Abstract
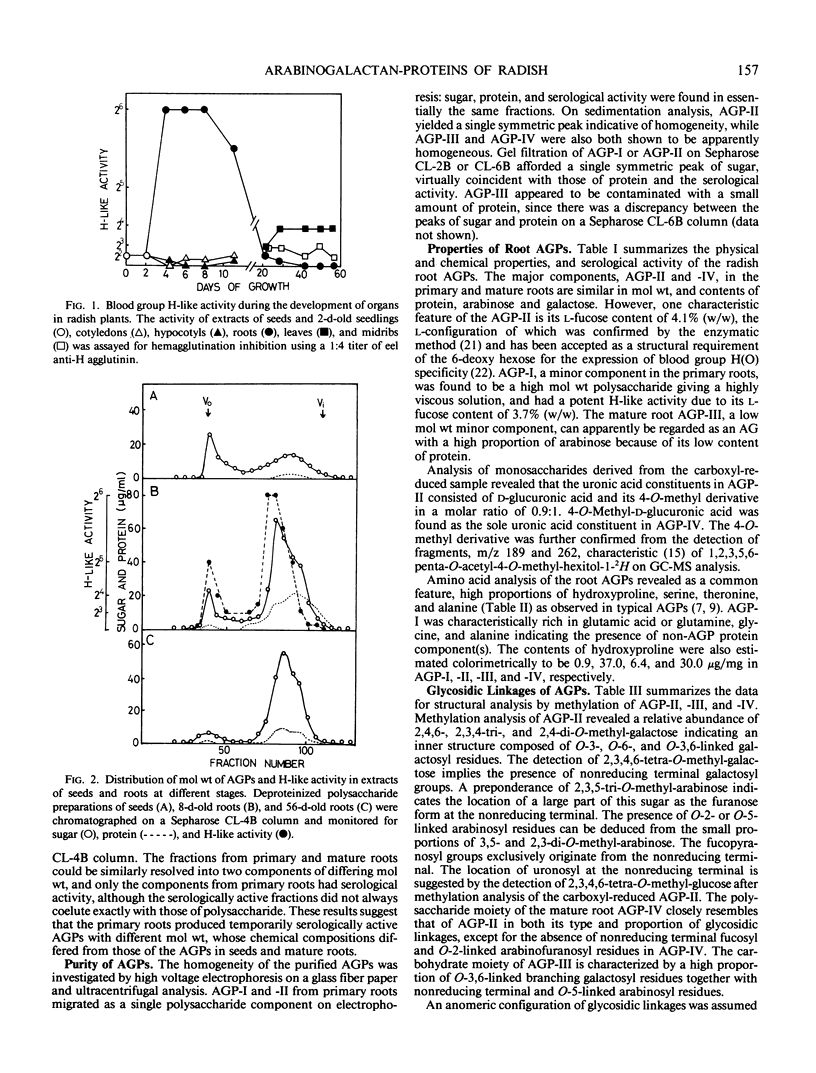
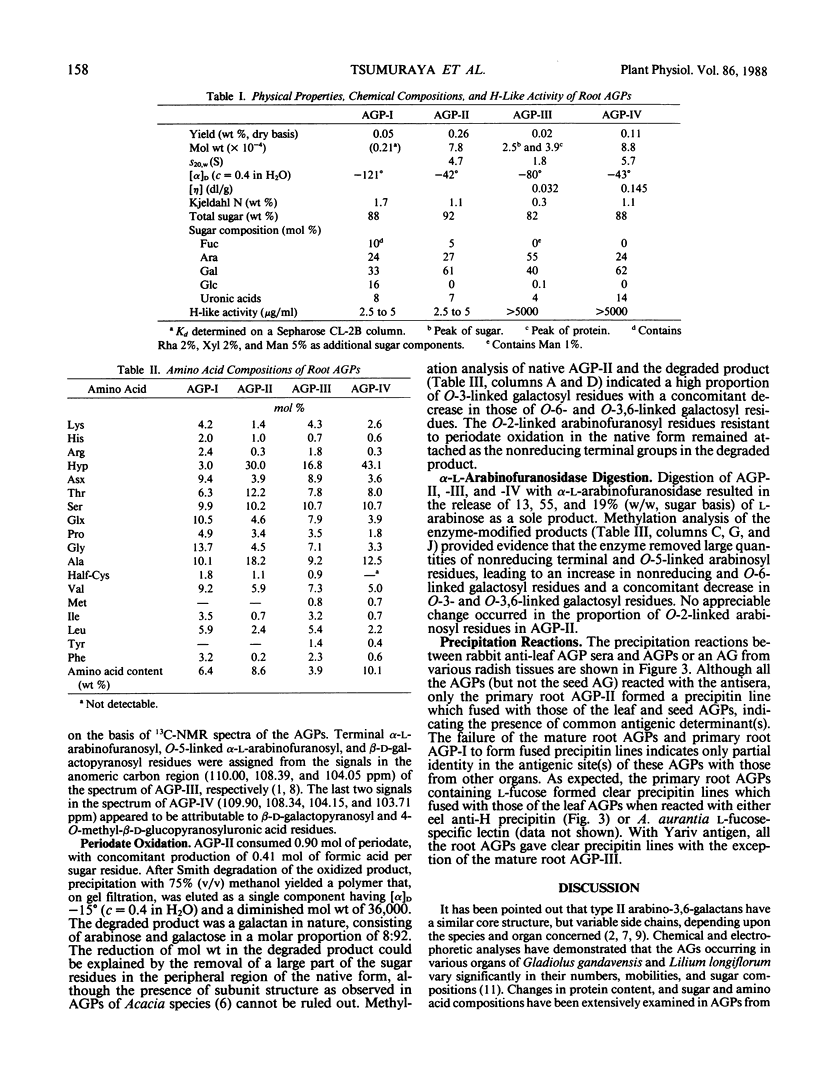
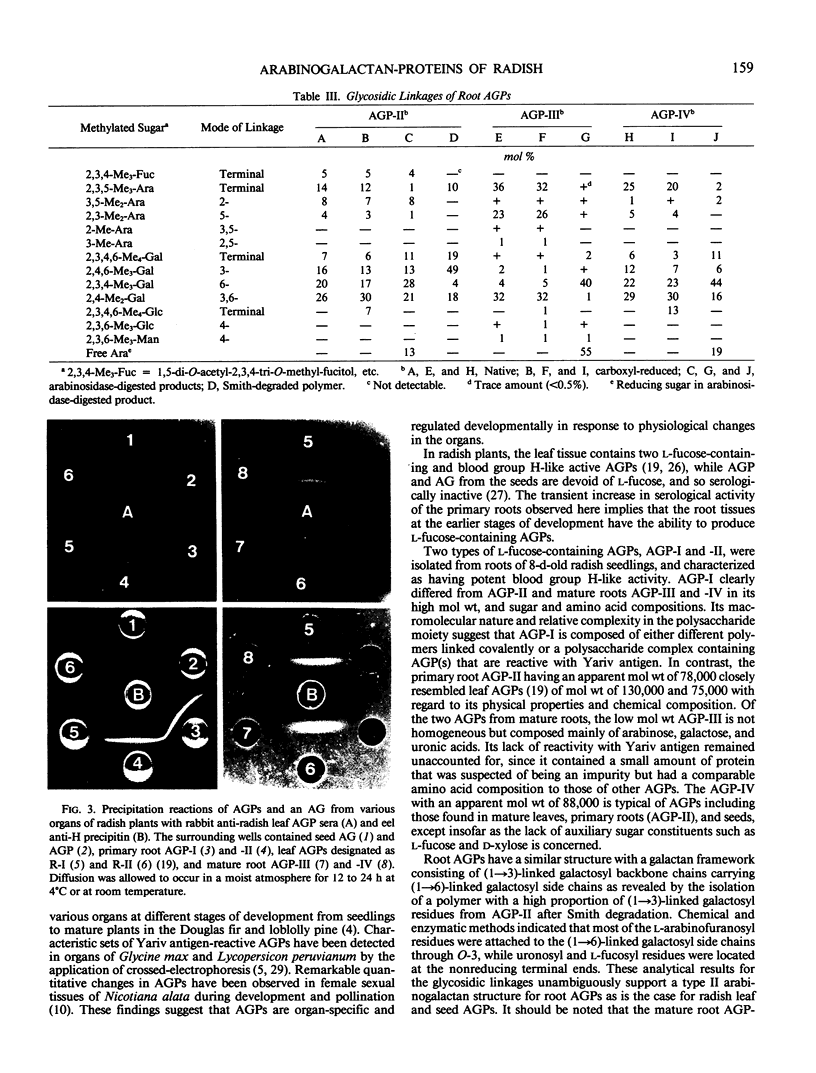
Organ-specific variations in blood group H-like activity were observed in developing radish plants. A temporary increase in serological activity was found to occur in the roots at the earlier stages of development. Arabinogalactan-proteins (AGPs) were isolated from primary and mature roots, and investigated for changes in their physicochemical properties, structure, and serological activities. These root AGPs were composed mainly of l-arabinose and d-galactose but were distinguishable from each other in their contents of l-fucose as well as of protein and hydroxyproline. The structures of the carbohydrate moieties of the root AGPs were essentially similar to those of AGPs isolated from seeds and mature leaves in that they consisted of consecutive (1→3)-linked β-d-galactosyl backbone chains having side chains of (1→6)-linked β-d-galactosyl residues, to which α-l-arabinofuranosyl residues were attached in the outer regions. One prominent feature of the primary root AGPs was that they contained appreciable amounts of l-fucose, which was presumably responsible for expression of the serological activity. In their immunological reactions with rabbit anti-radish leaf AGP antibody, the root AGPs were shown to share common antigenic determinant(s) with those of seed and leaf AGPs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gell A. C., Bacic A., Clarke A. E. Arabinogalactan-Proteins of the Female Sexual Tissue of Nicotiana alata: I. Changes during Flower Development and Pollination. Plant Physiol. 1986 Dec;82(4):885–889. doi: 10.1104/pp.82.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Hori H., Kaushal G. P., Elbein A. D. Fucosylation of membrane proteins in soybean cultured cells : effects of tunicamycin and swainsonine. Plant Physiol. 1985 Mar;77(3):687–694. doi: 10.1104/pp.77.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochibe N., Furukawa K. Purification and properties of a novel fucose-specific hemagglutinin of Aleuria aurantia. Biochemistry. 1980 Jun 24;19(13):2841–2846. doi: 10.1021/bi00554a004. [DOI] [PubMed] [Google Scholar]
- Murachi T., Suzuki A., Takahashi N. Evidence for glycoprotein nature of stem bromelain. Isolation of a glycopeptide. Biochemistry. 1967 Dec;6(12):3730–3736. doi: 10.1021/bi00864a015. [DOI] [PubMed] [Google Scholar]
- Schachter H., Sarney J., McGuire E. J., Roseman S. Isolation of diphosphopyridine nucleotide-dependent L-fucose dehydrogenase from pork liver. J Biol Chem. 1969 Sep 10;244(17):4785–4792. [PubMed] [Google Scholar]
- Springer G. F., Desai P. R. Monosaccharides as specific precipitinogens of eel anti-human blood-group H(O) antibody. Biochemistry. 1971 Sep 28;10(20):3749–3761. doi: 10.1021/bi00796a017. [DOI] [PubMed] [Google Scholar]
- Taylor R. L., Conrad H. E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry. 1972 Apr 11;11(8):1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]
- Uesaka E., Sato M., Raiju M., Kaji A. Alpha-l-arabinofuranosidase from Rhodotorula flava. J Bacteriol. 1978 Mar;133(3):1073–1077. doi: 10.1128/jb.133.3.1073-1077.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YARIV J., RAPPORT M. M., GRAF L. The interaction of glycosides and saccharides with antibody to the corresponding phenylazo glycosides. Biochem J. 1962 Nov;85:383–388. doi: 10.1042/bj0850383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holst G. J., Clarke A. E. Organ-Specific Arabinogalactan-Proteins of Lycopersicon peruvianum (Mill) Demonstrated by Crossed Electrophoresis. Plant Physiol. 1986 Mar;80(3):786–789. doi: 10.1104/pp.80.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]



