Lay Summary
Patient-reported outcomes (PROs) are especially important for people who receive left ventricular assist devices (LVADs) given their lifestyle changes, LVAD-focused self-care needs, and the potential for device-related adverse events.
Patient-Reported Outcomes Measurement Information System (PROMIS®) physical, mental and social health measures are advantageous for use in multiple chronic illnesses because they allow comparability of experiences across diseases/conditions.
Tracking changes in PRO outcomes across time in individual patients could provide patients and clinicians with the opportunity to identify positive and negative changes and ways to address them.
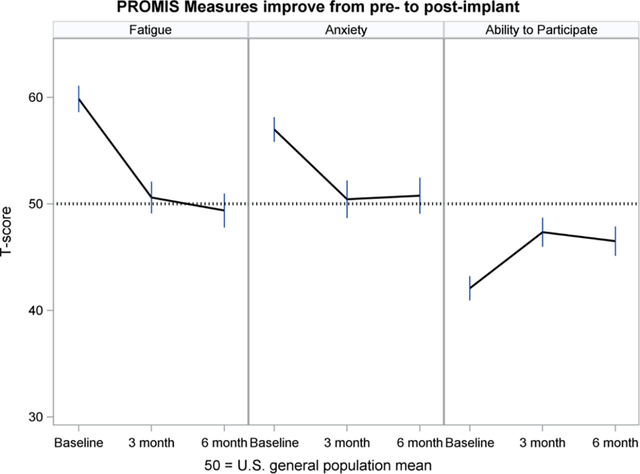
Study participants (n=272) completed 12 Patient-Reported Outcomes Measurement Information System (PROMIS®) physical, mental and social health measures (questionnaires) prior to implantation of a left ventricular assist device (LVAD) and again at 3 and 6 months post-implant. All but one PROMIS measure demonstrated significant improvement from pre-implant to 3 months; there was little change between 3 and 6 months. Because PROMIS measures were developed in the general population, patients with an LVAD, their caregivers and their clinicians can interpret the meaning of PROMIS scores in relation to the general population, helping them to monitor a return to normalcy in everyday life.
Graphical Abstract
Introduction
Longitudinal assessment of patient-reported outcomes (PROs) is highly relevant for people with cardiovascular disease.1 PROs may be especially important for the subset of advanced heart failure patients who receive left ventricular assist devices (LVADs) given their lifestyle changes, LVAD-focused self-care needs, and the potential for device-related adverse events. Understanding the full complement of LVAD effects on patients over time is important to patient selection, shared medical decision-making and future research.
Findings from a systematic review of 16 studies assessing PROs in LVAD populations2 and from subsequent studies3–10 revealed that LVADs were associated with improvement in several physical and mental domains of health-related quality of life (HRQOL). A comprehensive evaluation of PROs is critical to interpret response to treatment and understand change over time. Prior studies that have assessed PROs before and after LVAD implantation have used generic (e.g., EQ-5D-3L11, 12) and/or heart failure-specific (e.g., Kansas City Cardiomyopathy Questionnaire13, 14) measures of HRQOL.3–9 While these measures have provided important insights into change in HRQOL over time, some potentially important domains have not been assessed, including cognitive function and social health. In addition, some HRQOL concerns were measured using only single items, e.g., fatigue, anxiety, and depression. A more comprehensive assessment of PROs would help to better understand the full impact of LVAD support on patient HRQOL. Evaluating responsiveness validity (sensitivity to change) is an additional important attribute when considering the longitudinal use of a particular measure.15, 16
Given these unmet needs, the Mechanical Circulatory Support: Measures of Adjustment and Quality of Life (MCS A-QOL) study included a longitudinal evaluation of physical, mental and social health outcomes among patients pre- and post-LVAD. Health outcome measures used in MCS A-QOL were developed by the Patient-Reported Outcomes Measurement Information System (PROMIS®) to assess common, generic symptoms and experiences that apply to people in a variety of contexts or with a variety of diseases without needing to make attributions to a specific condition;17–21 all PROMIS measures are freely available(https://www.healthmeasures.net/). The PROMIS domain framework guided the development of multiple measures for physical, mental and social health.20 Physical health measures focused on physical function, symptoms, sleep function and sexual function; mental health measures focused on emotional distress, cognitive function and positive psychological function; and social health measures focused on social function (activities with others, and carrying out usual roles and responsibilities) and social relationships (connections with important others). Each PROMIS measure selected for MCS A-QOL was chosen for its relevance to patients with advanced heart failure who have undergone LVAD implantation.22 The aim of this report was to describe PROMIS physical, mental and social health outcomes from pre- to post-LVAD implant, and to examine responsiveness validity.15, 23
Methods
Sites and Sample
From October 2016 to February 2020 patients at 12 U.S. sites were enrolled in the hospital or outpatient clinic prior to planned LVAD implantation. The study was conducted in compliance with the ISHLT Ethics statement, was approved by Institutional Review Boards at all sites, and patients provided written informed consent. Study inclusion criteria were: (1) age ≥19 years, (2) scheduled for an initial (primary) continuous flow LVAD, (3) goal of LVAD therapy as follows: (a) “short-term” [heart transplant candidate listed with the United Network for Organ Sharing; UNOS], (b) “uncertain” heart transplant candidacy [possible heart transplant candidate, but not listed with UNOS], or (c) “long-term” [ineligible for heart transplantation, i.e., destination therapy], and (4) able to speak and understand English and provide self-report data on a computer touchscreen, standard computer and/or paper-based forms with minimal assistance.
Procedures
Prior to LVAD implant and at Months 3 and 6 post-LVAD participants completed a set of PROMIS and other PRO measures via self-administration (iPad in the clinic, or home computer using a personalized link sent by email, or paper questionnaires distributed in person or sent by mail) or in-person interview by the study coordinator, based on patient preference. The measures were presented in the same order to all participants. Participants were encouraged to complete PROs on-site; however, some chose to complete them at home. All participants were enrolled in person prior to the March 11, 2020, declaration of COVID-19 as a pandemic. Participants who were scheduled to complete questionnaires after this date (n=44) were encouraged to complete them at home. Windows for PRO completion were 30 days prior to implant, ±30 days for the Month 3 post-implant clinic visit, and ±60 days for the Month 6 visit. The set of PROs took approximately one hour to complete, and participants received a $20 gift card for each assessment. PROMIS measures were completed as computer-adaptive tests when administered by computer or as fixed-length short forms when administered on paper.18, 20 All other PROs were administered as fixed-length forms by any mode/method. Sociodemographic data were collected directly from study participants, while clinical data were either securely downloaded from the Society of Thoracic Surgeons Interagency Registry for Mechanically Assisted Circulatory Support (STS Intermacs) national database9 or collected from medical records by site research coordinators for participants not enrolled in the registry.
PROs, Clinician Ratings and Performance Tests
Table 1 summarizes information about the instruments administered in this study. The 12 PROMIS measures assessed Physical Health (Fatigue v1.0,24, 25 Physical Function v1.2,26, 27 Sleep Disturbance v1.0,28, 29 and Sleep-related Impairment v1.028, 29); Mental Health (Depression v1.0,30, 31 Anxiety v1.0,30 and Cognitive Function v1.0/v2.032, 33); and Social Health (Ability to Participate in Social Roles v2.0,34 Satisfaction with Social Roles and Activities v2.0,34 and Emotional, Informational and Instrumental Social Support v2.034). The PROs, clinician ratings and performance tests that were used as responsiveness indicators included the Kansas City Cardiomyopathy Questionnaire (KCCQ-12),13, 14 EQ-5D-3L,11, 12 PROMIS Global Health v.1.1,35 Global Ratings of Change,36, 37 NYHA Functional Classification,38 and 6-Minute Walk Test.39–42 All PROs and performance tests were scored according to published guidelines. PROMIS uses T-scores (mean=50, standard deviation=10).20 For most PROMIS instruments, a T-score of 50 is the average for the U.S. general population with a standard deviation of 10 based on calibration testing performed on large samples of the general population. A higher PROMIS T-score represents more of the concept being measured (Table 1).
Table 1.
Instruments Administered in the Study
| Type of Measure | Instrument | Description | Score Metrica | Meaning of a Higher Score |
|---|---|---|---|---|
| PROMIS® Measures Evaluated for Responsiveness | ||||
| Physical Health | ||||
| PROMIS® Fatigue v1.0 | Assesses fatigue from mild subjective feelings of tiredness to an overwhelming, debilitating, and sustained sense of exhaustion that is likely to decrease one’s ability to carry out daily activities, including the ability to work effectively and to function at one’s usual level in family or social roles. | IRT T-score | More fatigue | |
| PROMIS® Physical Function v1.2 | Assesses one’s ability to carry out activities that require physical actions, ranging from self-care (activities of daily living) to more complex activities that require a combination of skills, often within a social context. | IRT T-score | Better physical function | |
| PROMIS® Sleep Disturbance v1.0 | Assesses perceptions of sleep quality, sleep depth, and restoration associated with sleep; perceived difficulties and concerns with getting to sleep or staying asleep; and perceptions of the adequacy of and satisfaction with sleep | IRT T-score | Greater sleep disturbance | |
| PROMIS® Sleep-related Impairment v1.0 | Assesses perceptions of alertness, sleepiness, and tiredness during usual waking hours, and the perceived functional impairments during wakefulness associated with sleep problems or impaired alertness | IRT T-score | Greater sleep-related impairment | |
| Mental Health | ||||
| PROMIS® Depression v1.0 | Assesses negative mood, negative views of self, negative social cognition, and decreased positive affect and engagement | IRT T-score | More depression | |
| PROMIS® Anxiety v1.0 | Assesses fear (e.g., fearfulness, feelings of panic), anxious misery (e.g., worry, dread), hyperarousal (e.g., tension, nervousness, restlessness), and somatic symptoms related to arousal (e.g., racing or pounding heart, dizziness). | IRT T-score | More anxiety | |
| PROMIS® Cognitive Function v1.0/v2.0b | Assesses a person’s perception of functional abilities with regard to cognitive tasks in areas such as concentration, memory and mental acuity, including perceptions regarding change in one’s cognitive ability. | IRT T-score | Better cognitive function | |
| Social Health | ||||
| Social Function | ||||
| PROMIS® Ability to Participate in Social Roles and Activities v2.0 | Assesses the perceived ability to perform one’s usual social roles and activities. | IRT T-score | Fewer limitations (better ability to participate in social roles) | |
| PROMIS® Satisfaction with Social Roles and Activities v2.0 | Assesses satisfaction with performing one’s usual social roles and activities. | IRT T-score | Greater satisfaction. | |
| Social Relationships | ||||
| PROMIS® Social Support v2.0: Emotional Support Informational Support Instrumental Support | Three measures that assess functional aspects of supportive relationships: - Emotional (Perceived feelings of being cared for and valued as a person; having confidant relationships) - Informational (Perceived availability of helpful information or advice) - Instrumental (Perceived availability of assistance with material, cognitive or task performance) |
IRT T-score | More support | |
| Measures Used as Responsiveness Indicators (Anchors) | ||||
| Patient-Reported Outcomes | ||||
| Kansas City Cardiomyopathy Questionnaire (KCCQ-12) | Assesses heart failure-specific health-related quality of life (HRQOL); four subscales/domains and an overall summary score: - Physical Limitation - Symptom Frequency - Quality of Life - Social Limitation - Overall |
Sum score: 0 to 100 | Better HRQOL | |
| EuroQOL Five-Dimension, Three-Level (EQ-5D-3L) | Assesses generic health status; five dimensions: - Mobility - Self-care - Usual Activities - Pain/Discomfort - Anxiety/Depression Visual Analogue Scale (VAS) of Overall Health |
Ordinal scale for each dimension: no problems, some problems, extreme problems VAS: 0 to 100 |
Each dimension: extreme problems VAS: best imaginable health. |
|
| PROMIS® Global Health v1.1 | Evaluations of health in general; 4 items: - Quality of Life (02) - Mental Health (04) - Usual Social Activities and Roles (09) - Bothered by Emotional Problems (10) |
Ordinal scale: Poor to Excellent Poor to Excellent Poor to Excellent Always to Never |
Better Health | |
| Global Ratings of Change | Five retrospective assessments of change by the patient, who is asked to state whether they have experienced change from pre-implant to the present (3 months post-implant): - Physical Function - Quality of Sleep - Anxiety - Mood - Social Activities and Roles |
A lot better, A little better, About the same, A little bit worse, A lot worse | Worse change | |
| Clinician Rating | ||||
| NYHA Functional Classification | Clinician rating of how severely symptoms of heart failure limit physical activity | Ordinal scale: No limitation, Slight limitation, Marked limitation, Unable to carry on any physical activity without discomfort | Worse heart failure-related functional status | |
| Performance Test | ||||
| 6-Minute Walk Test | Assessment of functional capacity during a 6-minute walk in an enclosed hallway, which is free of traffic and distractions | Meters walked | Greater functional capacity | |
Score metric: Information about how the instrument is scored
Although two versions of this measure were used, the scoring was recalibrated to be comparable.
Item Response Theory (IRT) T-score: mean=50, standard deviation=10
sum score: aggregated item responses
Statistical methods
Missing data were evaluated to determine the most appropriate analytic strategies. Most sociodemographic and clinical characteristics had little to no missing data (0%–10%). Over 70% of study participants did not complete the 6-Minute Walk Test pre-implant, and over 50% did not complete it post-implant. Missing data for sociodemographic and clinical variables were excluded from analyses. The amount, reasons and patterns of missing PROMIS data were evaluated to determine if the missing data were missing completely at random (MCAR), missing at random (MAR), or missing not at random (MNAR).43 Six participant groups were created based on the time of the last assessment and reason for withdrawal/dropout.43, 44 Characteristics were compared across the six groups using a chi-square, Fisher’s exact test or analysis of variance F test. Completion of the PRO measures was also continuously monitored and there was no evidence of any pattern of missingness by measure.
Repeated measures analyses:
A mixed model framework for repeated measures data was implemented using all available data for 251 participants (92%) who had at least one PROMIS measurement.45, 46 If missing data are MCAR or MAR, a mixed model is advantageous as all available data can be used, i.e., analyses are not restricted to only participants with complete data over time.45, 46 Covariance pattern models were estimated with unstructured covariance parameters for the three repeated measures (pre-implant, and Months 3 and 6 post-implant). The Wald statistic was used as the Type 3 test of fixed effects, with a nominal significance level p<0.05. To evaluate the possibility of MNAR, pattern mixture models were implemented.43, 46 In these models, parameters were estimated within each missing pattern group which allows for different PROMIS scores and variability for each group at each assessment. The pattern mixture models and the models using pooled data were compared with a maximum likelihood ratio test and evaluated using a chi-square distribution with eight degrees of freedom. Analyses were conducted separately for each PROMIS measure.
Responsiveness indicators:
To evaluate PROMIS measures’ sensitivity to change (responsiveness), PROMIS change scores were mapped to change in other variables.47 Two sets of anchor change scores were calculated: the difference between pre-implant and Month 3, and the difference between Months 3 and 6. Three independent groups were formed for each anchor change score (better, no change, worse). For each KCCQ-12 domain, an increase (or decrease) of at least five points was considered better (or worse).48 For each EQ-5D-3L dimension, an improvement (or decline) of at least one level was considered better (or worse).49 For the EQ-5D-3L Visual Analogue Scale, an increase (or decrease) of at least ten points was considered better (or worse).50 For PROMIS global health items, an improvement (or decline) of at least one point was considered better (or worse). For each Global Rating of Change, “a lot better” and “a little better” were combined, as were “a lot worse” and “a little worse.” For NYHA, an improvement (or decline) of at least one class was considered better (or worse).49 For the 6-Minute Walk Test an increase (or decrease) in distance walked of at least 112 meters was considered better (or worse).51 The usefulness of each anchor was assessed by evaluating the correlation between PROMIS change scores and the anchor change categories.52 A Spearman correlation of 0.30 or greater defined an acceptable association.16 If a correlation did not meet this criterion, then that anchor was dropped from further analysis. The anchor change variables (better, no change, worse) were added to the repeated measures covariance pattern models.
Data collection, management and analysis were conducted with REDCap (Research Electronic Data Capture)53 and SAS/STAT software, Version 9.4.54
Results
Participants
A flow diagram for the MCS A-QOL study is shown in Figure 1. About one-quarter of eligible patients declined to participate (n=84). The majority who declined reported being too sick, too tired, or too stressed/anxious/depressed (n=54). Of the 272 enrolled participants, 89% completed the Baseline (pre-implant) assessment, and about half completed the Month 3 and Month 6 post-implant assessments. A total of 36 participants did not complete the study because they underwent heart transplantation (13% of enrolled), 38 participants died (14% of enrolled), and 20 were withdrawn for other reasons. Other missingness reasons at each assessment are also shown in Figure 1, with the most common reason being passive refusal, e.g., the participant agreed to complete assessments, but never did.
Figure 1.
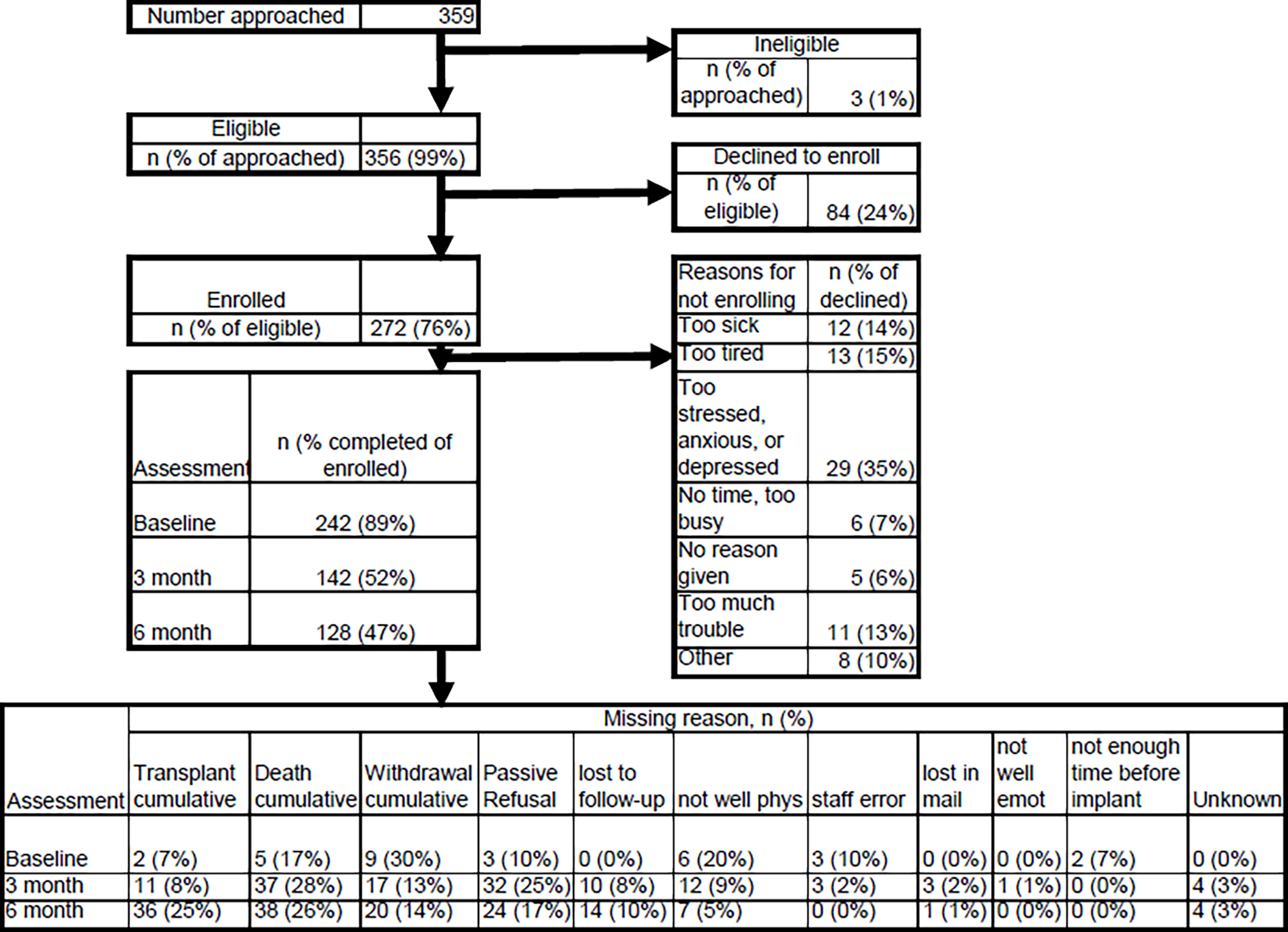
Flow Diagram for the MCS A-QOL Study
Table 2 summarizes the sociodemographic and clinical characteristics of all participants enrolled in MCS A-QOL (n=272), by the time of the last assessment and reason for withdrawal/dropout. There were 21 (8%) enrolled participants who did not complete any PROs (Group A), 84 (31%) who completed the pre-implant assessment only (Group B), and 94 (34%) who completed all three longitudinal assessments (Group F). The remaining 73 (27%) participants (Groups C, D, E) completed assessments at Month 3 and/or Month 6 post-implant, and nearly all of these participants completed the pre-implant assessment. The six groups were comparable on nearly all characteristics. Among the model sample (Groups B-F; n=251) the majority of participants had either a HeartWare HVAD (51%) or HeartMate III (39%) device.
Table 2.
Characteristics of Study Participants (n=272), by Time of Last PRO Assessment and Reason for Withdrawal/Dropout
| Group | ||||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | Groups B-F | ||
| No Assessments Completed (n=21) | Pre-Implant Assessment Only (n=84) | Withdrawn for Heart Transplant After Month 3 (n=23)a | Withdrawn/Dropped Out for Other Reason After Month 3 (n=16) | Month 6 Assessment Completed (n=34)b | All Pre- and Post-Implant Assessments Completed (n=94) | p-value | Model sample (n=251) | |
| Sociodemographic Characteristics | ||||||||
| Age in Years, mean (SD) | 61.0 (9.5) | 56.6 (11.2) | 50.7 (12.3) | 50.4 (17.7) | 58 (12.6) | 55.5 (12.0) | 0.030 | 55.4 (12.4) |
| Female | 3 (14%) | 26 (31%) | 3 (13%) | 4 (25%) | 10 (29%) | 21 (22%) | 0.396 | 64 (26%) |
| Married/committed partner | 11 (52%) | 50 (62%) | 13 (59%) | 4 (25%) | 23 (68%) | 57 (61%) | 0.096 | 147 (60%) |
| Ethnicity, Race | ||||||||
| Hispanic, any race | 1 (5%) | 13 (16%) | 2 (9%) | 3 (19%) | 1 (3%) | 6 (6%) | 0.090 (Non-Hispanic White vs. All Others) | 25 (10%) |
| Non-Hispanic, White | 16 (76%) | 42 (52%) | 15 (65%) | 9 (56%) | 22 (65%) | 67 (72%) | 155 (63%) | |
| Non-Hispanic, Black | 3 (14%) | 21 (26%) | 3 (13%) | 4 (25%) | 6 (18%) | 16 (17%) | 50 (20%) | |
| Non-Hispanic, Other | 1 (5%) | 5 (6%) | 3 (13%) | 0 (0%) | 5 (15%) | 4 (4%) | 17 (7%) | |
| Highest Education | ||||||||
| High School or Less | 6 (30%) | 30 (36%) | 11 (48%) | 8 (50%) | 14 (41%) | 29 (32%) | 0.514 (High School or Less vs. All Others) | 92 (37%) |
| Attended College/Tech School | 8 (40%) | 27 (33%) | 6 (26%) | 5 (31%) | 10 (29%) | 27 (29%) | 75 (30%) | |
| Associate/Bachelor’s Degree | 4 (20%) | 20 (24%) | 3 (13%) | 1 (6%) | 8 (24%) | 29 (32%) | 61 (25%) | |
| Graduate Degree | 2 (10%) | 6 (7%) | 3 (13%) | 2 (13%) | 2 (6%) | 7 (8%) | 20 (8%) | |
| Clinical Characteristics (prior to LVAD Implant) | ||||||||
| LVAD Implant Strategy | ||||||||
| Bridge to transplant/Short-term McS | 5 (38%) | 17 (21%) | 15 (65%) | 2 (13%) | 9 (26%) | 24 (26%) | 0.003 (Destination vs. All Others) | 67 (27%) |
| Possible bridge to transplant/Possible short-term MCS | 0 (0%) | 17 (21%) | 4 (17%) | 3 (20%) | 2 (6%) | 22 (24%) | 48 (19%) | |
| Destination therapy/Long-term MCS | 8 (62%) | 48 (59%) | 4 (17%) | 10 (67%) | 23 (68%) | 47 (51%) | 132 (53%) | |
| Etiology of Heart Failure | ||||||||
| Dilated cardiomyopathy | 9 (69%) | 48 (57%) | 17 (74%) | 11 (73%) | 22 (65%) | 65 (70%) | 0.494 (Dilated vs. All Others) | 163 (65%) |
| Ischemic cardiomyopathy | 2 (15%) | 21 (25%) | 5 (22%) | 3 (20%) | 7 (21%) | 22 (24%) | 58 (23%) | |
| Other | 2 (15%) | 15 (18%) | 1 (4%) | 1 (7%) | 5 (15%) | 6 (6%) | 28 (11%) | |
| NYHA Class | ||||||||
| Class I | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | 0.568 (I-III vs. IV) | 1 (0%) |
| Class II | 0 (0%) | 1 (1%) | 1 (4%) | 0 (0%) | 1 (3%) | 2 (2%) | 5 (2%) | |
| Class III | 1 (8%) | 16 (19%) | 7 (30%) | 2 (13%) | 6 (18%) | 18 (19%) | 49 (20%) | |
| Class IV | 11 (92%) | 67 (80%) | 15 (65%) | 13 (87%) | 26 (79%) | 72 (77%) | 193 (78%) | |
| Intermacs Profile | ||||||||
| 1 (critical cardiogenic shock) & 2 (progressive decline) | 8 (67%) | 39 (49%) | 6 (26%) | 5 (33%) | 9 (28%) | 25 (27%) | 0.686 (1–2 vs. 37) | 84 (35%) |
| 3 (stable, but inotrope-dependent) | 2 (17%) | 24 (30%) | 10 (43%) | 8 (53%) | 14 (44%) | 41 (45%) | 97 (40%) | |
| 4–7 (ambulatory advanced heart failure) | 2 (17%) | 16 (20%) | 7 (30%) | 2 (13%) | 9 (28%) | 25 (27%) | 59 (25%) | |
| Left Ventricular Ejection Fraction | ||||||||
| 30+ (normal/mild/moderate) | 0 (0%) | 3 (4%) | 1 (5%) | 1 (7%) | 4 (13%) | 3 (3%) | 0.334 (Severe vs. All Others) | 12 (5%) |
| 20–29 (moderate/severe) | 5 (38%) | 32 (40%) | 5 (26%) | 6 (43%) | 9 (28%) | 17 (20%) | 69 (28%) | |
| <20 (severe) | 8 (62%) | 45 (56%) | 13 (68%) | 7 (50%) | 19 (59%) | 67 (77%) | 151 (61%) | |
Pre-Implant Assessment missing for n=1 participant
Pre-Implant Assessment missing for n=9 participants; Month 3 Assessment missing for n=27
Entries in the table represent the number of participants (percentage), unless otherwise specified. Missing data were excluded.
MCS: mechanical circulatory support
PRO: Patient-Reported Outcome
p-value for comparison of Groups A through F
Repeated Measures Analyses
Groups B-F (n=251) were pooled for the longitudinal analyses. Covariance pattern models for 11 PROMIS measures (Fatigue, Physical Function, Sleep Disturbance, Sleep-related Impairment, Depression, Anxiety, Cognitive Function, Ability to Participate in Social Roles, Satisfaction with Social Roles and Activities, Emotional Social Support, and Instrumental Social Support) demonstrated statistically significant results (p<0.01) for the time effect (pre-implant, and Months 3 and 6 post-implant). Only one PROMIS measure (Informational Social Support) showed no change over time (p=0.277). Pattern mixture models to evaluate the possibility of MNAR data showed no significant change from the pooled data models (p>0.09 for all likelihood ratio test comparisons).
Figures 2–5 show least-squares means and 95% confidence intervals from the repeated measures models. There was a statistically significant improvement from Baseline (pre-implant) to Month 3 post-implant for 11 PROMIS measures, and no change between Months 3 and 6 post-implant. The direction of change (positive or negative) was consistent with the meaning of a high PROMIS score (more of the concept being measured). For example, Fatigue scores decreased (improved) from pre- to post-implant, whereas Physical Function increased (improved) over time. For all four PROMIS Physical Health measures (Figure 2), pre-implant mean scores were worse than the mean score of the general population (see horizontal reference line at 50), while post-implant scores often were nearly 50. For PROMIS Mental Health measures (Figure 3), Depression and Cognitive Function were nearly 50 at all three assessments, whereas Anxiety was higher (worse) pre-implant and then decreased to nearly 50 post-implant. For both PROMIS Social Function measures (Figure 4), pre-implant scores were lower (worse) than 50 and then improved post-implant but did not reach 50. For all three PROMIS Social Support measures (Figure 5), scores at all three assessments were higher (better) than 50.
Figure 2.
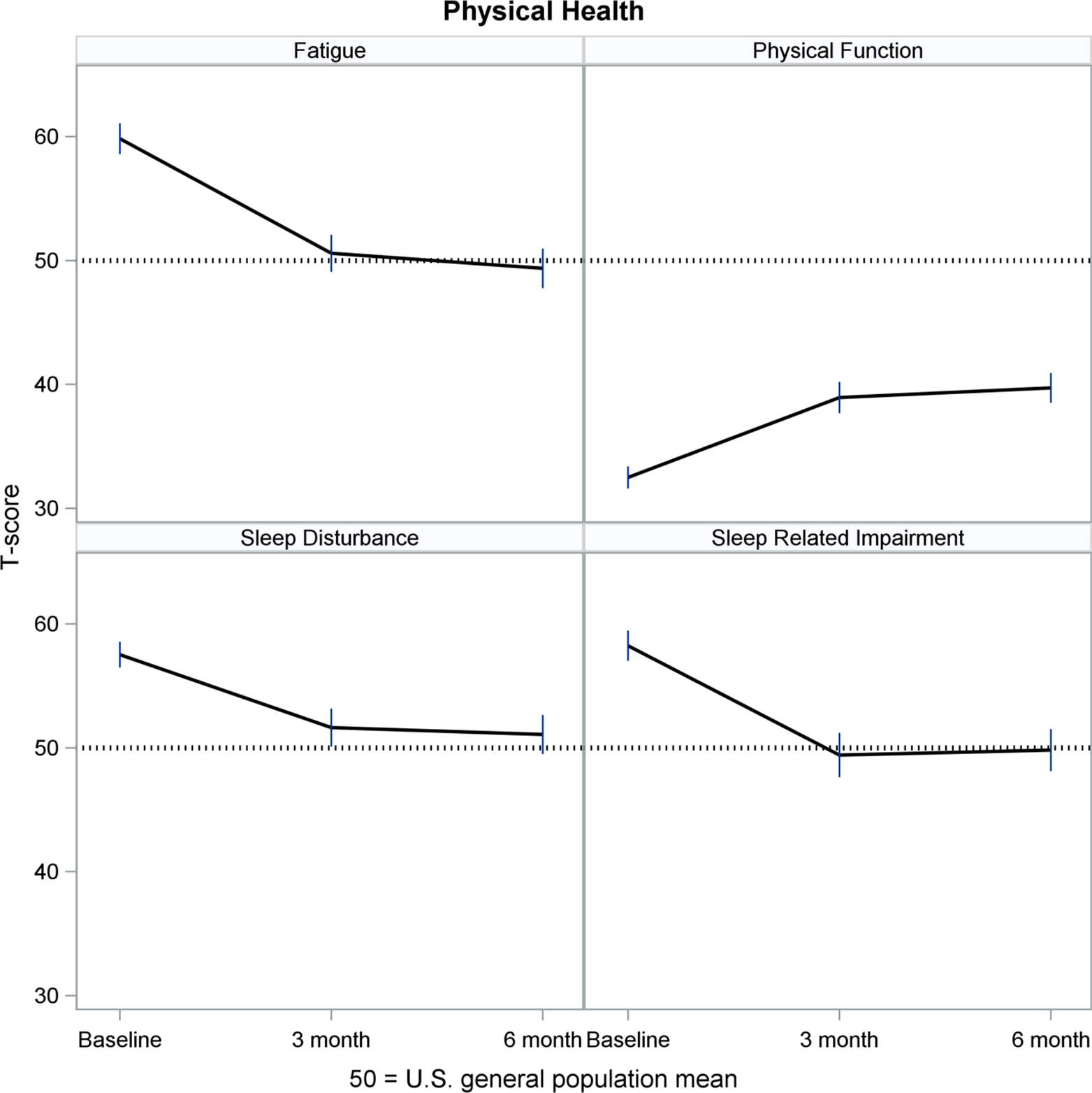
Physical Health Over Time
Figure 5.
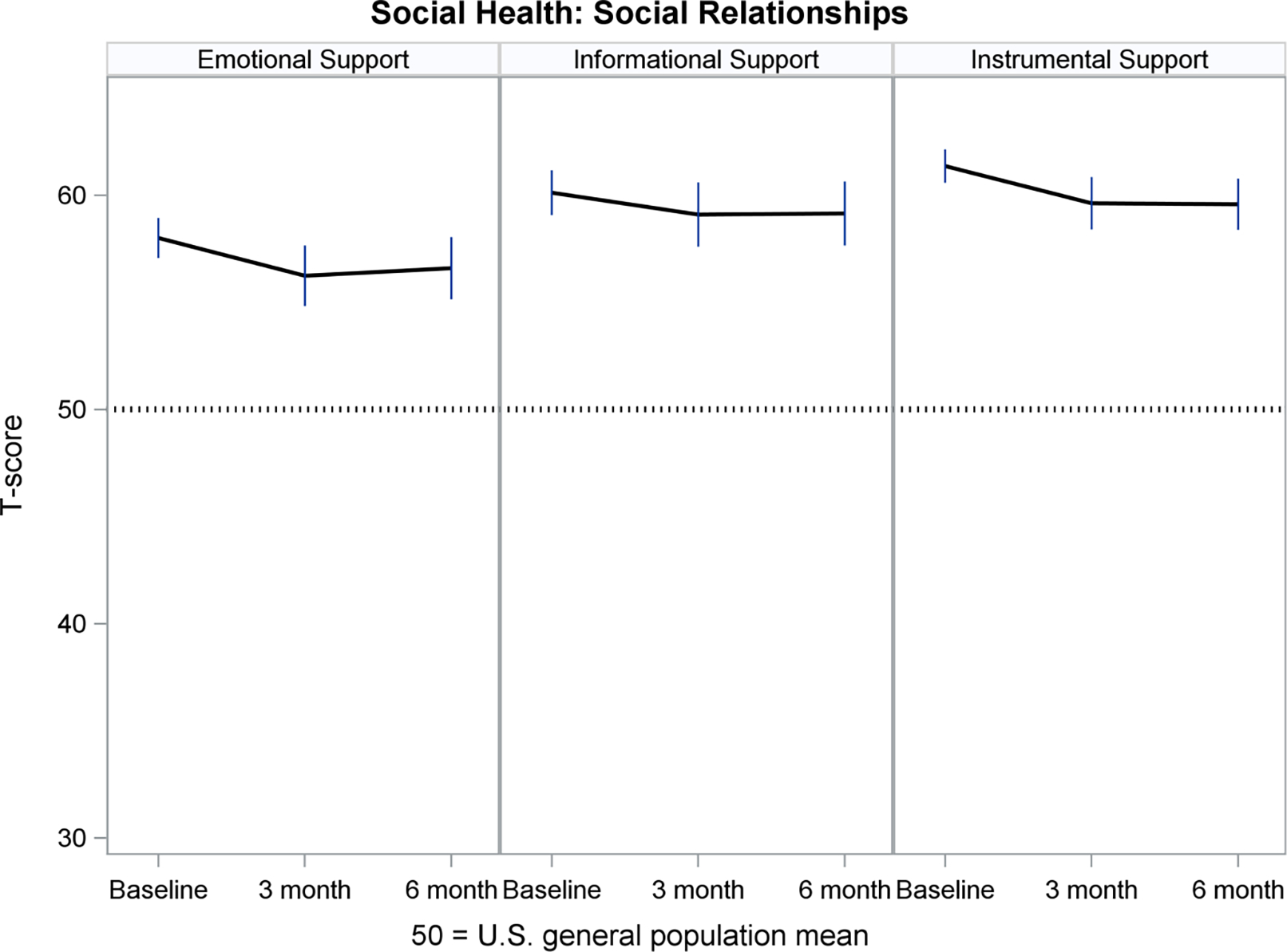
Social Health: Social Relationships Over Time
Figure 3.
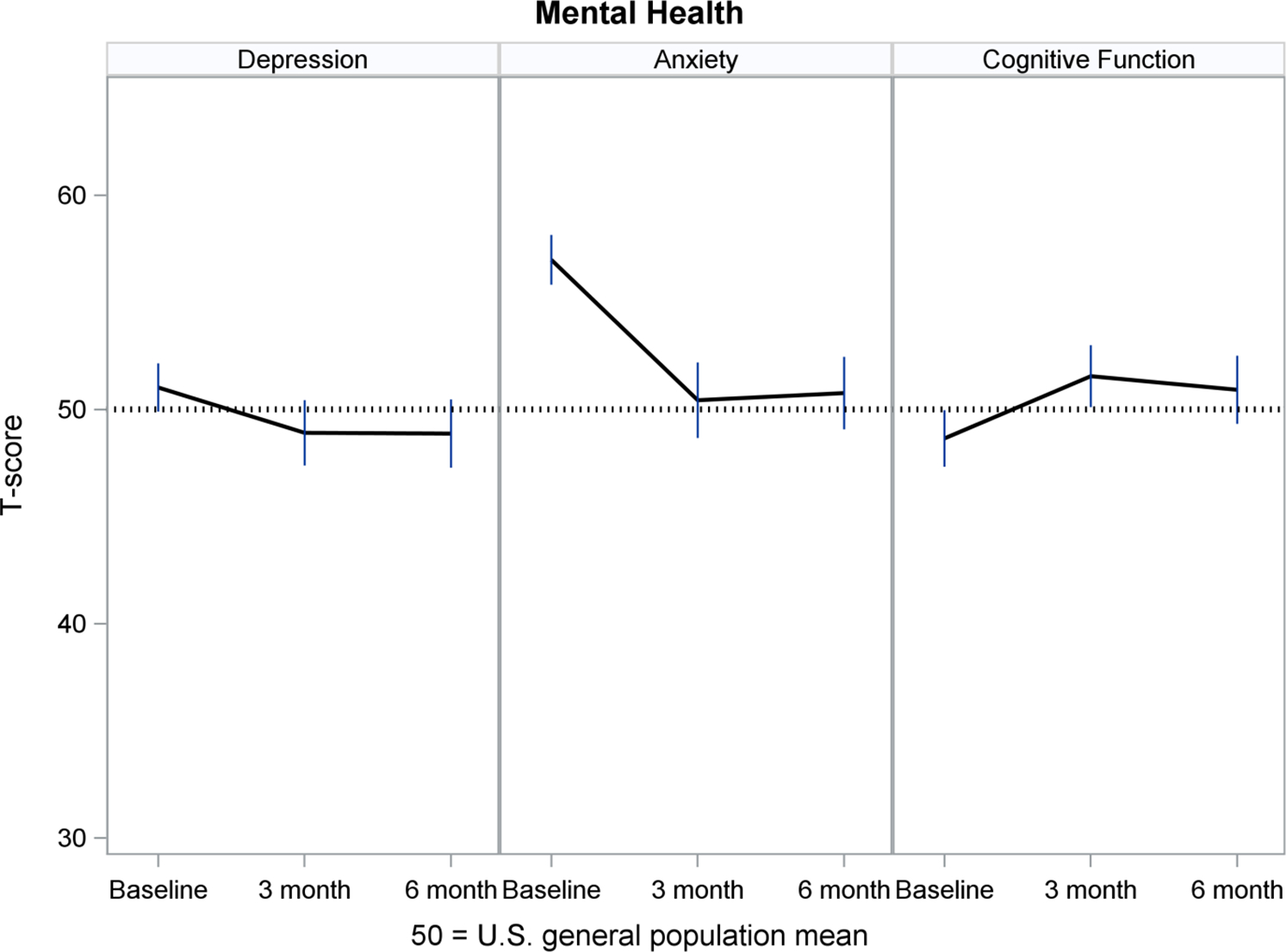
Mental Health Over Time
Figure 4.
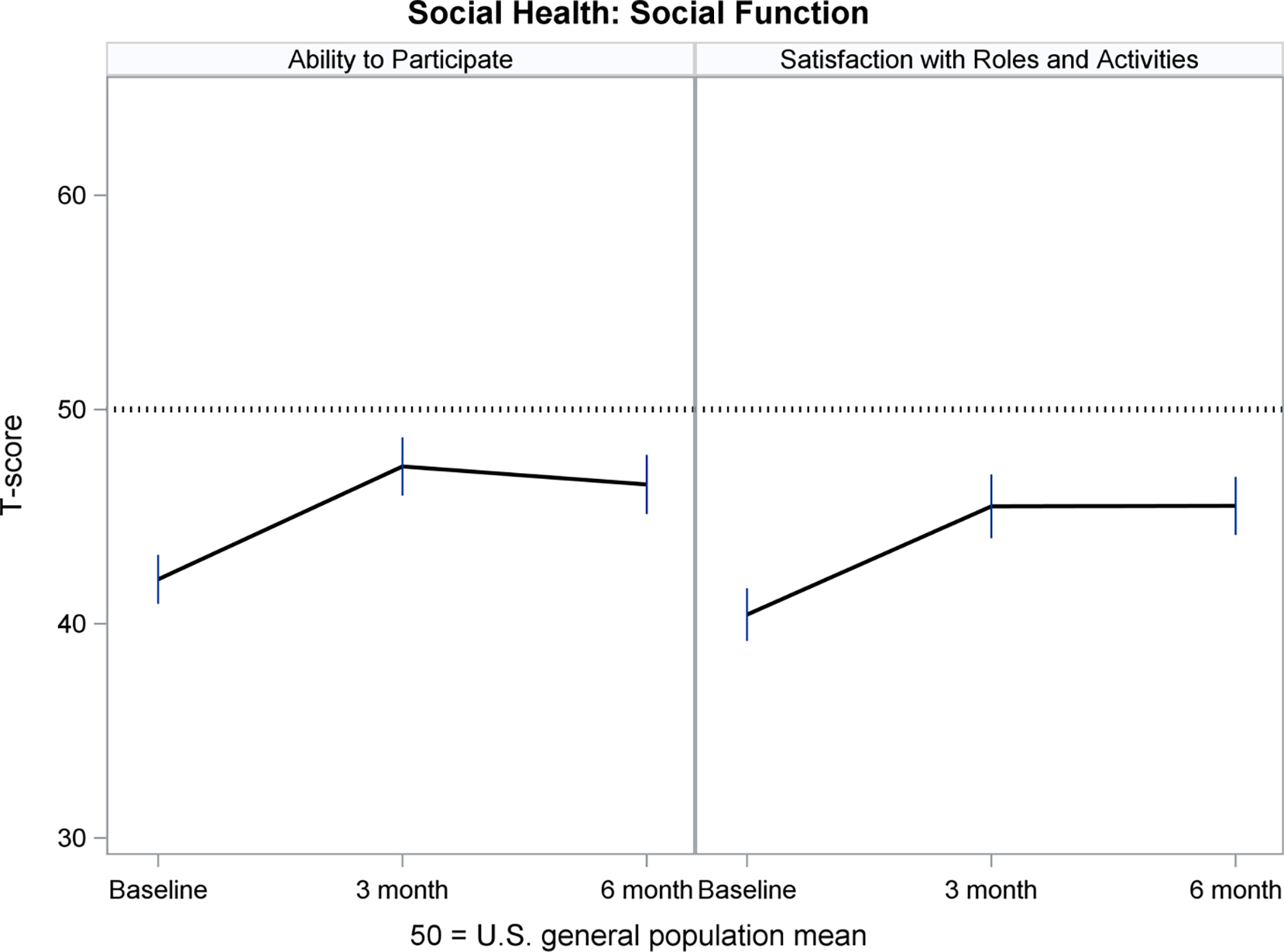
Social Health: Social Function Over Time
Responsiveness Analyses
The proportions of participants who were better, the same or worse on the responsiveness indicators (anchors) varied across anchors. Specifically, the majority of participants got better from pre-implant to Month 3 post-implant for the KCCQ-12 Quality of Life and Social Limitation, EQ-5D VAS, PROMIS Global Quality of Life and Usual Social Activities and Roles, Global Ratings of Change, NYHA, and 6-Minute Walk Test (range: 52%–82%). The proportions who got better for the remaining anchors (KCCQ-12 Physical Limitation, EQ-5D Usual Activities, Anxiety/Depression and Mobility, PROMIS Global Mental Health and Emotional Problems) ranged from 25% to 46%. The majority of participants stayed the same from Months 3 to 6 post-implant for EQ-5D Usual Activities, Anxiety/Depression and Mobility, PROMIS Global Emotional Problems, and NYHA (range: 55%–97%).
PROMIS least-squares means from the repeated measures models are shown in Table 3 for the anchor change variables. For most anchors, PROMIS mean changes were largest for participants who got better compared to those who stayed the same or got worse, e.g., the mean PROMIS Fatigue change was −13.7 for those who got better on EQ-5D-3L Usual Activities from pre-implant to Month 3 post-implant, −5.3 for those who stayed the same, and −3.5 for those who got worse. All anchor analyses for the change from pre-implant to Month 3 post-implant demonstrated statistically significant differences (p<0.05). About half of the anchor analyses for the change from Month 3 to Month 6 post-implant demonstrated significant differences.
Table 3.
Mean PROMIS® Change Scores, by Change in Responsiveness Indicator (Anchor)
| PROMIS Measure and Responsiveness Indicator (Anchor)1 | Mean PROMIS Change2 from Pre-Implant to 3 Months Post-Implant | Mean PROMIS Change2 from 3 to 6 Months Post-Implant | ||||||
|---|---|---|---|---|---|---|---|---|
| Better | Same | Worse | p-value3 | Better | Same | Worse | p-value3 | |
| PROMIS Fatigue | ||||||||
| EQ-5D-3L Usual Activities | −13.7 (1.6) | −5.3 (1.5) | −3.5 (3.1) | <0.001 | −3.4 (2.1) | −1.9 (1.4) | 2.8 (2.3) | 0.132 |
| 6-Minute Walk Test | −10.2 (1.8) | −1.4 (3.9) | 1.2 (4.0) | 0.017 | −7.5 (2.1) | 1.1 (2.0) | −2.1 (2.4) | 0.015 |
| PROMIS Physical Function | ||||||||
| KCCQ-12 Physical Limitations | 8.5 (1.2) | 4.6 (2.3) | 2.7 (1.3) | 0.005 | 2.1 (.9) | −1.6 (1.9) | −.2 (1.4) | 0.139 |
| EQ-5D-3L Mobility | 10.9 (1.3) | 3.7 (1.1) | .1 (2.2) | <0.001 | 5.1 (1.6) | 8 (.8) | −2.8 (1.8) | 0.005 |
| Global Rating of Change | 7.3 (.8) | 5.4 (2.4) | −.7 (2.1) | 0.003 | --- | --- | --- | --- |
| 6-Minute Walk Test | 7.5 (1.2) | −.4 (2.6) | −4.9 (3.1) | <0.001 | 2.1 (1.5) | −.9 (1.4) | 1.1 (1.7) | 0.307 |
| PROMIS Sleep Disturbance | ||||||||
| PROMIS Quality of Life | −6.5 (1.1) | −5.6 (2.1) | 1.3 (2.6) | 0.024 | −1.6 (1.8) | 1.4 (1.3) | −2.2 (1.8) | 0.189 |
| Global Rating of Change | −8.1 (1.2) | −5.3 (1.7) | −.8 (1.9) | 0.006 | --- | --- | --- | --- |
| PROMIS Sleep-related Impairment | ||||||||
| PROMIS Quality of Life | −11.0 (1.2) | −4.5 (2.4) | 6.1 (3.0) | <0.001 | −3.6 (2.0) | .2 (1.4) | 4.2 (2.0) | 0.024 |
| PROMIS Depression | ||||||||
| EQ-5D-3L Anxiety/Depression | −5.2 (1.6) | −2.1 (1.0) | 5.8 (2.7) | 0.002 | −5.5 (2.7) | .6 (1.1) | 1.3 (2.6) | 0.104 |
| PROMIS Emotional Problems | −7.1 (1.1) | −1.9 (1.1) | 4.6 (1.5) | <0.001 | −6.3 (1.3) | 1.0 (1.0) | 6.4 (1.9) | <0.001 |
| Global Rating of Change | −3.8 (1.0) | −2.4 (1.4) | 4.7 (2.2) | 0.003 | --- | --- | --- | --- |
| PROMIS Anxiety | ||||||||
| EQ-5D-3L Anxiety/Depression | −10.9 (2.2) | −5.0 (1.4) | .7 (3.5) | 0.013 | −6.1 (3.2) | 8 (1.3) | 3.8 (3.0) | 0.072 |
| PROMIS Emotional Problems | −14.2 (1.4) | −5.1 (1.4) | 4.0 (1.9) | <0.001 | −5.3 (1.6) | .9 (1.2) | 6.3 (2.4) | <0.001 |
| Global Rating of Change | −9.5 (1.3) | −7.3 (2.1) | 2.2 (2.4) | <0.001 | --- | --- | --- | --- |
| PROMIS Cognitive Function | ||||||||
| PROMIS Mental Health | 6.8 (1.3) | .4 (1.4) | −.7 (1.7) | <0.001 | 2.2 (1.9) | .3 (1.2) | −2.5 (1.6) | 0.144 |
| PROMIS Ability to Participate in Social Roles and Activities | ||||||||
| KCCQ-12 Social Limitation | 6.6 (1.2) | 2.2 (3.3) | .8 (2.8) | 0.107 | 2.7 (1.3) | −2.3 (2.4) | −3.0 (1.7) | 0.020 |
| EQ-5D-3L Usual Activities | 9.2 (1.6) | 3.1 (1.5) | −1.3 (3.2) | 0.003 | 1.2 (2.0) | −.5 (1.3) | −1.5 (2.2) | 0.644 |
| PROMIS Social Activities | 8.9 (1.3) | 3.4 (1.7) | −1.5 (2.0) | <0.001 | 4.5 (1.3) | .9 (1.3) | −5.5 (1.5) | <0.001 |
| Global Rating of Change | 9.2 (1.1) | 2.7 (1.8) | −2.5 (1.9) | <0.001 | --- | --- | --- | --- |
| PROMIS Satisfaction with Social Roles and Activities | ||||||||
| PROMIS Social Activities | 9.5 (1.4) | 2.4 (1.9) | −4.5 (2.2) | <.001 | 4.3 (1.2) | .5 (1.1) | −4.4 (1.3) | <.001 |
Better, Same and Worse categories were defined by changes in the anchor (responsiveness indicator); see Methods
Mean Change: Least-squares mean (standard error)
p-value for overall comparison of three categories (Better, Same, Worse)
---: Global Rating of Change was not measured at 6 months
Discussion
Findings from the MCS A-QOL longitudinal study may be useful to describe the benefits of LVAD implantation. The study assessed a wide range of physical, mental and social health outcomes and the results showed how outcomes were affected by LVAD from pre- to post-implant. Specifically, the PROMIS measures captured large improvements from pre-implant to three months post-LVAD implant and showed little change between three and six months. These findings demonstrate the dramatic very early positive impact of LVAD on PROs, which was also reported by others.8, 55 In MCS A-QOL PROMIS measures were also responsive to change in other measures commonly used with LVAD patients, e.g., PROMIS mean changes improved (or worsened) for participants who improved (or worsened) on KCCQ-12 and EQ-5D-3L subscales.
PROMIS measures have been used in diverse populations, but only a few studies evaluated their usefulness in heart failure populations,24, 56–59 including those who undergo heart transplantation.60, 61 MCS A-QOL findings build upon the only other study to use PROMIS measures in an LVAD population.62 However, that study was cross-sectional and was limited by the use of only three PROMIS measures and variable times for data collection relative to implant date. This MCS A-QOL report evaluated the use of 12 PROMIS measures (four physical health, three mental health, and five social health) administered pre- and post-implant in LVAD patients at 12 sites.
PROMIS measures are advantageous for use in populations with multiple chronic illnesses, which allows comparability of experiences across diseases.20 Hence, patients with an LVAD, their caregivers and their clinicians can interpret the meaning of PROMIS scores in relation to the general population, helping them to monitor a return to normalcy in everyday life. For example, in MCS A-QOL, PROMIS Fatigue, Sleep Disturbance, Sleep-related Impairment and Anxiety measures prior to implant were worse than the general population mean, while post-implant scores improved to the general population mean. PROMIS Physical Function and Social Function measures improved from pre- to post-implant, although scores did not reach the general population mean. PROMIS Depression and Cognitive Function were near the general population mean from pre- to post-implant, and PROMIS Social Support measures were higher than the general population pre- and post-implant.
Use of PROMIS measures may enhance shared decision-making as patients consider the option of LVAD implantation and may support surveillance of HRQOL-related problems that may occur after implant, which can guide targeted therapies. For example, reduction in sleep disturbance and improvement in the ability to participate in social roles and activities, which occurred from pre-implant to Month 3 post-implant in MCS A-QOL, may be useful to describe benefits of LVAD implantation when considering this treatment option. Notably, tracking changes in PRO outcomes across time in individual patients could provide clinicians with the opportunity to identify positive and negative changes and address them with patients. A large study of patients with heart failure demonstrated the feasibility of integrating PRO assessments into outpatient clinic visits; however, the authors also identified barriers related to completion of PRO measures by patients and incorporation of PROs into clinical practice by clinicians.63 Patients reported that PRO measures (including PROMIS measures) were useful in identifying concerns, but also indicated that their value was reduced by lack of discussion with clinicians.64 More work is needed to enhance implementation of PROs into clinical practice.
There are some limitations to this study. The sample lacked diversity, as the majority were male, non-Hispanic White, well-educated and married; however, this is comparable to LVAD recipients in the U.S. Generalizability is limited because patients who were too ill did not enroll in the study, and the proportion of participants who were transplanted at 6 months (13%) was somewhat higher than registry data (8%).65
Participants were only evaluated up to six months post-LVAD implant; longer follow-up is needed to better understand PRO changes over time. Missing data are a common challenge in clinical research. While only 94 participants (35%) completed all three assessments (pre-implant, and Months 3 and 6 post-implant) in MCS A-QOL, implementation of state-of-the-science methods for missing data, including pattern mixture models for MNAR,45, 46 allowed 251 participants (92%) to be included in longitudinal modeling. The anchor-based analyses, however, were based only on survivors.
This longitudinal report from MCS A-QOL expands the body of evidence for the validity of PROMIS measures by establishing that they are sensitive to clinical change and changes in health status in people with an LVAD. This suggests that these measures can be used in clinical practice and in longitudinal observational research and clinical trials, alongside heart failure-specific measures, to provide a more comprehensive assessment of the impact of advanced heart failure and its surgical treatment on patients. Subsequent reports from MCS A-QOL will include the development of an LVAD-specific set of PRO measures.
Supplementary Material
Acknowledgements
This study was funded by the National Heart, Lung and Blood Institute of the National Institutes of Health; grant # R01-HL130502.
Footnotes
Financial Conflict of Interest Statement
The authors report the following relationships: Grants or contracts (CSL: NIH; QED: NIH, Medical Research Foundation; JS: Natera; BR: PCORI, UCSD); consulting fees (LAA: ACI Clinical, Amgen, Boston Scientific, Cytokinetics, Novartis; JS: Medtronic; JL: Abbott, AstraZeneca, Alleviant, Boston Scientific, Merck, CVRx, VWave, Edwards; EDA: Abiomed, Novartis, Abbott, Ionis Pharmaceuticals, Sana Biotechnology, Medtronic, Lexeo Pharmaceuticals, Cytokinetics; KLG: Amgen); Payment or honoraria (LAA: UpToDate; JJT: CareDx, Medtronic, Paragonix; MSK: Medtronic; BR: TEACH faculty development program; KLG: AHA); Payment for expert testimony (EDA: Astra Zeneca); Meeting/travel support (MSK: Abbott; KLG: ISHLT, AHA); Data Safety Monitoring Board or Advisory Board (JJT: CareDx, Medtronic, Abiomed, Takeda, Abbott; MSK: Medtronic; JKK: Carmat and Xeltis clinical trials); Leadership or fiduciary role (EDA: Papillon Therapeutics, ResQ Pharmaceuticals); JKK: ISHLT Research Foundation, International Society for Heart & Lung Transplantation; KLG: ISHLT); Other (LAA: Circulation: Heart Failure, Associate Editor; DC: President-elect, PROMIS Health Organization; JKK: Director of the Data Center for STS-Intermacs Registry for Mechanical Circulatory Support)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rumsfeld J, Alexander K, Goff DJ, Graham M, Ho P, Masoudi F, et al. Cardiovascular health: The importance of measuring patient-reported health status: A scientific statement from the american heart association. Circulation. 2013;127:2233–2249 [DOI] [PubMed] [Google Scholar]
- 2.Brouwers CJ, Denollet J, de Jonge N, Caliskan K, Kealy J, Pedersen SS. Patient-reported outcomes in left ventricular assist device therapy: A systematic review and recommendations for clinical research and practice. Circulation. Heart failure. 2011;4:714–723 [DOI] [PubMed] [Google Scholar]
- 3.Grady KL, Naftel D, Stevenson L, Amanda Dew M, Weidner G, Pagani FD, et al. Overall quality of life improves to similar levels after mechanical circulatory support regardless of severity of heart failure before implantation. The Journal of heart and lung transplantation. 2014;33:412–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grady KL, Naftel DC, Myers S, Dew MA, Weidner G, Spertus JA, et al. Change in health-related quality of life from before to after destination therapy mechanical circulatory support is similar for older and younger patients: Analyses from intermacs. The Journal of heart and lung transplantation. 2015;34:213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grady KL, Wissman SW, Naftel DC, Myers S, Gelijins A, Moskowitz A, et al. Age and gender differences and factors related to change in health-related quality of life from before to 6 months after left ventricular assist device implantation: Findings from interagency registry for mechanically assisted circulatory support. The Journal of heart and lung transplantation. 2016;35:777–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White-Williams C, Fazeli-Wheeler P, Myers S, Kirklin J, Pamboukian S, Naftel D, et al. Hrqol improves from before to 2 years after mcs, regardless of implant strategy: Analyses from intermacs. The Journal of Heart and Lung Transplantation. 2016;35:S25 [Google Scholar]
- 7.Stehlik J, Estep JD, Selzman CH, Rogers JG, Spertus JA, Shah KB, et al. Patient-reported health-related quality of life is a predictor of outcomes in ambulatory heart failure patients treated with left ventricular assist device compared with medical management: Results from the roadmap study (risk assessment and comparative effectiveness of left ventricular assist device and medical management). Circulation: Heart Failure. 2017;10:e003910. [DOI] [PubMed] [Google Scholar]
- 8.Cowger JA, Naka Y, Aaronson KD, Horstmanshof D, Gulati S, Rinde-Hoffman D, et al. Quality of life and functional capacity outcomes in the momentum 3 trial at 6 months: A call for new metrics for left ventricular assist device patients. The Journal of Heart and Lung Transplantation. 2018;37:15–24 [DOI] [PubMed] [Google Scholar]
- 9.Kormos RL, Cowger J, Pagani FD, Teuteberg JJ, Goldstein DJ, Jacobs JP, et al. The society of thoracic surgeons intermacs database annual report: Evolving indications, outcomes, and scientific partnerships. J Heart Lung Transplant. 2019;38:114–126 [DOI] [PubMed] [Google Scholar]
- 10.Lee CS, Mudd JO, Lyons KS, Denfeld QE, Jurgens CY, Aouizerat BE, et al. Heart failure symptom biology in response to ventricular assist device implantation. The Journal of cardiovascular nursing. 2019;34:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Group TE. Euroqol-a new facility for the measurement of health-related quality of life. Health policy. 1990;16:199–208 [DOI] [PubMed] [Google Scholar]
- 12.EuroQol. Eq-5d-3l user guide. Rotterdam, The Netherlands: EuroQol Research Foundation; 2018. [Google Scholar]
- 13.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the kansas city cardiomyopathy questionnaire: A new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255 [DOI] [PubMed] [Google Scholar]
- 14.Spertus JA, Jones PG. Development and validation of a short version of the kansas city cardiomyopathy questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8:469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aaronson N, Alonso J, Burnam A, Lohr KN, Patrick DL, Perrin E, et al. Assessing health status and quality-of-life instruments: Attributes and review criteria. Qual Life Res. 2002;11:193–205 [DOI] [PubMed] [Google Scholar]
- 16.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. Journal of clinical epidemiology. 2008;61:102–109 [DOI] [PubMed] [Google Scholar]
- 17.DeWalt DA, Rothrock N, Yount S, Stone AA. Evaluation of item candidates: The promis qualitative item review. Med Care. 2007;45:S12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeve BB, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, et al. Psychometric evaluation and calibration of health-related quality of life item banks: Plans for the patient-reported outcomes measurement information system (promis). Medical Care. 2007;45:S22–S31 [DOI] [PubMed] [Google Scholar]
- 19.Castel LD, Williams KA, Bosworth HB, Eisen SV, Hahn EA, Irwin DE, et al. Content validity in the promis social-health domain: A qualitative analysis of focus-group data. Qual Life Res. 2008;17:737–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The patient-reported outcomes measurement information system (promis) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riley WT, Rothrock N, Bruce B, Christodolou C, Cook K, Hahn EA, et al. Patient-reported outcomes measurement information system (promis) domain names and definitions revisions: Further evaluation of content validity in irt-derived item banks. Qual Life Res. 2010;19:1311–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grady KL, Magasi S, Hahn EA, Buono S, McGee EC, Jr., Yancy C. Health-related quality of life in mechanical circulatory support: Development of a new conceptual model and items for self-administration. J Heart Lung Transplant. 2015;34:1292–1304 [DOI] [PubMed] [Google Scholar]
- 23.Borneman MJ. Criterion validity. In: Salkind NJ, ed. Encyclopedia of research design Thousand Oaks, CA: SAGE Publications, Inc.; 2012:292–296. [Google Scholar]
- 24.Cella D, Lai Jin-Shei, Jensen SE, Chistodoulou C, Junghaenel DU, Reeve BB, Stone AA. Promis fatigue item bank had clinical validity across diverse chronic conditions. Journal of Clinical Empidemiology. 2016;73:128–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai J-S, Cella D, Choi S, Junghaenel DU, Christodoulou C, Gershon R, et al. How item banks and their application can influence measurement practice in rehabilitation medicine: A promis fatigue item bank example. Archives of physical medicine and rehabilitation. 2011;92:S20–S27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rose M, Bjorner JB, Becker J, Fries J, Ware J. Evaluation of a preliminary physical function item bank supported the expected advantages of the patient-reported outcomes measurement information system (promis). Journal of clinical epidemiology. 2008;61:17–33 [DOI] [PubMed] [Google Scholar]
- 27.Rose M, Bjorner JB, Gandek B, Bruce B, Fries JF, Ware JE Jr. The promis physical function item bank was calibrated to a standardized metric and shown to improve measurement efficiency. Journal of clinical epidemiology. 2014;67:516–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buysse DJ YL, Moul DE, Germain A, Stover A, Dodds NE, Johnston KL, Shablesky-Cade MA, Pilonkis PA. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33:781–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu L BD, Germain A, Moul DE, Stover A, Dodds NE, Johnston KL, Pilkonis PA. Development of short forms from the promis™ sleep disturbance and sleep-related impairment item banks. Behavioral Sleep Medicine. 2012;10:6–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D, et al. Item banks for measuring emotional distress from the patient-reported outcomes measurement information system (promis®): Depression, anxiety, and anger. Assessment. 2011;18:263–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pilkonis PA, Yu L, Dodds NE, Johnston KL MC, SM L. Validation of the depression item bank from the patient-reported outcomes measurement information system (promis) in a three-month observational study. Journal of Psychiatry Research. 2014;56:112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai JS, Wagner LI, Jacobsen PB, Cella D. Self-reported cognitive concerns and abilities: Two sides of one coin? Psychooncology. 2014;23:1133–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.PROMIS. Promis cognitive function scoring manual. 2019
- 34.Hahn EA, DeWalt DA, Bode RK, Garcia SF, DeVellis RF, Correia H, et al. New english and spanish social health measures will facilitate evaluating health determinants. Health Psychol. 2014;33:490–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (promis) global items. Qual Life Res. 2009;18:873–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–415 [DOI] [PubMed] [Google Scholar]
- 37.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56:395–407 [DOI] [PubMed] [Google Scholar]
- 38.Association CCotNYH. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. Boston: Little, Brown & Co; 1994. [Google Scholar]
- 39.Foray A, Williams D, Reemtsma K, Oz M, Mancini D. Assessment of submaximal exercise capacity in patients with left ventricular assist devices. Circulation. 1996;94:II222–226 [PubMed] [Google Scholar]
- 40.Cahalin LP, Mathier MA, Semigran MJ, Dec GW, DiSalvo TG. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110:325–332 [DOI] [PubMed] [Google Scholar]
- 41.Guazzi M, Dickstein K, Vicenzi M, Arena R. Six-minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: A comparative analysis on clinical and prognostic insights. Circulation: Heart Failure. 2009;2:549–555 [DOI] [PubMed] [Google Scholar]
- 42.Grady KL, Stevenson LW, Pagani FD, Teuteberg J, Pamboukian SV, Birks E, et al. Beyond survival: Recommendations from intermacs for assessing function and quality of life with mechanical circulatory support. The Journal of heart and lung transplantation. 2012;31:1158–1164 [DOI] [PubMed] [Google Scholar]
- 43.Little RJA, Rubin DB. Statistical analysis with missing data. John Wiley & Sons, Inc.; 2002. [Google Scholar]
- 44.Troxel AB, Fairclough DL, Curran D, Hahn EA. Statistical analysis of quality of life with missing data in cancer clinical trials. Stat Med. 1998;17:653–666 [DOI] [PubMed] [Google Scholar]
- 45.Brown H, Prescott R Applied mixed models in medicine. West Sussex, England: John Wiley & Sons Ltd.; 1999. [Google Scholar]
- 46.Fairclough DL. Design and analysis of quality of life studies in clinical trials. Boca Raton: Chapman & Hall/CRC; 2002. [Google Scholar]
- 47.Cella D, Bullinger M, Scott C, Barofsky I. Group vs individual approaches to understanding the clinical significance of differences or changes in quality of life. Mayo Clin Proc. 2002;77:384–392 [DOI] [PubMed] [Google Scholar]
- 48.Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the kansas city cardiomyopathy questionnaire in clinical trials and clinical care: Jacc state-of-the-art review. J Am Coll Cardiol. 2020;76:2379–2390 [DOI] [PubMed] [Google Scholar]
- 49.Yost KJ, Cella D, Chawla A, Holmgren E, Eton DT, Ayanian JZ, et al. Minimally important differences were estimated for the functional assessment of cancer therapy-colorectal (fact-c) instrument using a combination of distribution- and anchor-based approaches. J Clin Epidemiol. 2005;58:1241–1251 [DOI] [PubMed] [Google Scholar]
- 50.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in eq-5d utility and vas scores in cancer. Health Qual Life Outcomes. 2007;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flynn KE, Lin L, Moe GW, Howlett JG, Fine LJ, Spertus JA, et al. Relationships between changes in patient-reported health status and functional capacity in outpatients with heart failure. Am Heart J. 2012;163:88–94.e83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yost KJ, Eton DT. Combining distribution-and anchor-based approaches to determine minimally important differences: The facit experience. Evaluation & the health professions. 2005;28:172–191 [DOI] [PubMed] [Google Scholar]
- 53.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (redcap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sas/stat 15.1 user’s guide: High-performance procedures. Cary, NC: SAS Institute Inc.; 2018. [Google Scholar]
- 55.Lee CS, Gelow JM, Chien CV, Hiatt SO, Bidwell JT, Denfeld QE, et al. Implant strategy-specific changes in symptoms in response to left ventricular assist devices. The Journal of cardiovascular nursing. 2018;33:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flynn KE DM, Lin L, Fawzy M, Graham FL, Hahn EA, Hays RD, Kormos RL, Liu H, McNulty M, Weinfurt KP. Reliability and construct validity of promis® measures for patients with heart failure who undergo heart transplant. Quality of Life Research 2015;24:2591–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carney RM, Freedland KE, Steinmeyer BC, Rubin EH, & Rich MW Clinical predictors of depression treatment outcomes in patients with coronary heart disease. Psychosomatic Research. 2016;88:36–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gebrie MH. An analysis of beck depression inventory 2nd edition (bdi-ii). Global Journal of Endocrinological Metabolism. 2018;2: 1–5 [Google Scholar]
- 59.Freeland K, Steinmeyer BC, Carney RM, Rubin EH, Rich MW. Use of the promis(r) depression scale and the beck depression inventory in patients with heart failure. Health Psychology. 2019;88:36–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hahn EA, Beaumont JL, Pilkonis PA, Garcia SF, Magasi S, DeWalt DA, et al. The promis satisfaction with social participation measures demonstrated responsiveness in diverse clinical populations. J Clin Epidemiol. 2016;73:135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schalet BD HR, Jensen SE, Beaumont JL, Fries JF, Cella D. Validity of promis physical function measured in diverse clinical samples. Journal of Clinical Epidemiology. 2016;73:112–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ayers BC, Wood EL, Bruckel J, Ling V, Kutyifa S, McNitt H, Vidula J, Alexis S, Prasad IG Promising new tool correlates well with the kansas city cardiomyopathy questionnaire in left ventricular assist device patients. The Journal of Heart and Lung Transplantation. 2019;38:S438 [Google Scholar]
- 63.Stehlik J, Rodriguez-Correa C, Spertus JA, Biber J, Nativi-Nicolau J, Zickmund S, et al. Implementation of real-time assessment of patient-reported outcomes in a heart failure clinic: A feasibility study. Journal of cardiac failure. 2017;23:813–816 [DOI] [PubMed] [Google Scholar]
- 64.Mondesir FL, Zickmund SL, Yang S, Perry G, Galyean P, Nativi-Nicolau J, et al. Patient perspectives on the completion and use of patient-reported outcome surveys in routine clinical care for heart failure. Circulation: Cardiovascular Quality and Outcomes. 2020;13:e007027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Molina EJ, Shah P, Kiernan MS, Cornwell WK 3rd, Copeland H, Takeda K, et al. The society of thoracic surgeons intermacs 2020 annual report. Ann Thorac Surg. 2021;111:778–792 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


