Abstract
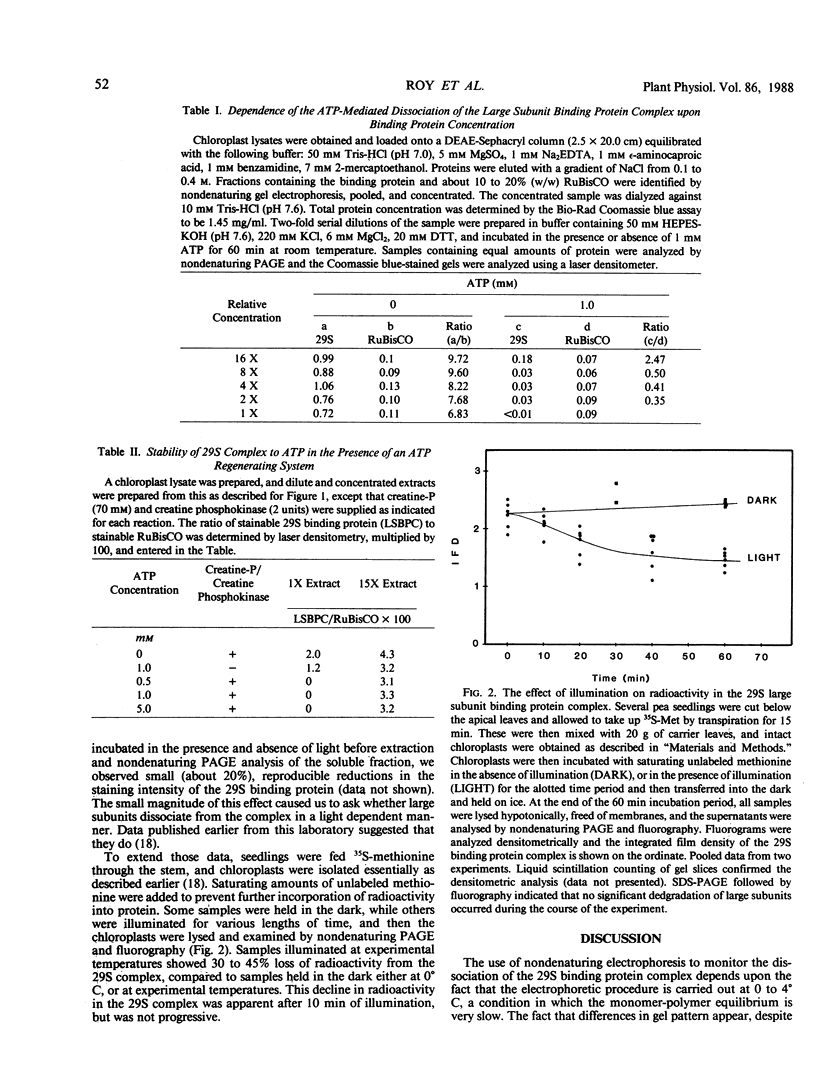
We are studying the stability of the binding protein which associates with newly synthesized large subunits of ribulose bisphosphate carboxylase. In chloroplast extracts, it has been shown that a dodecameric complex of the large subunit binding protein dissociates extensively into binding protein monomers and 7S (117 kilodaltons) large subunit-containing complexes in the presence of ATP. The concentrations of ATP which bring this about are quite low, prompting some investigators to suggest that the dodecameric complex might not exist in vivo. We have found, however, that in concentrated chloroplast extracts, at protein concentrations which are closer to those which occur in organello, the dissociation of the binding protein complex by ATP is much less extensive. For this reason, we have tested the stability of the binding protein in organello, by illuminating chloroplasts followed by lysis and polyacrylamide gel electrophoresis of the extracts. Radioactive large subunits associated with the dodecameric binding protein dissociated extensively in the light. The results are consistent with the idea that the high molecular weight form of the binding protein can function as a reservoir of large subunits which can be tapped in vivo, in a reaction dependent on light and ATP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barraclough R., Ellis R. J. Protein synthesis in chloroplasts. IX. Assembly of newly-synthesized large subunits into ribulose bisphosphate carboxylase in isolated intact pea chloroplasts. Biochim Biophys Acta. 1980 Jun 27;608(1):19–31. doi: 10.1016/0005-2787(80)90129-x. [DOI] [PubMed] [Google Scholar]
- Bloom M. V., Milos P., Roy H. Light-dependent assembly of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1013–1017. doi: 10.1073/pnas.80.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon S., Wang P., Roy H. Inhibition of ribulose bisphosphate carboxylase assembly by antibody to a binding protein. J Cell Biol. 1986 Oct;103(4):1327–1335. doi: 10.1083/jcb.103.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Post-translational transport into intact chloroplasts of a precursor to the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6110–6114. doi: 10.1073/pnas.75.12.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmingsen S. M., Ellis R. J. Purification and properties of ribulosebisphosphate carboxylase large subunit binding protein. Plant Physiol. 1986 Jan;80(1):269–276. doi: 10.1104/pp.80.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lennox C. R., Ellis R. J. The carboxylase-large-subunit-binding protein: photoregulation and reversible dissociation. Biochem Soc Trans. 1986 Feb;14(1):9–11. doi: 10.1042/bst0140009. [DOI] [PubMed] [Google Scholar]
- Milos P., Roy H. ATP-released large subunits participate in the assembly of RuBP carboxylase. J Cell Biochem. 1984;24(2):153–162. doi: 10.1002/jcb.240240206. [DOI] [PubMed] [Google Scholar]
- Nivison H. T., Jagendorf A. T. Factors permitting prolonged translation by isolated pea chloroplasts. Plant Physiol. 1984 Aug;75(4):1001–1008. doi: 10.1104/pp.75.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy H., Bloom M., Milos P., Monroe M. Studies on the assembly of large subunits of ribulose bisphosphate carboxylase in isolated pea chloroplasts. J Cell Biol. 1982 Jul;94(1):20–27. doi: 10.1083/jcb.94.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seftor R. E., Jensen R. G. Causes for the Disappearance of Photosynthetic CO(2) Fixation with Isolated Spinach Chloroplasts. Plant Physiol. 1986 May;81(1):81–85. doi: 10.1104/pp.81.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]