, S., Yu, L. M., Freeman, J., Chadwick, A., Vaccari, C., Waite, F., Rosebrock, L., Petit, A., Vanderslott, S., Lewandowsky, S., Larkin, M., Innocenti, S., McShane, H., Pollard, A. J., & Loe, B. S. (2021). Injection fears and COVID-19 vaccine hesitancy. Psychological Medicine, 11, 1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia, F., Imperato, C., Boursier, V., Franceschini, C., Schimmenti, A., & Musetti, A. (2023). The role of defense styles and psychopathological symptoms on adherence to conspiracy theories during the COVID-19 pandemic. Scientific Reports, 13, 3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso, G., Lucifora, C., Perconti, P., & Plebe, A. (2019). Evaluating mentalization during driving. In VEHITS 2019 - Proceedings of the 5th International Conference on Vehicle Technology and Intelligent Transport Systems. [Google Scholar]
- Grasso, G. M., Lucifora, C., Perconti, P., & Plebe, A. (2020). Integrating human acceptable morality in autonomous vehicles. In International Conference on Intelligent Human Systems Integration (pp. 41-45). Springer. [Google Scholar]
- Haaker, J., Maren, S., Andreatta, M., Merz, C. J., Richter, J., Richter, S. H., Shira, M. D., Lange, M. D., Jüngling, K., Nees, F., Seidenbecher, T., Fullana, M. A., Carsten T. W., Lonsdorf, T. B. (2019). Making translation work: Harmonizing cross-species methodology in the behavioral neuroscience of Pavlovian fear conditioning. Neuroscience and Biobehavioral Reviews, 107, 329-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidt, J., McCauley, C., & Rozin, P. (1994). Individual differences in sensitivity to disgust: A scale sampling seven domains of disgust elicitors. Personality and Individual Differences, 16, 701-713. [Google Scholar]
- Larson, H. J., Gakidou, E., & Murray, C. J. L. (2021). The Vaccine-Hesitant Moment. New England Journal of Medicine, 387, 58-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledda, C., Costantino, C., Liberti, G., & Rapisarda, V. (2022). The Italian Version of the Adult Vaccine Hesitancy Scale (aVHS) for the Working-Age Population: Cross-Cultural Adaptation, Reliability, and Validity. Vaccines, 10, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf, T. B., Menz, M. M., Andreatta, M., Fullana, M. A., Golkar, A., Haaker, J., Heitland, I., Hermann, A., Kuhn, M., Kruse, O., Meir Drexler, S., Meulders, A., Nees, F., Pittig, A., Richter, J., Römer, S., Shiban, Y., Schmitz, A., Straube, B., Vervliet, B., Wendt, J., Baas, J. M. P., & Merz, C. J. (2017). Don't fear 'fear conditioning': Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neuroscience and Biobehavioral Reviews, 77, 247-285 [DOI] [PubMed] [Google Scholar]
- Lucifora, C., Grasso, G. M., Nitsche, M. A., D’Italia, G., Sortino, M., Salehinejad, M. A., Falzone, A., Avenanti, A., & Vicario, C. M. (2022). Enhanced fear acquisition in individuals with evening chronotype: A virtual reality fear conditioning/extinction study. Journal of Affective Disorders, 311, 344-352. [DOI] [PubMed] [Google Scholar]
- Lucifora, C., Grasso, G. M., Perconti, P., & Plebe, A. (2020). Moral dilemmas in self-driving cars [Dilemmi morali nelle automobili a guida autonoma]. Rivista Internazionale di Filosofia e Psicologia, 11, 238–250. [Google Scholar]
- Maren, S., & Holmes, A. (2016). Stress and Fear Extinction. Neuropsychopharmacology, 41, 58-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens, R., Götz, F. M., Golino, H. F., Roozenbeek, J., Schneider, C. R., Kyrychenko, Y., Kerr, J. R., Stieger, S., McClanahan, W. P., Drabot, K., He, J., & van der Linden, S. (2023). The Misinformation Susceptibility Test (MIST): A psychometrically validated measure of news veracity discernment. Behavior Research Methods. 10.3758/s13428-023-02124-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marković, V., Vicario, C. M., Yavari, F., Salehinejad, M. A., & Nitsche, M. A. (2021). A Systematic Review on the Effect of Transcranial Direct Current and Magnetic Stimulation on Fear Memory and Extinction. Frontiers in Human Neuroscience, 15, 655947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad, M. R., & Quirk, G. J. (2012). Fear extinction as a model for translational neuroscience: Ten years of progress. Annual Review of Psychology, 63, 129-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad, M. R., Wright, C. I., Orr, S. P., Pitman, R. K., Quirk, G. J., & Rauch, S. L. (2007). Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry, 62, 446–454. [DOI] [PubMed] [Google Scholar]
- Murphy, J., Vallières, F., Bentall, R. P., Shevlin, M., McBride, O., Hartman, T. K., ... Hyland, P. (2021). Psychological characteristics associated with COVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nature Communication, 12, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, K. M. & Davis, M.(2007). Mechanisms of Fear Extinction. Molecular Psychiatry, 12(2), 120–150. [DOI] [PubMed] [Google Scholar]
- Ney, L. J., Vicario, C. M., Nitsche, M. A., & Felmingham, K. L. (2021). Timing matters: Transcranial direct current stimulation after extinction learning impairs subsequent fear extinction retention. Neurobiology of Learning and Memory, 177, 107356. [DOI] [PubMed] [Google Scholar]
- Pedrabissi, L., & Santinello, M. (1989). Verifica della validità dello STAI forma Y di Spielberger [Verification of the validity of the STAI, Form Y, by Spielberger]. Giunti Organizzazioni Speciali, 191-192, 11–14. [Google Scholar]
- Petzold, M. B., Bendau, A., Plag, J., Pyrkosch, L., Mascarell Maricic, L., Betzler, F., Rogoll, J., Große, J., & Ströhle, A. (2020). Risk, resilience, psychological distress, and anxiety at the beginning of the COVID-19 pandemic in Germany. Brain and Behavior, 10, e01745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps, E. A., Delgado, M. R., Nearing, K. I., & LeDoux, J. E. (2004). Extinction learning in humans: Role of the amygdala and vmPFC. Neuron, 43, 897–905. [DOI] [PubMed] [Google Scholar]
- Rabinak, C. A., Mori, S., Lyons, M., Milad, M. R., & Phan, K. L. (2017). Acquisition of CS-US contingencies during Pavlovian fear conditioning and extinction in social anxiety disorder and posttraumatic stress disorder. Journal of Affective Disorders, 207, 76-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum, L. (2021). Escaping Catch-22 — Overcoming Covid vaccine hesitancy. New England Journal of Medicine, 384, 1367-1371. [DOI] [PubMed] [Google Scholar]
- Sekizawa, Y., Hashimoto, S., Denda, K., Ochi, S., & So, M. (2022). Association between COVID-19 vaccine hesitancy and generalized trust, depression, generalized anxiety, and fear of COVID-19. BMC Public Health, 22, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, A. (2022, December 23). China estimates COVID surge is infecting 37 million people a day - Bloomberg News. Reuters. Retrieved from https://www.reuters.com/business/healthcare-pharmaceuticals/china-estimates-covid-surge-is-infecting-37-million-people-day-bloomberg-news-2022-12-23/
- van der Linden, S. (2022). Misinformation: Susceptibility, spread, and interventions to immunize the public. Nature Medicine, 28, 460-467. [DOI] [PubMed] [Google Scholar]
- Vicario, C. M., Nitsche, M. A., Felmingham, K. (2017). Forgetting fear associations through tES: Which memory process might be critical? Translational Psychiatry, 7, e1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario, C. M., Nitsche, M. A., Hoysted, I., Yavari, F., Avenanti, A., Salehinejad, M. A., & Felmingham, K. L. (2020). Anodal transcranial direct current stimulation over the ventromedial prefrontal cortex enhances fear extinction in healthy humans: A single-blind sham-controlled study. Brain Stimulation, 13, 489-491. [DOI] [PubMed] [Google Scholar]
- Vicario, C. M., Salehinejad, M. A., Felmingham, K., Martino, G., & Nitsche, M. A. (2019). A systematic review on the therapeutic effectiveness of non-invasive brain stimulation for the treatment of anxiety disorders. Neuroscience and Biobehavioral Review, 96, 219-231. [DOI] [PubMed] [Google Scholar]
- WMA (2013). World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA, 310(20), 2191–2194. [DOI] [PubMed] [Google Scholar]
- Zhang, H., Li, Y., Peng, S., Jiang, Y., Jin, H., & Zhang, F. (2022). The Effect of Health Literacy on COVID-19 Vaccine Hesitancy among community population in China: The Moderating Role of Stress. Vaccine, 40, 4473–4478. [DOI] [PMC free article] [PubMed] [Google Scholar]