Abstract
Objective:
The aim was to analyze the incidence trend and annual average incidence change of type 1 diabetes (T1DM) in the population <18 years of age in Malatya province.
Materials and Methods:
Medical files of patients followed up with T1DM in pediatric endocrinology clinics were reviewed. The data for the child census was taken from the Turkish Statistical Institute (TUIK), and T1DM incidence was analyzed according to the calendar year, gender, and age groups. Recently diagnosed T1DM patients per 100 000 children per year were calculated. In addition, the trend in annual incidence change over the period 2007-2019 was analyzed.
Results:
The mean incidence of T1DM during the 13 years was 13.1/105 child years (13.8/105 child years for girls and 12.4/105 child years for boys). During the 13-year follow-up period, a significant increasing trend in the incidence of T1DM was detected. The average annual percent change (AAPC) was 8.3%. According to age groups, the average AAPC was 8.1% between 0 and 4 years old, 9.4% between 5 and 9 years old, 12.1% between 10 and 14 years old, and 30.1% between 15 and 17 years old.
Conclusion:
The incidence of T1DM in children under 18 years of age in Malatya, one of the largest cities in the Eastern Anatolia region of Turkey, was determined as 13.1/105 child years in the last 13 years and the average annual increase rate was 8.3%.
Keywords: Type 1 diabetes mellitus, epidemiology, incidence, pediatrics, trends
What is already known on this topic?
The incidence and prevalence of type 1 diabetes mellitus are increasing among children globally.
Type 1 diabetes mellitus in children peaks in the 5-9 and 10-14 age groups.
Type 1 diabetes mellitus usually peaks in autumn and winter.
What this study adds on this topic?
In our study, the incidence of type 1 diabetes mellitus is 13.1/100 000 on average in the period 2007-2019.
During this 13-year period, a significant upward trend in the incidence of type 1 diabetes mellitus was observed.
The annual percent change was 8.3%.
Introduction
Type 1 diabetes mellitus (T1DM) is an important chronic, systemic, and metabolic disease affecting children and adolescents. The incidence of T1DM shows an increasing trend among children worldwide. In addition, the reported rates of T1DM incidence vary greatly between different populations. These variations are explained by differences in ethnicity, geographical zone, and industrial improvement levels.1-7 The incidence of T1DM is higher in children, but the onset of T1DM can occur at any age.8 The global epidemiology of childhood-onset T1DM is well defined, with estimates updated biannually in the International Diabetes Federation (IDF) Diabetes Atlas.9 In a systematic review, the authors reported the epidemiology of the incidence of T1D in young adults (>15 years) compared with childhood-onset T1D (<15 years).7
Multinational studies such as DIAMOND, EURODIAB, and SERACH study are available to determine the incidence and prevalence of T1DM. According to the first multinational report published in the 2000s (involving 50 countries), the incidence of T1DM in children <14 years of age was 26/105 child years. The global incidence of T1DM in children <15 years of age is quite variable, and it is very high in Finland and relatively low in China.10,11
Turkey is located between Europe and Asia. Reports on childhood T1DM incidence and incidence trends in Turkey are insufficient and vary between 7.2/105 and 16.7/105 child years.12-15 All but one of these studies were limited to short evaluation periods, and 3 of the 4 reports were regional reports. These limitations make it difficult to estimate the trend of T1D incidence in this part of the world. In addition, it has been reported that the incidence of T1D varies approximately 2-fold across the country and Malatya is among the provinces with the highest incidence rate in Turkey. The reasons for these differences and increases have not been clearly determined yet. Therefore, based on population data in the 13 years from 2007 to 2019, this study focused on determining the annual incidence of T1DM, the incidence trend, and the mean annual percentage change in incidence in children <18 years of age in the city of Malatya, located in the eastern region of Turkey.
Materials and Methods
Study Setting and Participants
This study included children <18 years of age who were newly diagnosed with T1DM between January 1, 2007, and December 31, 2019. There are 2 pediatric endocrinology centers in Malatya, and this study was conducted jointly by these 2 centers. Pediatric patients diagnosed with diabetes are sent to these pediatric endocrinology departments and regularly recorded in their health records. Children under the age of 18 in Turkey are included in the scope of general health insurance. Hence, diagnosis, treatment, and rehabilitation services are provided free of charge for all children. All newly diagnosed children with T1DM residing in Malatya, except refugees, were included in the study. Children with diabetes are followed up in the pediatric endocrinology centers at least once a year. Cases temporarily residing in Malatya at the time of diagnosis of T1DM were excluded from the study, while cases diagnosed outside Malatya with a permanent residence address in Malatya were included. The population data was taken from the population registration system based on the address reported by the Turkish Statistical Institute (TUIK).16
Data Collection and Definitions
The study was conducted as a retrospective file review. Exclusion criteria were cases with syndromic diabetes, maturity-onset diabetes of the young (MODY diabetes), type 2 diabetes, neonatal diabetes, and transient hyperglycemia secondary to diseases such as pancreatitis, steroid use, Cushing's syndrome, chemotherapy-induced hyperglycemia, and lipodystrophy. The diagnosis of T1DM for children is based on the ISPAD Clinical Practice Consensus Guideline.17 Children with diabetes were categorized into 4 groups according to age: <5, 5-9, 10-14, and 15-17 years old. Furthermore, the study time was classified into 3 periods: 2007-2010, 2011-2014, and 2015-2019. In the T1DM diagnosis, birth dates were used to determine any seasonal differences. In addition, the trend in incidence change over the 13-year observation period was examined.
Ethics Committee Approval
Ethical approval for the study was given by the Ethics Committee of Malatya Training and Research Hospital (approval number: 23536505-604.02).
Statistical Analysis
Incidence rates in the study were calculated as the number of cases per 100 000 person-years with a 95% CI estimated using the normalized approach. To assess the effect of time (years) on the incidence of T1DM, a Poisson regression model with age and gender groups as factors were used. P < .05 was considered statistically significant. Statistical analyses were performed with the Statistical Package for Social Sciences for Windows, version 17.0 (SPSS Inc. Chicago, Ill, USA). Trend analyses for incidence rates were calculated using Joinpoint Trend Analysis Software, version 4.9.0.0 (March 2021). CIs for trend analysis and average annual percent change (AAPC) were estimated for the entire 13-year follow-up from 2007 to 2019.
Results
Demographic Characteristics
A total of 395 children aged <18 years were diagnosed with T1DM during the 13-year study period. Of the cases, 203 (51.4%) were female, and 192 (48.6%) were male, with a male/female ratio of 0.95:1. The mean ± SD age at diagnosis was 8.8 ± 4.4 years, although the age range, as expected, was very broad (0.6-17.9 years). The age at diagnosis was similar (P = .567) in girls (8.7 ± 4.3 years) and boys (9.0 ± 4.3 years). Of the cases, 35.9% were in the 5-9 years age group, 33.2% in the 10-14 age group, 23% in the 0-4 age group, and 7.8% in the 15-17 age group. When evaluated according to the seasons of admission, 116 (29.4%) children with diabetes were diagnosed in autumn, 112 (28.4%) in winter, 87 (22.0%) in summer, and 80 (20.3%) in spring. Of the children with diabetes, 107 (27.1%) were born in summer, 104 (26.3%) in autumn, 96 (24.3%) in winter, and 88 (22.3%) in spring.
Incidence Rates
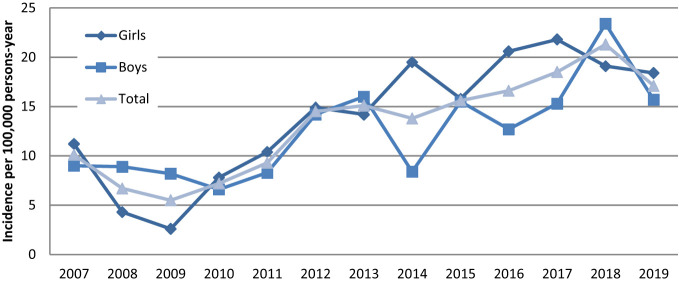
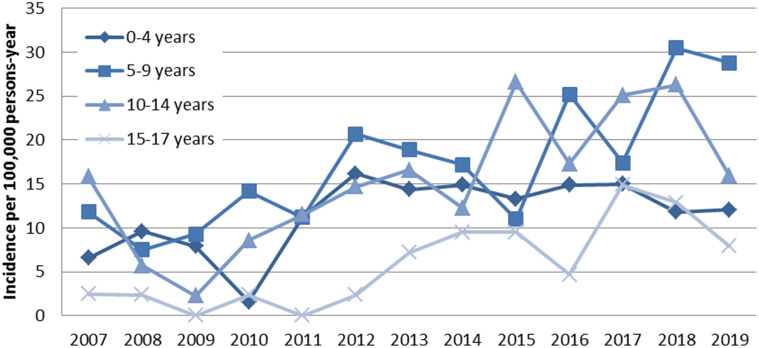
Census data for Malatya covering 2007-2019 were obtained from the Turkish Statistical Institute population registration system. 14 Based on these data, the 13-year mean incidence of T1DM was 13.1/105 (95% CI: 11.8-14.4) child years. In 2018, the annual incidence was the highest, while in 2009, it was the lowest. The T1DM incidence trend of boys and girls by years is shown in Figure 1, while the change in the incidence trend by age group over the years is given in Figure 2.
Figure 1.
Change in type 1 diabetes incidence proportions by sex between 2007 and 2019.
Figure 2.
Change in type 1 diabetes incidence proportions by age groups between 2007 and 2019.
When the study period was divided into 3, the mean annual incidence of T1DM was 7.3/105 (95% CI: 5.6-9.1) child years between 2007 and 2010, 13.2/105 (95% CI: 10.8-15.5) child years between 2011 and 2014, and 17.9/105 (95% CI: 15.3-20.3) child years between 2015 and 2019. The annual incidence of T1DM between the ages of 0-14 years was calculated as 14.6/105 (95% CI: 12.1-17.2) child years. This rate was 15.8/105 (95% CI: 13.1-18.4) child years in girls and 13.6/105 (95% CI: 11.6-15.3) child years in boys. When our study cohort was evaluated according to age groups, higher incidence rates were observed in the 5-9 age group (Table 1). When evaluated according to sex, the mean incidence was 13.8/105 (95% CI: 11.9-15.7) child years in girls, slightly higher than the incidence found for boys which was 12.4/105 (95% CI: 10.6-14.2), although this was not significant (P = .30) (Table 1).
Table 1.
Incidence Rates and Adjusted Incidence Rate Ratios of Type 1 Diabetes Among Children Aged <18 Years Per 100 000 Person-Year by Sex and Age Groups between June 1, 2007 and December 31, 2019
| Cases | Populationa | IR per 105 (95% CI) | IR rate (95% CI) |
P | |
|---|---|---|---|---|---|
| Sex | |||||
| Girls | 203 | 14 66 542 | 13.8 (11.94-15.75) |
Ref | |
| Boys | 192 | 15 42 588 | 12.4 (10.69-14.21) |
0.90 (0.70-1.10) |
.30 |
| Age Group | |||||
| 0-4 years | 91 | 7 87 038 | 11.6 (9.19-13.94) |
Ref | |
| 5-9 years | 142 | 827 093 | 17.2 (14.34-20.0) |
1.49 (1.13-1.95) |
.003 |
| 10-14 years | 131 | 865 949 | 15.1 (12.54-17.72) |
1.31 (0.99-1.73) |
.048 |
| 15-17 years | 31 | 529 050 | 5.8 (3.8-7.9) |
0.51 (0.33-0.77) |
<.0001 |
| Study Period Chronological Subgroups | |||||
| 2007-2010 | 70 | 948 615 | 7.3 (5.65-9.11) |
Ref | |
| 2011-2014 | 123 | 929 380 | 13.2 (10.89-15.57) |
1.79 (1.33-2.44) |
<.0001 |
| 2015-2019 | 202 | 1 131 135 | 17.9 (15.39-20.32) |
2.42 (1.83-3.22) |
<.0001 |
| Total | 395 | 3 009 130 | 13.1 (11.83-14.42) |
1.80 (1.38-2.33) |
<.0001 |
CI, confidence interval; IR, incidence rate; ref, reference category.
aThirteen-year aggregated annual population.
Incidence trends
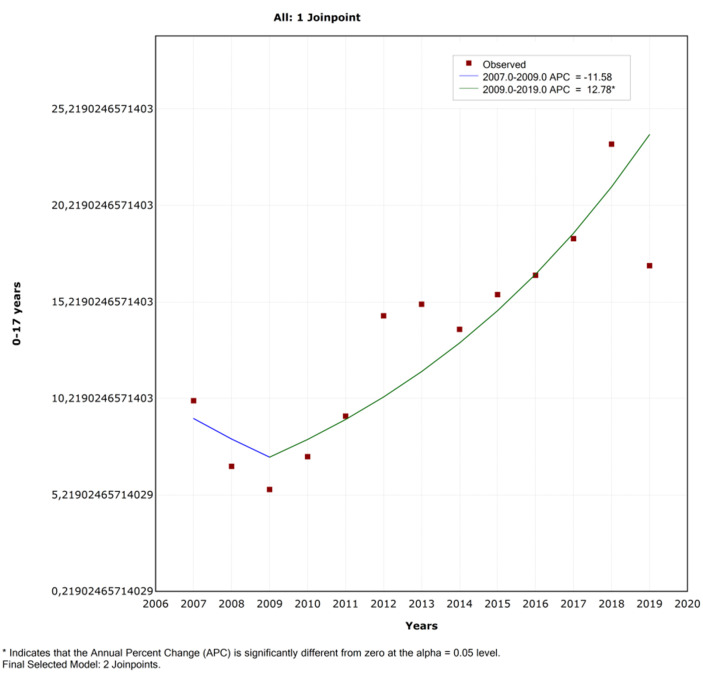
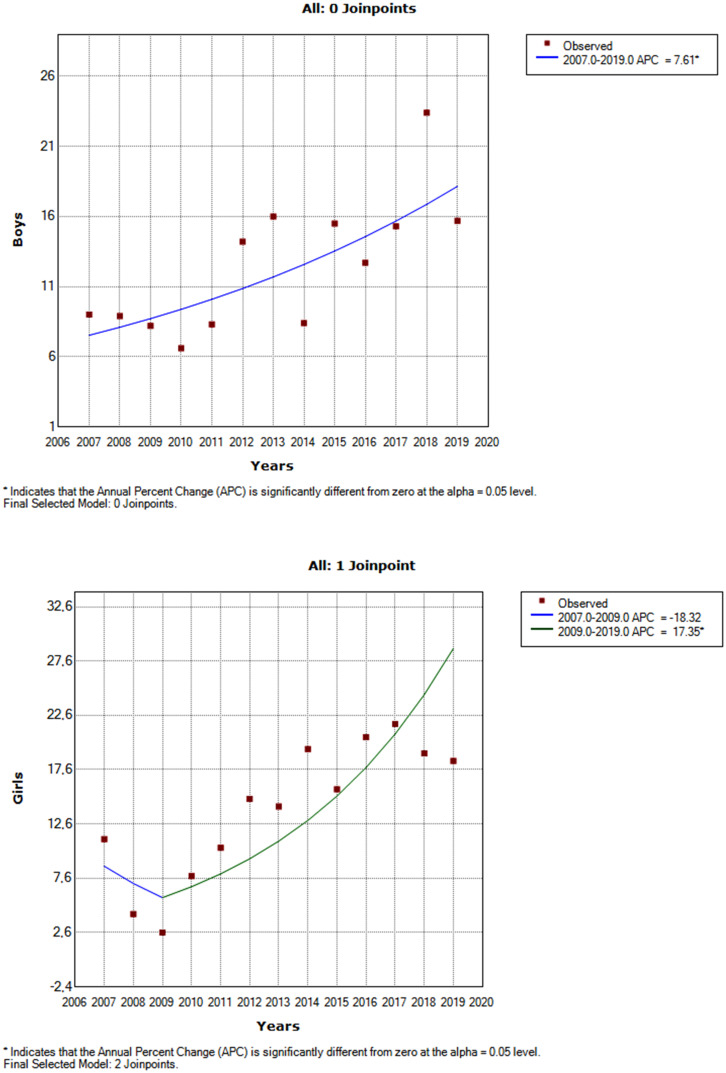
Using Joinpoint analysis, a significant increasing trend in the incidence of T1DM was detected over a total observation period of 13 years. The AAPC was 8.3% (Table 2 and Figure 3). As seen in Table 2, subgroup analysis showed a significant increasing trend in girls after 2009. Additionally, a significant increasing trend was observed in boys without Joinpoint; the AAPC was 7.61% (95% CI: 3.3-12.2). The graphs by sex can be seen in Figure 4. Comparison of the 4 age groups indicated that the increase in T1DM diagnosis trend was significant for each group (Table 2). The AAPC was 30.1%, 12.1%, 9.41%, and 8.1% for the age groups 15-17 years, 10-14 years, 5-9 years, and 0-4 years, respectively.
Table 2.
Results of the Trend Analysis Incidence of Type 1 Diabetes Among Children Aged <18 Years with Joinpoint Regression by Sex and Age Groups between June 1, 2007 and December 31, 2019
| Average Annual Rate | Trend 1 | Trend 2 | AAPC | Cl | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | APC | P | Years | APC | P | ||||||
| Gender | Girls | 13.89 | 2007-2009 | −18.3 | .481 | 2009-2019 | 17.4* | <.001 | 10.5** | 0.4 to 21.6 | .032 |
| Boys | 12.48 | 2007-2019 | 7.61* | .002 | 7.61** | 3.3 to 12.2 | .002 | ||||
| Age | 0-4 years | 11.5 | 2007-2019 | 8.10* | .089 | 8.1** | −1.4 to 18.5 | .039 | |||
| 5-9 years | 17.1 | 2007-2019 | 9.41* | <.001 | 9.41** | 4.6 to 14.5 | <.001 | ||||
| 10-14 years | 15.1 | 2007-2019 | 12.1* | .015 | 12.1** | 2.7 to 22.4 | .015 | ||||
| 15-17 years | 5.8 | 2007-2019 | 30.1* | .023 | 30.1** | 4.4 to 62.1 | .023 | ||||
| Total | 0-17 years | 13.1 | 2007-2009 | −11.6 | .647 | 2009-2019 | 12.8* | <.001 | 8.3** | −1.1 to 18.6 | .045 |
AAPC, average annual percent change; APC, annual percent change.
* APC is significantly different from zero at the alpha = 0.05 level.
**AAPC is significantly different from zero at the alpha = 0.05 level.
Figure 3.
Joinpoint regression analysis of type 1 diabetes incidence between 2007 and 2019 in children <18 years. APC, annual percent change.
Figure 4.
Joinpoint regression analysis of type 1 diabetes incidence by sex between 2007 and 2019. APC, annual percent change.
Discussion
There are few reports on the incidence trend of 1DM in Turkish children.15 The T1DM incidence trend in Turkish children is one of the most important results of our community-based study. The mean annual T1DM incidence, incidence trends, and AAPC were calculated over a period of 13 years (2007-2019) in children under 18 years in Malatya, one of the largest cities in Eastern Anatolia. This study, while no significant change was found in the incidence trend in girls between 2007 and 2009, a significant increase was found between 2009 and 2019. Similarly, while no significant change was found in the incidence trend in all cases between 2007 and 2009, a significant increase was found between 2009 and 2019. A significant increasing trend of T1D was detected in the total 13-year observation period; the average annual percent change was 8.3%.
Reports have shown high incidence rates of T1DM in children in Scandinavian countries such as Sweden, Finland, and Scotland.2,18-20 Various studies have shown an increased incidence of T1DM.7,21-23 According to the EURODIAB study published in 2012, the incidence of T1DM was 36.6/105 in Sweden, 33.9/105 in England (Northern Ireland), 17.5/105 in Austria, 19.3/105 in the Czech Republic, 25.1/105 in Denmark, 23.7/105 in Germany (North Rhine-Westphalia), 32.8/105 in Norway, 13.1/105 in Switzerland, and 12.1/105 in Spain.4
Demirbilek et al12 found the incidence of T1DM in children to be 7.2/105 person-years in a single-center study in Diyarbakır, located in Southeastern Anatolia. In a nationwide study on the incidence of T1DM in Turkish children and adolescents, regional differences in incidence rates were reported in Turkey, which is divided into 5 geographical regions. In this nationwide survey, data of people with diabetes were taken from the national health insurance registry for calculating the incidence, which was 10.8/105.13 Recently, in 2018, a T1DM incidence study was conducted in a region including İstanbul, which has a high rate of urbanization and industrialization. The data of children with T1DM attending pediatric endocrinology units in this region were prospectively collected from hospital records; the incidence of T1DM was 8.99/105.14
Some studies in populations with a high incidence of T1DM have reported a slowing of the increase in the incidence rate over the last decade, while overall, the global increase continues.9,22,23 Research in Canada by Fox et al6 showed a slightly increased incidence of T1DM in the 11 years between 2002 and 2013. Reports3,5,23-25 have found that the increase in the incidence rate of T1DM differs significantly across regions in Europe, with the highest increase in incidence rates reported in Central Eastern European countries. In contrast, much lower increases were reported in the Scandinavian countries and the Czech Republic. Between 1989 and 2013, the EURODIAB study5 found a 3.4% annual increase in the incidence of T1DM in children <15 years of age. In the most recent study15 conducted in Turkey, the incidence rate of T1DM was found to be 16.7/105, with an average annual increase of 7.8%.
In our study, while the mean annual incidence of T1DM was 13.1/105 over 13 years, it was 7.3/105 between 2007 and 2010, 13.2/105 between 2011 and 2014, and 17.8/105 between 2015 and 2019. The 2015-2019 incidence was 2.4 times higher than that of the 2007-2010 period. This study reports a significant increase in the incidence of T1DM at an average rate of 8.3% per year (95% CI: −1.1 to 18.6) over the 13-year observation period. In our country, the annual incidence rates were found to be higher in the study by Esen and Okdemir15 (the only study in our country) and in our study compared to the European and American studies.6,5,15,22 Our study is presented in Table 3 in comparison with these studies. These increases were found in all age groups and in both genders. While a statistically insignificant decrease was detected in female cases between 2007 and 2009, an annual average increase of 10.5% (95% CI: 0.4-21.6) was observed between 2009 and 2019. No reason could be suggested to explain the decrease in the earlier period.
Table 3.
| AAPC | |||
|---|---|---|---|
| Country/City | Year | Rate of Increase (%) | 95% CI |
| Turkey, Malatya* | 2007-2019 | 8.3 | −1.1 to 18.6% |
| Turkey, Elazığ | 2009-2019 | 7.8 | 2.7 to 13.1% |
| Finland, whole nation | 1989-2013 | 2.7 | 2.4 to 3.0% |
| Czechia, whole nation | 1989-2013 | 4.7 | 4.3 to 5.0% |
| Norway, whole nation | 1989-2013 | 2.1 | 1.7 to 2.4% |
| Switzerland, whole nation | 1989-2013 | 3.2 | 2.6 to 3.7% |
| UK, Oxford | 1989-2013 | 1.2 | 0.7 to 1.7% |
| Italy, Marche | 1989-2013 | 0.8 | −0.3 to 2.0% |
| Spain, Catalonia | 1989-2013 | 0.5 | 0.0 to 0.9% |
| Canada, British Columbia | 2002-2013 | 1.3 | 0.0 to 2.5% |
| United States** | 2001–2009 | 1.8 | 1.0 to 2.6% |
AAPC, average annual percent change; T1DM, type 1 diabetes mellitus.
*Our study.
**Colorado, Ohio, South Carolina, and Washington.
These results suggested that the incidence of T1DM is increasing in this region of Turkey. Our study shows that the incidence of T1DM in Malatya is similar to countries such as Switzerland and Spain. Based on the limited number of studies, Turkey appears to be in the group of countries where the incidence rate of T1DM among children is moderate. However, the average annual rate of increase was higher than in other European countries. While 70.5% of the population in Turkey was in the provincial and district centers in 2007, this proportion increased to 92.8% in 2019.26 Urbanization can be considered a significant risk factor for T1DM.15,27,28 Factors such as viral infections, unhealthy eating habits, insufficient physical activity, and little use of sunlight, which are more common in densely populated urban communities, may contribute to the development of T1DM in children. Although environmental factors such as high socioeconomic status and increasing urbanization have been suggested to play a role in the increase in T1DM incidence rate, there has been no significant change in socioeconomic, demographic, or public health in Malatya during the 13-year period. Since refugee children were not included in the study and there was no internal migration, genetic factors cannot explain this increase.
The increased incidence of T1DM in children from nations with unchanged genetic makeup is evidence of an important influence of environmental factors as well as genetic factors in the etiology of T1DM. The incidence of T1DM tends to increase in children who migrate from low-frequency countries to high-frequency countries, are born in a new country, or migrate at a young age (younger than 10 years), providing evidence for the influence of environmental factors.27,29,30 In genetically vulnerable people, some environmental factors can trigger the immune system and lead to damage to the beta cells in the pancreas that produce insulin.27,31,32 However, in our study, specific environmental triggers that are postulated to cause the development of T1DM, such as various infections, exposure to microorganisms, gut microbiota profiles, use of probiotics, early cow's milk initiation, exposure to some chemicals, and fluctuating micronutrient intake were not assessed or identified.
Even though the mean annual incidence rates in girls and boys in our cohort were similar, female predominance was observed in the 0-4, 5-9, and 10-14 age groups, while the 15-17 age group was dominated by boys. Male dominance at this age may be due to boys starting puberty later than girls. In general, there is a slight male dominance in high-frequency countries and female dominance in low-frequency countries.21,33-35
In previous reports, different peak ages were found in the incidence of T1DM.2,4,36 It is known that the incidence of T1DM usually peaks in the peripubertal period (10-14 age group). In our cohort, the highest incidence was in the 5-9 age group, and the second highest incidence was found in the 10-14 age group in both genders. The lowest incidence was in the 15-17 group which also exhibited the highest average annual increase rate, while the lowest rate of increase was in the 0-4 age group. The World Health Organization examined standardized incidence information on T1DM in the 1990-1999 DIAMOND study.2 This study showed that children aged 5-9 years were at higher risk of developing T1DM than children aged 0-4. Some countries have reported2,35,36 the highest incidence in the 5-9 age group, while in other countries, this was found in children aged 10-14. In our study, the incidence of T1DM was higher, especially in the winter and autumn seasons. Similar to our results, some countries show higher incidence rates during winter.37,38 Furthermore, the development of pancreatic islet autoimmunity varies seasonally, although a possible association between the season of birth and risk of T1DM has also been reported. Winter birth appears to be protective against T1DM, suggesting that very early environmental factors are important in T1DM.11,39 Some studies40,41 have reported that children born in summer and spring have a higher potential to develop T1DM than those born in other seasons. It has been suggested that seasonally variable environmental factors, such as viral infections and the amount of ultraviolet light available, affect the development of T1DM by affecting immunity and metabolism.42 In our study, children born in the winter and spring seasons had lower T1DM incidence rates. Our data support that the season of birth may have an impact on the development of T1D in children <18 years.
The most important limitation of the study is its local design, and the results cannot, therefore, reflect the entire region. Since the incidence of T1DM varies from country to country and from region to region within a country, our results provide limited information for Turkey. Different ethnic characteristics and heterogeneous socioeconomic status can partially explain these differences.
Conclusion
Incidence of T1DM in children under 18 years of age in Malatya, a largest city in eastern Turkey, was found to be 13.1/105 children/years in the period 2007-2019, and the average annual increase in incidence rate was 8.3%. Considering this increase in the incidence of T1DM, it is crucial to monitor the incidence trends, ideally on a national scale, and identify possible trigger factors in order to develop preventive strategies for individual regions and the country as a whole.
Footnotes
Ethics Committee Approval: Ethical approval for the study was given by the Ethics Committee of Malatya Training and Research Hospital (approval number: 23536505-604.02).
Informed Consent: Written informed consent was obtained from the patients who agreed to take part in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – I.D.; Design – I.D.; Supervision – I.D., A.A., N.C.; Funding – I.D., A.A.; Materials – I.D., A.A., N.C.; Data collection and/or Processing – I.D., N.C., E.O.; Analysis and/or Interpretation – I.D., A.A., E.C., N.C., E.O., L.K.; Literature Review – I.D., A.A.; Writing – I.D., A.A., E.C., N.C., E.O., L.K.; Critical Review – I.D., A.A., E.C., N.C., E.O., L.K.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: This study received no funding.
References
- 1. Variation and trends in incidence of childhood diabetes in Europe. EURODIAB ACE study group. Lancet. 2000;355(9207):873 876. Erratum in: Lancet. 2000;356(9242): 1690. [PubMed] [Google Scholar]
- 2. DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med. 2006;23(8):857 866. ( 10.1111/j.1464-5491.2006.01925.x) [DOI] [PubMed] [Google Scholar]
- 3. Harding JL, Wander PL, Zhang X, et al. The incidence of adult-onset type 1 diabetes: a systematic review from 32 countries and regions. Diabetes Care. 2022;45(4):994 1006. ( 10.2337/dc21-1752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patterson CC, Gyürüs E, Rosenbauer J, et al. Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55(8):2142 2147. ( 10.1007/s00125-012-2571-8) [DOI] [PubMed] [Google Scholar]
- 5. Patterson CC, Harjutsalo V, Rosenbauer J, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989-2013: a multicentre prospective registration study. Diabetologia. 2019;62(3):408 417. ( 10.1007/s00125-018-4763-3) [DOI] [PubMed] [Google Scholar]
- 6. Fox DA, Islam N, Sutherland J, Reimer K, Amed S. Type 1 diabetes incidence and prevalence trends in a cohort of Canadian children and youth. Pediatr Diabetes. 2018;19(3):501 505. ( 10.1111/pedi.12566) [DOI] [PubMed] [Google Scholar]
- 7. Diaz-Valencia PA, Bougnères P, Valleron AJ. Global epidemiology of type 1 diabetes in young adults and adults: a systematic review. BMC Public Health. 2015;15(1):255. ( 10.1186/s12889-015-1591-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391(10138):2449 2462. ( 10.1016/S0140-6736(18)31320-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. International Diabetes Federation. IDF Diabetes Atlas. 10th ed. Brussels: , Belgium, 2021. Available at: https://www.diabetesatlas.org. Accessed 02 Jun 2023. [Google Scholar]
- 10. Harjutsalo V, Sund R, Knip M, Groop PH. Incidence of type 1 diabetes in Finland. JAMA. 2013;310(4):427 428. ( 10.1001/jama.2013.8399) [DOI] [PubMed] [Google Scholar]
- 11. Weng J, Zhou Z, Guo L, et al. Incidence of type 1 diabetes in China, 2010-13: population based study. BMJ. 2018;360:j5295. ( 10.1136/bmj.j5295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Demirbilek H, Özbek MN, Baran RT. Incidence of type 1 diabetes mellitus in Turkish children from the southeastern region of the country: a regional report. J Clin Res Pediatr Endocrinol. 2013;5(2):98 103. ( 10.4274/Jcrpe.954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yeşilkaya E, Cinaz P, Andıran N, et al. First report on the nationwide incidence and prevalence of Type 1 diabetes among children in Turkey. Diabet Med. 2017;34(3):405 410. ( 10.1111/dme.13063) [DOI] [PubMed] [Google Scholar]
- 14. Poyrazoğlu Ş, Bundak R, Yavaş Abalı Z, et al. Incidence of Type 1 diabetes in children aged below 18 years during 2013-2015 in Northwest Turkey. J Clin Res Pediatr Endocrinol. 2018;10(4):336 342. ( 10.4274/jcrpe.0025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Esen I, Okdemir D. Trend of type 1 diabetes incidence in children between 2009 and 2019 in Elazig, Turkey. Pediatr Diabetes. 2020;21(3):460 465. ( 10.1111/pedi.12984) [DOI] [PubMed] [Google Scholar]
- 16. Turkish Statistical Institute. Available at: https://biruni.tuik.gov.tr/medas/. Accessed December 17, 2020. [Google Scholar]
- 17. Mayer-Davis EJ, Kahkoska AR, Jefferies C, et al. ISPAD Clinical Practice Consensus Guidelines 2018: definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes. 2018;19(suppl 27):7 19. ( 10.1111/pedi.12773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cardwell CR, Carson DJ, Patterson CC. Higher incidence of childhood-onset type 1 diabetes mellitus in remote areas: a UK regional small-area analysis. Diabetologia. 2006;49(9):2074 2077. ( 10.1007/s00125-006-0342-0) [DOI] [PubMed] [Google Scholar]
- 19. Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371(9626):1777 1782. ( 10.1016/S0140-6736(08)60765-5) [DOI] [PubMed] [Google Scholar]
- 20. Patterson CC, Rosenbauer J, Neu A. Incidence trends for childhood type 1 diabetes during 1989-2013 in 24 European registries participating in the EURODIAB study. In Pediatric diabetes: Special Issue: Abstracts for the 42nd Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD), Valencia, Spain/International Society for pediatric and Adolescent Diabetes (ISPAD). Sperling MA, editor in chief. Copenhagen: Wiley Blackwell. Munksgaard, 2016. [Google Scholar]
- 21. Zhao Z, Sun C, Wang C, et al. Rapidly rising incidence of childhood type 1 diabetes in Chinese population: epidemiology in Shanghai during 1997-2011. Acta Diabetol. 2014;51(6):947 953. ( 10.1007/s00592-014-0590-2) [DOI] [PubMed] [Google Scholar]
- 22. Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of Type 1 and Type 2 diabetes among youths, 2002-2012. N Engl J Med. 2017;376(15):1419 1429. ( 10.1056/NEJMoa1610187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gregory GA, Robinson TIG, Linklater SE, et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. 2022;10(10):741 760. ( 10.1016/S2213-8587(22)00218-2) [DOI] [PubMed] [Google Scholar]
- 24. Skrivarhaug T, Stene LC, Drivvoll AK, Strøm H, Joner G, Norwegian Childhood Diabetes Study Group. Incidence of type 1 diabetes in Norway among children aged 0-14 years between 1989 and 2012: has the incidence stopped rising? Results from the Norwegian childhood diabetes registry. Diabetologia. 2014;57(1):57 62. ( 10.1007/s00125-013-3090-y) [DOI] [PubMed] [Google Scholar]
- 25. Berhan Y, Waernbaum I, Lind T, Möllsten A, Dahlquist G, Swedish Childhood Diabetes Study Group. Thirty years of prospective nationwide incidence of childhood type 1diabetes: the accelerating increase by time tends to level off in Sweden. Diabetes. 2011;60(2):577 581. ( 10.2337/db10-0813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. T UIK. Turkish statistical institute. Available at: https://data.tuik.gov.tr/Kategori/GetKategori?p=Population-and-Demography-109. Address Based Population Registration System, Population by Province, Age Group and Sex, 04 February 2020. Date of last access 12th November 2020. [Google Scholar]
- 27. Ilonen J, Lempainen J, Veijola R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol. 2019;15(11):635 650. ( 10.1038/s41574-019-0254-y) [DOI] [PubMed] [Google Scholar]
- 28. Szalecki M, Wysocka-Mincewicz M, Ramotowska A, et al. Epidemiology of type 1 diabetes in Polish children: a multicentre cohort study. Diabetes Metab Res Rev. 2018;34(2). ( 10.1002/dmrr.2962) [DOI] [PubMed] [Google Scholar]
- 29. Hussen HI, Persson M, Moradi T. The trends and the risk of type 1 diabetes over the past 40 years: an analysis by birth cohorts and by parental migration background in Sweden. BMJ Open. 2013;3(10):e003418. ( 10.1136/bmjopen-2013-003418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oilinki T, Otonkoski T, Ilonen J, Knip M, Miettinen PJ. Prevalence and characteristics of diabetes among Somali children and adolescents living in Helsinki, Finland. Pediatr Diabetes. 2012;13(2):176 180. ( 10.1111/j.1399-5448.2011.00783.x) [DOI] [PubMed] [Google Scholar]
- 31. Zhou H, Sun L, Zhang S, Zhao X, Gang X, Wang G. Evaluating the causal role of gut microbiota in type 1 diabetes and its possible pathogenic mechanisms. Front Endocrinol. 2020;11:125. ( 10.3389/fendo.2020.00125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xia Y, Xie Z, Huang G, Zhou Z. Incidence and trend of type 1 diabetes and the underlying environmental determinants. Diabetes Metab Res Rev. 2019;35(1):e3075. ( 10.1002/dmrr.3075) [DOI] [PubMed] [Google Scholar]
- 33. Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, Laporte R, Tuomilehto J. The diabetes mondiale (diamond) project group. Incidence of childhood type 1 diabetes worldwide. Diabetes Care. 2000;23(10):1516 1526. ( 10.2337/diacare.23.10.1516) [DOI] [PubMed] [Google Scholar]
- 34. Svensson J, Carstensen B, Mortensen HB, Borch-Johnsen K, Danish Study Group of Childhood Diabetes. Early childhood risk factors associated with type 1 diabetes-is gender important? Eur J Epidemiol. 2005;20(5):429 434. ( 10.1007/s10654-005-0878-1) [DOI] [PubMed] [Google Scholar]
- 35. Serban V, Brink S, Timar B, et al. An increasing incidence of type 1 diabetes mellitus in Romanian children aged 0 to 17 years. J Pediatr Endocrinol Metab. 2015;28(3-4):293 298. ( 10.1515/jpem-2014-0364) [DOI] [PubMed] [Google Scholar]
- 36. Shaltout AA, Wake D, Thanaraj TA, et al. Incidence of type 1 diabetes has doubled in Kuwaiti children 0-14 years over the last 20 years. Pediatr Diabetes. 2017;18(8):761 766. ( 10.1111/pedi.12480) [DOI] [PubMed] [Google Scholar]
- 37. Patterson CC, Gyürüs E, Rosenbauer J, et al. Seasonal variation in month of diagnosis in children with type 1 diabetes registered in 23 European centers during 1989-2008: little short-term influence of sunshine hours or average temperature. Pediatr Diabetes. 2015;16(8):573 580. ( 10.1111/pedi.12227) [DOI] [PubMed] [Google Scholar]
- 38. Svensson J, Lyngaae-Jørgensen A, Carstensen B, Simonsen LB, Mortensen HB, Danish Childhood Diabetes Registry. Long-term trends in the incidence of type 1 diabetes in Denmark: the seasonal variation changes over time. Pediatr Diabetes. 2009;10(4):248 254. ( 10.1111/j.1399-5448.2008.00483.x) [DOI] [PubMed] [Google Scholar]
- 39. Kahn HS, Morgan TM, Case LD, et al. Association of type 1 diabetes with month of birth among U.S. youth: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(11):2010 2015. ( 10.2337/dc09-0891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Songini M, Casu A, Ashkenazi I, Laron Z. Seasonality of birth in children (0-14 years) and young adults (0-29 years) with type 1 diabetes mellitus in Sardinia differs from that in the general population. The Sardinian collaborative group for epidemiology of IDDM. J Pediatr Endocrinol Metab. 2001;14(6):781 783. ( 10.1515/jpem.2001.14.6.781) [DOI] [PubMed] [Google Scholar]
- 41. Vaiserman AM, Carstensen B, Voitenko VP, et al. Seasonality of birth in children and young adults (0-29 years) with type 1 diabetes in Ukraine. Diabetologia. 2007;50(1):32 35. ( 10.1007/s00125-006-0456-4) [DOI] [PubMed] [Google Scholar]
- 42. Stanescu DE, Lord K, Lipman TH. The epidemiology of type 1 diabetes in children. Endocrinol Metab Clin North Am. 2012;41(4):679 694. ( 10.1016/j.ecl.2012.08.001) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a