Abstract
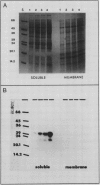
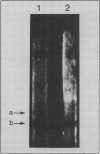
Thalassiosira pseudonana Husedt (Hasle and Heimdal) clone 3H was grown in axenic culture in artificial seawater medium containing 10−8 molar Na275SeO3. Biochemical distribution of radiolabeled Se was determined by solvent extraction techniques, gel filtration, and polyacrylamide gel electrophoresis. Of the total cellular Se, 51% was protein bound. Two soluble macromolecules of 21 and 29 kilodaltons contained 75Se. These results are the first to provide evidence of specific Se-containing compounds in a photosynthetic organism. Glutathione peroxidase (GSH-Px) activity was measured in cell-free extracts and on nondenaturing polyacrylamide gels by a glutathione-reductase coupled assay. Two enzymes showing GSH-Px activity were present. One enzyme was active with H2O2 and tert-butyl hydroperoxide (tBOOH); consistent with known Sedependent GSH-Pxs, but the other enzyme was only active with tBOOH. Co-migration of the H2O2-active GSH-Px and 75Se on nondenaturing polyacrylamide gels provides evidence that T. pseudonana contains a Sedependent GSH-Px. The molecular weight of one of the 75Se-labeled macromolecules is identical with the weight of previously characterized GSH-PX subunits. We conclude that the obligate requirement for Se in Thalassiosira pseudonana is due in part to the presence of the selenoenzyme glutathione peroxidase.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler E., West C. Red cell glutathione peroxidase polymorphism in Afro-Americans. Am J Hum Genet. 1974 Mar;26(2):255–258. [PMC free article] [PubMed] [Google Scholar]
- Brown T. A., Shrift A. Identification of Selenocysteine in the Proteins of Selenate-grown Vigna radiata. Plant Physiol. 1980 Oct;66(4):758–761. doi: 10.1104/pp.66.4.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C. K., Tappel A. L. Response of glutathione peroxidase to dietary selenium in rats. J Nutr. 1974 Apr;104(4):444–451. doi: 10.1093/jn/104.4.444. [DOI] [PubMed] [Google Scholar]
- Flohe L., Günzler W. A., Schock H. H. Glutathione peroxidase: a selenoenzyme. FEBS Lett. 1973 May 15;32(1):132–134. doi: 10.1016/0014-5793(73)80755-0. [DOI] [PubMed] [Google Scholar]
- Flohé L., Zimmermann R. The role of GSH peroxidase in protecting the membrane of rat liver mitochondria. Biochim Biophys Acta. 1970 Nov 3;223(1):210–213. doi: 10.1016/0005-2728(70)90149-0. [DOI] [PubMed] [Google Scholar]
- Gennity J. M., Bottino N. R., Zingaro R. A., Wheeler A. E., Irgolic K. J. The binding of selenium to the lipids of two unicellular marine algae. Biochem Biophys Res Commun. 1984 Jan 13;118(1):176–182. doi: 10.1016/0006-291x(84)91083-0. [DOI] [PubMed] [Google Scholar]
- HUNTER F. E., Jr, LEVY J. F., FINK J., SCHUTZ B., GUERRA F., HURWITZ A. Studies on the mechanism by which anaerobiosis prevents swelling of mitochondria in vitro: effect of electron transport chain inhibitors. J Biol Chem. 1959 Aug;234(8):2176–2186. [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane L. C. A simple method for stabilizing protein-sulfhydryl groups during SDS-gel electrophoresis. Anal Biochem. 1978 Jun 1;86(2):655–664. doi: 10.1016/0003-2697(78)90792-3. [DOI] [PubMed] [Google Scholar]
- Overbaugh J. M., Fall R. Characterization of a Selenium-Independent Glutathione Peroxidase From Euglena gracilis. Plant Physiol. 1985 Feb;77(2):437–442. doi: 10.1104/pp.77.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacheck I., Cabib E. A simple procedure for protein determination by the Lowry method in dilute solutions and in the presence of interfering substances. Anal Biochem. 1981 Nov 1;117(2):311–314. doi: 10.1016/0003-2697(81)90784-3. [DOI] [PubMed] [Google Scholar]
- Rotruck J. T., Pope A. L., Ganther H. E., Swanson A. B., Hafeman D. G., Hoekstra W. G. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973 Feb 9;179(4073):588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Smith J., Shrift A. Phylogenetic distribution of glutathione peroxidase. Comp Biochem Physiol B. 1979;63(1):39–44. doi: 10.1016/0305-0491(79)90231-1. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. Selenium-dependent enzymes. Annu Rev Biochem. 1980;49:93–110. doi: 10.1146/annurev.bi.49.070180.000521. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. Some selenium-dependent biochemical processes. Adv Enzymol Relat Areas Mol Biol. 1979;48:1–28. doi: 10.1002/9780470122938.ch1. [DOI] [PubMed] [Google Scholar]