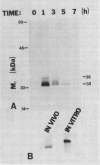
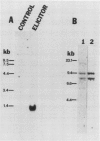
Abstract
Chitinase, which catalyzes the hydrolysis of β-1,4 N-acetylglucosamine linkages of the fungal cell wall polymer chitin, is a component of the inducible defenses of plants. We show that chitinase synthesis is stimulated in bean (Phaseolus vulgaris L.) cell suspension cultures treated with fungal cell wall elicitors and in hypocotyls in response to infection with the fungus Colletotrichum lindemuthianum. Chitinase cDNA clones were isolated by antibody screening of a λgt11 cDNA library containing sequences complementary to poly A+ RNA from elicited cells. The identity of these clones was confirmed by nucleotide sequence analysis and comparison of the deduced amino acid sequence with that determined for the amino-terminal sequence of bean chitinase. Elicitor causes a very rapid activation of chitinase transcription with a 10-fold stimulation after 5 minutes and 30-fold increase within 20 minutes. This leads to a marked, transient accumulation of chitinase transcripts with maximum levels 2 hours after elicitor treatment, concomitant with the phase of rapid enzyme synthesis. Chitinase transcripts also markedly accumulate in wounded and infected hypocotyls. Chitinase cDNA sequences hybridize to several genomic fragments suggesting there are several chitinase genes in the bean genome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell J. N., Ryder T. B., Wingate V. P., Bailey J. A., Lamb C. J. Differential accumulation of plant defense gene transcripts in a compatible and an incompatible plant-pathogen interaction. Mol Cell Biol. 1986 May;6(5):1615–1623. doi: 10.1128/mcb.6.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglie K. E., Gaynor J. J., Broglie R. M. Ethylene-regulated gene expression: molecular cloning of the genes encoding an endochitinase from Phaseolus vulgaris. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6820–6824. doi: 10.1073/pnas.83.18.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer C. L., Bell J. N., Ryder T. B., Bailey J. A., Schuch W., Bolwell G. P., Robbins M. P., Dixon R. A., Lamb C. J. Co-ordinated synthesis of phytoalexin biosynthetic enzymes in biologically-stressed cells of bean (Phaseolus vulgaris L.). EMBO J. 1985 Feb;4(2):285–289. doi: 10.1002/j.1460-2075.1985.tb03627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K., Cramer C. L., Bolwell G. P., Dixon R. A., Schuch W., Lamb C. J. Rapid transient induction of phenylalanine ammonia-lyase mRNA in elicitor-treated bean cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6731–6735. doi: 10.1073/pnas.82.20.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg S. M., Weinberger C., Ong E. S., Cerelli G., Oro A., Lebo R., Thompson E. B., Rosenfeld M. G., Evans R. M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985 Dec 19;318(6047):635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M. A., Lamb C. J. Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol Cell Biol. 1987 Jan;7(1):335–341. doi: 10.1128/mcb.7.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdy M. C., Lamb C. J. Chalcone isomerase cDNA cloning and mRNA induction by fungal elicitor, wounding and infection. EMBO J. 1987 Jun;6(6):1527–1533. doi: 10.1002/j.1460-2075.1987.tb02396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohnen D., Shinshi H., Felix G., Meins F. Hormonal regulation of beta1,3-glucanase messenger RNA levels in cultured tobacco tissues. EMBO J. 1985 Jul;4(7):1631–1635. doi: 10.1002/j.1460-2075.1985.tb03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rapid switching of plant gene expression induced by fungal elicitor. Science. 1985 Mar 8;227(4691):1240–1243. doi: 10.1126/science.227.4691.1240. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinshi H., Mohnen D., Meins F. Regulation of a plant pathogenesis-related enzyme: Inhibition of chitinase and chitinase mRNA accumulation in cultured tobacco tissues by auxin and cytokinin. Proc Natl Acad Sci U S A. 1987 Jan;84(1):89–93. doi: 10.1073/pnas.84.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somssich I. E., Schmelzer E., Bollmann J., Hahlbrock K. Rapid activation by fungal elicitor of genes encoding "pathogenesis-related" proteins in cultured parsley cells. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2427–2430. doi: 10.1073/pnas.83.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]