Abstract
Oral submucous fibrosis (OSMF) is a chronic, progressive, insidious premalignant disease with multifactorial etiology affecting any part of the oral cavity and sometimes the pharynx by triggering a rapid onset of trismus and dysphagia due to stiffness at the lips, cheek, pharynx, and upper oesophageal region. Submucous fibrosis resembles many auto-immune, dermatological, mucocutaneous, and fibrotic lesions that include scleroderma, amyloidosis, iron deficiency anemia, and systemic or generalized fibromatosis clinically and histologically. Several authors established an association between oral submucous fibrosis and scleroderma with predominant oral manifestations on the basis of similarity in clinical and histological characteristics despite different pathogenesis and prognostic aspects. Scleroderma or systemic sclerosis is an autoimmune connective tissue disorder clinically manifested as fibrosis of the skin, blood vessels, and visceral organs with or without the involvement of the oral cavity. Thus, understanding the disease mechanism, appropriate early diagnosis, and clinical management of these two entities play an important role in disease prognosis and treatment outcomes. The present review was carried out to briefly present a concise overview of the etiopathogenesis, clinical, histological, diagnosis, and management aspects of OSMF and scleroderma based on the available literature, with special emphasis on similarities and differences between these two entities subsequently aiding in appropriate treatment planning.
Keywords: scleroderma, submucous fibrosis, oral premalignancy, inflammation, hyalinization, collagen disease, autoimmune, antibodies
Introduction and background
Collagen is a major structural protein that represents the primary fibrous component of the extracellular matrix including skin, muscle, tendon, bone, and cartilage. The synthesis and degradation of collagen is an active process and any alterations in the collagen structure or mechanism will affect the oral tissues. The common connective tissue disorders with characteristic oral manifestations are systemic lupus erythematosus (SLE), systemic sclerosis/scleroderma (SSc), rheumatoid arthritis (RA), and Sjögren syndrome (SS), which often impose challenges in diagnosis and treatment owing to their uncertain presentation and unpredictable prognosis [1-3]. Several authors have established an association between SSc and oral submucous fibrosis (OSMF) based on a similarity in clinical and histological characteristics despite different pathogenesis and prognostic aspects [4-6].
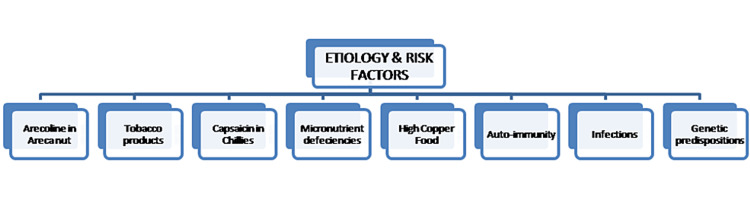
OSMF is an insidious, progressive, long-standing disease affecting any part of the oral cavity. Sometimes, the pharynx is often associated with a juxta-epithelial inflammatory reaction followed by a fibroelastic transformation of the lamina propria and epithelial atrophy histopathologically, preceded by vesicle formation, thus triggering the rapid onset of trismus, severe pain, and dysphagia due to stiffness in the cheeks, lips, perioral region, pharynx, and upper esophageal region. It is a chronic, pre-cancerous condition commonly seen among middle-aged groups with higher prevalence in the Southeast Asian subcontinent, including India. Although etiopathogenesis is not fully established, a multifactorial etiology is proposed, with areca nut as the chief causative agent while smokeless tobacco, chilies, micronutrient deficiencies, vitamin deficiencies, malnutrition, toxic copper levels in food products, autoimmunity, and genetic predisposition are often considered contributing risk factors [7-9].
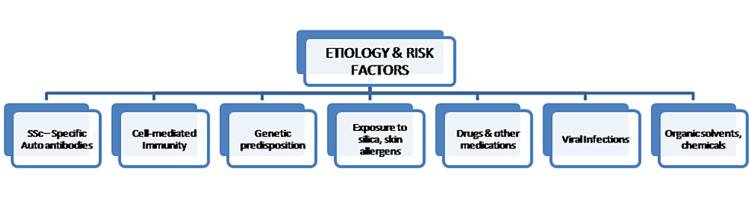
Scleroderma (SSc) is an uncommon connective tissue disorder affecting the skin and internal organs with a wide array of oral manifestations predominantly associated with fibrotic manifestations and widening of the periodontal ligament space, and neurological systems often resemble OSMF, which was previously termed idiopathic scleroderma of the mouth although SSc primarily includes sclerosis of the skin and other extremities rather than the oral counterpart. OSMF is believed to be a localized collagen disease of the oral cavity, whereas scleroderma or systemic sclerosis is a heterogeneous collagen disorder characterized by thickening of the skin, extensive fibrosis, abnormal nail fold capillaries, and vasculopathy because of increased deposition of the extracellular matrix caused by fibroblast dysfunction with excessive production of autoantibodies. The aetiopathogenesis of SSc is not fully established; however, antibodies directed against the epithelium, with a strong association with SSc-specific autoantibodies, and cell-mediated immunity were suggested based on immunological studies [10-13].
The differential diagnosis of scleroderma with an oral manifestation is ascertained as OSMF, which challenges the clinicians due to similar clinical and histopathological findings. The review was carried out to briefly present a concise overview of the etiopathogenesis, clinical, histological, diagnosis, and management aspects of OSMF and scleroderma based on the available literature, with special emphasis on similarities and differences between these two entities subsequently aiding in appropriate treatment planning.
Literature overview
Our review of the literature included the following findings: 1. Desa JV (1957) revealed that scleroderma preliminarily affected the skin (cutaneous) with occasional oral mucosa manifestations while OSMF affects the oro-pharyngeal regions with no established cutaneous or visceral spread [14]; 2. Binnie WH and Cawson RA (1972) concluded a definitive lack of evidence of a connection between OSMF and progressive systemic sclerosis despite similar clinical and histological features likely due to the sharing of a defect of collagen maturation [15]; 3. Morawetz et al (1987) observed a similarity between OSMF and scleroderma clinically and histopathologically; however, they detected that OSMF is not characterized by serological changes as found in scleroderma [16]; 4. Wood RE and Lee P (1988) demonstrated that the oral manifestations of systemic sclerosis appeared as a characteristic “purse string” furrow and a widening of the periodontal ligament space [17]; 5. Rajendran R (1994) suggested that the major histocompatibility complex (MHC)-mediated immunological instability is effective in both disease entities [7]; 6. Rao NR et al. (2020) proposed an inter-professional approach for the early diagnosis and appropriate management of both oral and systemic symptoms of OSMF, to reduce mortality and morbidity rates [6].
Review
Methodology
A structured literature search for articles written in the English language in PubMed/MEDLINE, EBSCOhost, Google Scholar, Scopus, IEEE Xplore Digital Library, and Web of Science databases was conducted by using the following MeSH terms: “Oral Submucous Fibrosis” OR Oral Scleroderma” AND “Dental”, “Systemic Sclerosis” AND “Autoimmune diseases” "Collagen disorders, Oral" OR “Premalignant Conditions” OR “Connective tissue” OR "All Metadata", “Oral Manifestations, Dermatology”, “Fibrosis, Mucocutaneous”, “Oral Precancer, Arecoline”, “Histopathology, OSMF” AND “Oro-facial manifestations, Scleroderma”.
Epidemiological considerations
OSMF is one of the most common premalignant conditions of the oral cavity, affecting more than five million people globally, and is predominantly seen among the South and Southeast Asian subcontinent population with a prevalence of 0.2-2.3% in males and 1.2-4.6% in females. It is common among 11 to 60-year-olds in India, presenting with about 2.3-7.6% malignant transformation depending on diagnostic criteria and follow-up interval [1,2,7-9].
Steen V et al., Weeding et al., and Hill et al. showed an increased prevalence of SSc among Blacks (race and ethnic predilection), with an annual estimated incidence of 19% per million per year and an overall prevalence of 250 per million approximately, and globally predominantly seen among females compared to males (1:3), with wide-ranging ages with peaks from 30 to 50 years [18-20]. Morphea, a plaque form of localized scleroderma affecting mostly women with an incidence of three cases per 100,000 individuals every year, is more prevalent in adults while linear scleroderma affects mostly children less than 12 years of age [21].
Khan AM et al., Jain A et al., Kumar KK et al., and Hazarey VK et al., in their epidemiological studies, observed higher OSMF instances among the young to middle-aged groups and school-going children, with a higher male predilection owing to habit addiction [22-25].
Oral submucous fibrosis: etiology, risk factors, and etiopathogenesis
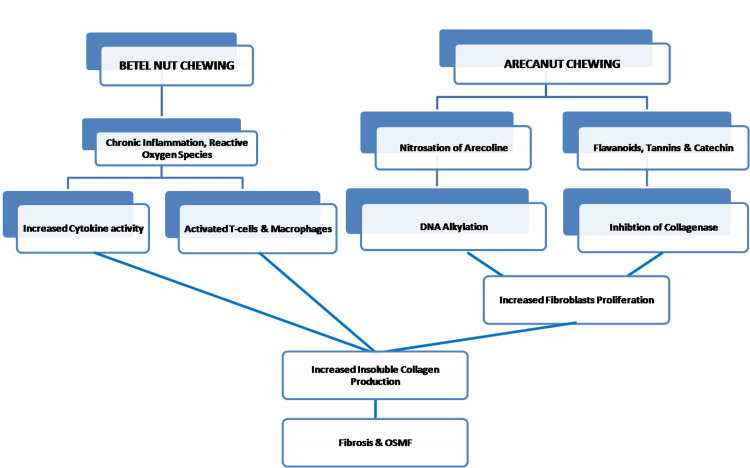
Figures 1, 2 show the etiology, risk factors, and etiopathogenesis of OSMF.
Figure 1. Etiology and risk factors of oral submucous fibrosis.
Image Credit: Dr. Sreedevi J
Figure 2. Etiopathogenesis of OSMF.
Image Credit: Dr. Sreedevi J
OSMF: oral submucous fibrosis
Scleroderma: etiology, risk factors, and etiopathogenesis
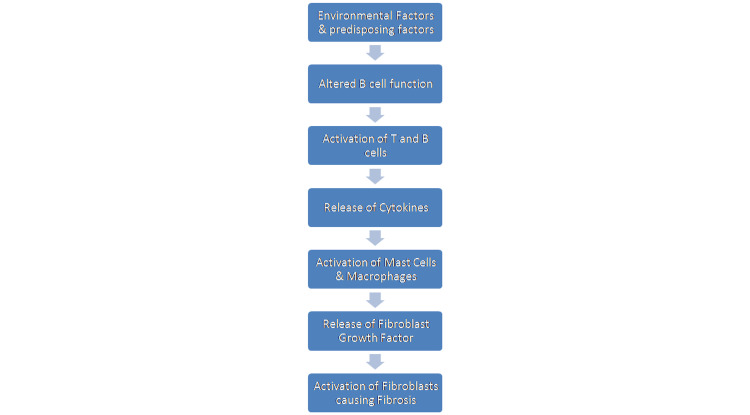
Figures 3, 4 show the etiology, risk factors, and etiopathogenesis of scleroderma.
Figure 3. Etiology and risk factors of scleroderma.
Image Credit: Dr. Sreedevi J
Figure 4. Etiopathogenesis of scleroderma.
Image Credit: Dr. Sreedevi J
Clinical features of OSMF and scleroderma
Table 1 shows the clinical features of OSMF and scleroderma [26].
Table 1. Clinical features of OSMF and scleroderma.
OSMF: oral submucous fibrosis; TMJ: temporomandibular joint
| OSMF | SCLERODERMA | |
| INTRAORAL FEATURES | ||
| Oral mucosa | Diffuse blanching (marble stone) | Pale and firm |
| Pigmentation | Less | Less |
| Papilla | Less | - |
| Uvula | Shrunken (inverted hockey stick appearance) | - |
| Erosion and ulceration | Seen | - |
| Tongue | Restrictive movements | Stiff |
| Periodontal ligament space widening | Absent | Present |
| Resorption of alveolar bone | Absent | Present |
| Epithelial atrophy | Present | - |
| Salivation | Reduced | Reduced |
| Buccal mucosa | Thick fibrous bands | - |
| Telangiectasia | - | Present |
| Teeth | Missing | Increased decay and missing |
| Gingival recession | Absent | Present |
| Capillaroscopy | - | Detection of abnormal nail fold capillaries |
| EXTRAORAL FEATURES | ||
| Mouth opening | Reduced | Reduced |
| Lips | Thinning | Retraction |
| Cheeks | Sunken with multiple folds | - |
| Deglutition | Difficulty experienced | - |
| Weight loss | Extreme | - |
| Atrophy of musculature | Present | - |
| Ankylosis of TMJ | - | Present |
| Dysphagia | Present | Present |
| Hard and soft palate | Fibrosis | Fibrosis |
Histopathological features of OSMF and SSc
Table 2 shows the histopathological features of OSMF and SSc [1-5,27-34]
Table 2. Histopathological features of OSMF and SSc.
OSMF: oral submucous fibrosis; SSc: scleroderma
| OSMF | SSc: associated oral manifestation features | |
| Characteristic Findings | 1. Sub-epithelial inflammatory reaction | 1. Perivascular inflammatory infiltrate |
| 2. Juxta-epithelial hyalinization degenerative and atrophic changes in muscular layer fibrosis | 2. Increased extracellular matrix, thickened blood vessels, loss of dermal papillary structures, fibrosis | |
| Histological Stages | Stages - inflammation, hyalinization, fibrosis, malignant transformation | Stages - inflammation, vasculopathy, fibrosis/sclerosis |
| Epithelium | Atrophic epithelium showing dysplastic changes at the later stages with loss of rete ridges | Normal or atrophic/flattened superficial layer with loss of rete ridges. Nodular collection of lymphocytes at the dermal-subcutaneous junction |
| Connective tissue stroma | 1. Granulation, degenerative, and atrophic changes in the muscular layer. 2. Fibrosis - complete collagen hyalinization, obliterated blood vessels, lymphocytes, and plasma cells, dense band of collagen bundles. 3. Malignant transformation - dysplasia, erythroplakia, and changes into squamous cell carcinoma | 1. Vasculopathy - edematous endothelial cells Submucosal hyalinization with thickening of blood vessels. Later stages show the disappearance of subcutaneous fat and severe sclerosis at the walls (intima) of the blood vessels. 2. Fibrosis/Sclerosis-thickening& hyalinization of the collagen fibers in the skin. 3. Deposition of collagen fibers (sclerosis) at the reticular layer. 4. Atrophy, degeneration, and drop out of adnexal structures “entrapped” by excessive collagen deposition |
Laboratory diagnosis
Table 3 lists the laboratory diagnosis.
Table 3. Laboratory diagnosis.
| Oral submucous fibrosis | Scleroderma | |
| Hemoglobin | Decreased | Normal |
| Serum Iron | Decreased | Normal |
| Serum protein | Decreased | Normal |
| Vitamin B 12 complex | Decreased | Normal |
| Folic acid | Decreased | Normal |
| ESR | Increased | Increased |
| Cu, Zn, Albumin, Mucoproteins | Decreased | Normal |
| T lymphocyte count | Decreased | Normal |
| Eosinophils | Normal | Decreased |
Immunological markers
Serological analysis in OSMF shows increased serum levels of IgA, IgD, IgE, Beta-2-microglobulin, and HLA typing (A10, B7, and DR3) [2,6-9]. Many immunochemistry studies have revealed CK2, CD3, and CD4-positive cells at the epithelial, juxta-epithelial, and basilar regions. Altered expression of tumor suppressor and promoter genes (p53, bcl-2, bax, and ki-67), mutations in the adenomatous polyposis coli gene along with an increased level of vimentin, plasminogen activator inhibitor-1, Heat Shock Protein (HSP-47), keratinocyte growth factor-1, and cystatin C in OSMF patients also indicates the increased risk of carcinomatous changes [2,7-9].
Clinical chemical investigation of SSc includes transaminase (alanine transaminase - ALT, aspartate aminotransferase - AST), cholestasis parameters (λ-GT, AP), lactate dehydrogenase (LDH), creatinine, creatinine kinase (CK), and aldolase. In advanced stages, elevated levels of creatinine >10% indicate renal impairment with multiple organ involvement.
Cytological studies
Cytogenetic studies on OSMF revealed increased AgNOR (silver binding nucleolar organizer region) proliferative activity, and salivary marker studies revealed higher levels of S-100A7, peroxidases, lactic acid dehydrogenases, and 2-hydroxy 8-deoxy-guanosine with decreased superoxide dismutase, vitamins, antioxidants, and micronutrients in saliva indicating the malignant risk potential of the disease.
SSc-specific autoantibody Scl-70, anti-topoisomerase 1, or ACA (anti-centromere antibody) are considered the primary serological marker in SSc. The presence of systemic disease markers in SSc such as anti-nuclear factor, gammaglobulin, or immunological factors, anti-histone antibodies, increased collagen procollagen type III serum levels, and a decrease in anti-phospholipid antibodies are indicators of localized scleroderma [35-39].
Management
The following are certain management modalities [2,23,40-47].
Oral Submucous Fibrosis
Habit cessation involves the complete cessation of the habit or reducing the usage of areca nut (primary etiological agent). Topical treatment includes oral physiotherapy, local corticosteroids, intralesional injection of several enzymes such as hyaluronidase, colchicine with hyaluronidase, collagenase, antifibrotic cytokines, and other intercellular substances to decrease proliferation and abnormal deposition of collagen fibers. Systemic treatment includes micronutrient supplement therapy, antioxidants such as b-carotene, vitamins, lycopene, curcumins, Spirulina and aloe vera, immunomodulatory drugs, and systemic corticosteroids. Surgery with rehabilitative management includes submucosal resection and complete striping of fibrous bands, coronoidectomy, bilateral temporalis myotomy, followed by reconstruction with partial thickness skin grafts, buccal palatal graft, microvascular free radial forearm flaps, tongue flaps, and nasolabial flaps at the resected fibrotic site. Use of diode laser, KTP-532, CO2 laser, and ErCr: YSGG laser surgeries to promote healthy wound healing and reduce scar formation and trismus. Stem cell therapy through the intralesional injection of autologous bone marrow stem cells has shown a better prognosis by significantly increasing the mouth opening and reducing discomfort.
Scleroderma
Topical treatment includes topical corticosteroids, tacrolimus, phototherapy, and calcipotriol with PUVA (Psoralen ultraviolet A) cream, which has shown a better response rate in active diseases. Systemic treatment includes local scleroderma-systemic corticosteroids, methotrexate, D-penicillamine, oral vitamin D, psoralen-UVA (PUVA), phenytoin, cyclosporine, and interferon-alpha therapy. For diffuse scleroderma, the immunosuppressive drugs cyclophosphamide and mycophenolate mofetil are used. Calcium channel blockers were given for the management of CREST ((calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) syndrome in the early stages followed by Iloprost or bosentan for topical digit ulcers. In severe disease with extensive systemic organ involvement, oxygen therapy, anticoagulants, antibiotics, angiotensin-converting enzyme (ACE) inhibitors, diuretics, and corticosteroids were recommended to avoid multiorgan failure. For surgical treatment of severe cases with involvement of facial structures, palliative reconstruction surgery and physical therapy with ultraviolet radiation are beneficial modalities.
Conclusions
OSMF possesses a significant rate of morbidity and mortality owing to its progressive masticatory difficulties with subsequent nutritional deficiencies and increased risk of the potential for malignant transformation. It has been well-established that dense fibrosis, decreased vascularity, persistent habit, chronic irritation, prolonged altered cytokine activity, and alkaloid secretions bring about surface epithelial changes and malignant transformation in OSMF. Localized scleroderma or morphea classically presents with benign and self-limited evolution when confined to the underlying tissue; however, the overall mortality and morbidity are high in systemic sclerosis, both in local and diffuse variants, representing a 27% to 72% death rate caused largely by cardiovascular and pulmonary involvement. Thus, appropriate assessment, determination of risk factors, early diagnosis, and intervention along with palliative care are essential to improve the overall prognosis, survival rate, and treatment outcomes and enhance the quality of life.
The authors have declared that no competing interests exist.
References
- 1.Oral manifestations of autoimmune connective tissue diseases. Pandey A, Pandey M, Pandey VP, Ravindran V. Indian J Rheumatol. 2018;13:264–272. [Google Scholar]
- 2.Oral submucous fibrosis: an overview of a challenging entity. Gupta S, Jawanda MK. Indian J Dermatol Venereol Leprol. 2021;87:768–777. doi: 10.25259/IJDVL_371_20. [DOI] [PubMed] [Google Scholar]
- 3.Autoimmunity in systemic sclerosis: current concepts. Boin F, Rosen A. Curr Rheumatol Rep. 2007;9:165–172. doi: 10.1007/s11926-007-0012-3. [DOI] [PubMed] [Google Scholar]
- 4.Deficiency in collagen and fibronectin phagocytosis by human buccal mucosa fibroblasts in vitro as a possible mechanism for oral submucous fibrosis. Tsai CC, Ma RH, Shieh TY. J Oral Pathol Med. 1999;28:59–63. doi: 10.1111/j.1600-0714.1999.tb01997.x. [DOI] [PubMed] [Google Scholar]
- 5.Oral submucous fibrosis: review on aetiology and pathogenesis. Tilakaratne WM, Klinikowski MF, Saku T, Peters TJ, Warnakulasuriya S. Oral Oncol. 2006;42:561–568. doi: 10.1016/j.oraloncology.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Oral submucous fibrosis: a contemporary narrative review with a proposed inter-professional approach for an early diagnosis and clinical management. Rao NR, Villa A, More CB, Jayasinghe RD, Kerr AR, Johnson NW. J Otolaryngol Head Neck Surg. 2020;49:3. doi: 10.1186/s40463-020-0399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oral submucous fibrosis: etiology, pathogenesis, and future research. Rajendran R. https://pubmed.ncbi.nlm.nih.gov/7867145/ Bull World Health Organ. 1994;72:985–996. [PMC free article] [PubMed] [Google Scholar]
- 8.Oral submucous fibrosis and its dermatological relation. Ali FM, Patil A, Patil K, Prasant MC. Indian Dermatol Online J. 2014;5:260–265. doi: 10.4103/2229-5178.137772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oral submucous fibrosis: newer proposed classification with critical updates in pathogenesis and management strategies. Passi D, Bhanot P, Kacker D, Chahal D, Atri M, Panwar Y. Natl J Maxillofac Surg. 2017;8:89–94. doi: 10.4103/njms.NJMS_32_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pathophysiology of systemic sclerosis: state of the art in 2014. Dumoitier N, Lofek S, Mouthon L. Presse Med. 2014;43:0–78. doi: 10.1016/j.lpm.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Oral and periodontal manifestations associated with systemic sclerosis: a case series and review. Jagadish R, Mehta DS, Jagadish P. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3459512/ J Indian Soc Periodontol. 2012;16:271–274. doi: 10.4103/0972-124X.99275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. van den Hoogen F, Khanna D, Fransen J, et al. Ann Rheum Dis. 2013;72:1747–1755. doi: 10.1136/annrheumdis-2013-204424. [DOI] [PubMed] [Google Scholar]
- 13.Idiopathic scleroderma of the mouth. Report of three cases. Su I. AMA Arch Otolaryngol. 1954;59:330–332. doi: 10.1001/archotol.1954.00710050342011. [DOI] [PubMed] [Google Scholar]
- 14.LXXXVII submucous fibrosis of the palate and cheek. DeSa JV. Ann Otol Rhinol Laryngol. 1957;66:1143–1159. doi: 10.1177/000348945706600420. [DOI] [PubMed] [Google Scholar]
- 15.A new ultrastructural finding in oral submucous fibrosis. Binnie WH, Cawson RA. Br J Dermatol. 1972;86:286–290. doi: 10.1111/j.1365-2133.1972.tb02230.x. [DOI] [PubMed] [Google Scholar]
- 16.Oral submucous fibrosis. Morawetz G, Katsikeris N, Weinberg S, Listrom R. Int J Oral Maxillofac Surg. 19871;16:609–614. doi: 10.1016/s0901-5027(87)80115-7. [DOI] [PubMed] [Google Scholar]
- 17.Analysis of the oral manifestations of systemic sclerosis (scleroderma) Wood RE, Lee P. Oral Surg Oral Med Oral Radiol. 1988;65:172–178. doi: 10.1016/0030-4220(88)90161-2. [DOI] [PubMed] [Google Scholar]
- 18.Severe organ involvement in systemic sclerosis with diffuse scleroderma. Steen VD, Medsger TA. Arthritis Rheum. 2000;43:2437–2444. doi: 10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 19.Cancer and scleroderma. Weeding E, Casciola-Rosen L, Shah AA. Rheum Dis Clin North Am. 2020;46:551–564. doi: 10.1016/j.rdc.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Risk of cancer in patients with scleroderma: a population based cohort study. Hill CL, Nguyen AM, Roder D, Roberts-Thomson P. Ann Rheum Dis. 2003;62:728–731. doi: 10.1136/ard.62.8.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Histopathologic spectrum of morphea. Chiu YE, Abban CY, Konicke K, Segura A, Sokumbi O. Am J Dermatopathol. 2021;43:1–8. doi: 10.1097/DAD.0000000000001662. [DOI] [PubMed] [Google Scholar]
- 22.Effect of areca nut chewing and maximal mouth opening in school going children in Ahmedabad. Khan AM, Sheth MS, Purohit RR. Indian J Med Paediatr Oncol. 2016;37:239–341. doi: 10.4103/0971-5851.195734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oral submucous fibrosis in paediatric patients: a systematic review and protocol for management. Jain A, Saumya T. Int J Surg Oncol. 2019;2019:3497136. doi: 10.1155/2019/3497136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oral submucous fibrosis: a clinico-histopathological study in Chennai. Kiran Kumar K, Saraswathi TR, Ranganathan K, Uma Devi M, Elizabeth J. Indian J Dent Res. 2007;18:106–111. doi: 10.4103/0970-9290.33785. [DOI] [PubMed] [Google Scholar]
- 25.Oral submucous fibrosis: study of 1000 cases from central India. Hazarey VK, Erlewad DM, Mundhe KA, Ughade SN. J Oral Pathol Med. 2007;36:12–17. doi: 10.1111/j.1600-0714.2006.00485.x. [DOI] [PubMed] [Google Scholar]
- 26.Localized scleroderma: clinical spectrum and therapeutic update. Careta MF, Romiti R. An Bras Dermatol. 2015;90:62–73. doi: 10.1590/abd1806-4841.20152890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Familial occurrence frequencies and relative risks for systemic sclerosis (scleroderma) in three United States cohorts. Arnett FC, Cho M, Chatterjee S, Aguilar MB, Reveille JD, Mayes MD. Arthritis Rheum. 2001;44:1359–1362. doi: 10.1002/1529-0131(200106)44:6<1359::AID-ART228>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 28.Pathogenesis of oral submucous fibrosis. Khan S, Chatra L, Prashanth SK, Veena KM, Rao PK. J Cancer Res Ther. 2012;8:199–203. doi: 10.4103/0973-1482.98970. [DOI] [PubMed] [Google Scholar]
- 29.Betel chewing and dietary habits of chewers without and with submucous fibrosis and with concomitant oral cancer. Seedat HA, van Wyk CW. https://pubmed.ncbi.nlm.nih.gov/3194806/ S Afr Med J. 1988;74:572–575. [PubMed] [Google Scholar]
- 30.Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Distler JH, Jüngel A, Huber LC, et al. Arthritis Rheum. 2007;56:311–322. doi: 10.1002/art.22314. [DOI] [PubMed] [Google Scholar]
- 31.Histological features of localized scleroderma 'en coup de sabre': a study of 16 cases. Taniguchi T, Asano Y, Tamaki Z, et al. J Eur Acad Dermatol Venereol. 2014;28:1805–1810. doi: 10.1111/jdv.12280. [DOI] [PubMed] [Google Scholar]
- 32.Sine scleroderma, limited cutaneous, and diffused cutaneous systemic sclerosis survival and predictors of mortality. De Almeida Chaves S, Porel T, Mounié M, et al. Arthritis Res Ther. 2021;23:295. doi: 10.1186/s13075-021-02672-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clinical and functional staging of oral submucous fibrosis. Haider SM, Merchant AT, Fikree FF, Rahbar MH. Br J Oral Maxillofac Surg. 2000;38:12–15. doi: 10.1054/bjom.1999.0062. [DOI] [PubMed] [Google Scholar]
- 34.Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. LeRoy EC, Black C, Fleischmajer R, et al. https://pubmed.ncbi.nlm.nih.gov/3361530/ J Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- 35.College of Rheumatology preliminary classification criteria for systemic sclerosis: addition of severe nailfold capillaroscopy abnormalities markedly increases the sensitivity for limited scleroderma (letter) Arthritis Rheum. 2001;44:735–736. doi: 10.1002/1529-0131(200103)44:3<735::AID-ANR125>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 36.Association between grading of oral submucous fibrosis with frequency and consumption of areca nut and its derivatives in a wide age group: a multicentric cross sectional study from Karachi, Pakistan. Hosein M, Mohiuddin S, Fatima N. J Cancer Prev. 2015;20:216–222. doi: 10.15430/JCP.2015.20.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Recent concepts of etiopathogenesis and management of oral submucous fibrosis: a review of literature. Singh P. J Evol Med Dent Sci. 2015;28:13728–13742. [Google Scholar]
- 38.Localized scleroderma in adults and children. Clinical and laboratory investigations on 239 cases. Marzano AV, Menni S, Parodi A, Borghi A, Fuligni A, Fabbri P, Caputo R. https://pubmed.ncbi.nlm.nih.gov/12695134/ Eur J Dermatol. 2003;13:171–176. [PubMed] [Google Scholar]
- 39.Biomarkers in systemic sclerosis. Castro SV, Jimenez SA. https://www.springer.com/journal/40699. Biomark Med. 2010;4:133–147. doi: 10.2217/bmm.09.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Current protocols in the management of oral submucous fibrosis: an update. Arakeri G, Rai KK, Boraks G, et al. J Oral Pathol Med. 2017;46:418–423. doi: 10.1111/jop.12583. [DOI] [PubMed] [Google Scholar]
- 41.Scleroderma and the oral health implications. Derbi HA, Borromeo G. Advances in Dentistry & Oral Health. 2018;7:555716. [Google Scholar]
- 42.Oral submucous fibrosis: a review of the current management and possible directions for novel therapies. Warnakulasuriya S, Kerr AR. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:232–241. doi: 10.1016/j.oooo.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 43.Comparative study of the efficacy of herbal antioxdants oxitard and aloe vera in the treatment of oral submucous fibrosis. Patil S, Halgatti V, Maheshwari S, Santosh BS. J Clin Exp Dent. 2014;6:265. doi: 10.4317/jced.51424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Resolution of severe digital ulceration during a course of Bosentan therapy. Snyder MJ, Jacobs MR, Grau RG, Wilkes DS, Knox KS. Ann Intern Med. 2005;142:802–803. doi: 10.7326/0003-4819-142-9-200505030-00029. [DOI] [PubMed] [Google Scholar]
- 45.Systemic sclerosis/scleroderma: a treatable multisystem disease. Hinchcliff M, Varga J. https://pubmed.ncbi.nlm.nih.gov/18953973/ Am Fam Physician. 2008;78:961–968. [PubMed] [Google Scholar]
- 46.Update on management of morphea (localized scleroderma) in children. George R, George A, Kumar TS. Indian Dermatol Online J. 2020;11:135. doi: 10.4103/idoj.IDOJ_284_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.PUVA-cream photochemotherapy for the treatment of localized scleroderma. Grundmann-Kollmann M, Ochsendorf F, Zollner TM, Spieth K, Sachsenberg-Studer E, Kaufmann R, Podda M. J Am Acad Dermatol. 2000;43:675–678. doi: 10.1067/mjd.2000.105503. [DOI] [PubMed] [Google Scholar]