Abstract
Objective:
The 7-month Support, Educate, Empower (SEE) personalized glaucoma coaching program was previously shown to improve glaucoma medication adherence by 21-percentage points. This study’s goal was to assess the impact of the SEE program on Self Determination Theory (SDT) metrics and other patient-centered outcome measures.
Participants & methods:
Glaucoma patients (≥40 years old, taking ≥1 medication) self-reporting poor medication adherence were recruited at the University of Michigan. Eight surveys (with 10 subscales) were completed before and after the 7-month SEE program. Three surveys assessed changes in SDT (Treatment Self-regulation Questionnaire, Healthcare-Climate Questionnaire, Perceived Competence) while the other assessed participants’ Glaucoma Knowledge, Glaucoma Medication Self efficacy, Glaucoma-related distress, Perceived benefits, confidence asking and getting questions answered.
Results:
Thirty-nine participants completed the SEE program. Significant improvements were in 7 subscales, including all three SDT tenets of competence (mean change = 0.9, standard deviation = ± 1.2, adjusted p = 0.0002), autonomy (0.5, ± 0.9, 0.044), and relatedness (p = 0.002). Glaucoma-related distress (−2.0, ± 3.2, 0.004), confidence asking questions (1.1, ± 2.0, 0.008), and confidence getting questions answered (1.0, ± 2.0, 0.009) also improved. Glaucoma-related distress was correlated with perceived competence (r=−0.56, adjusted p = 0.005) and an increase in perceived competence was associated with a decrease in glaucoma-related distress (β= −0.43, 95% CI −0.67 – −0.20, adjusted p = 0.007).
Conclusion:
The SEE program improved participants’ autonomous motivation, perceived support, perceived competence, glaucoma-related distress, and competence. These results point to the promising potential of SDT-guided behavioral interventions in improving patient-centered metrics.
Keywords: Electronically monitored medication adherence, glaucoma, patient-centered outcome measures
Precis
Self-determination theory (SDT) guided behavioral interventions are effective in improving several patient-centered metrics, including their glaucoma-related distress. However, whether improvement in patient-centered metrics can drive an improvement in medication-taking behavior remains to be seen.
Despite availability of effective treatment options, glaucoma continues to remain a leading cause of blindness in the United States. One important reason is that fewer than 50% of patients take medications as recommended.1,2 Multiple reasons have been reported for poor medication adherence, including low health literacy, low self-efficacy, prohibitive cost, and medication side effects.3 Regardless of the reason, poor adherence to glaucoma medication has not only been associated with worse clinical outcomes but also with worse patient-centered factors such as vision-related quality of life.4–9
Consequently, finding an effective intervention that can improve patients’ adherence rates has become an urgent priority for ophthalmologists. Two systematic reviews, including a Cochrane Review, have found that the most successful glaucoma medication adherence interventions provided patient-centered care in the form of in-person and individualized counseling sessions alongside medication reminders.10–17 These results corroborate the findings of many studies across multiple disciplines that found patient-centered, autonomy-supportive counselling approaches, such as motivational interviewing, were effective in prompting and maintaining health-promoting behaviors.18–22
Self-Determination Theory (SDT) provides a conceptual framework for understanding how and why these interventions work. SDT postulates that people engage in lasting and meaningful behavioral changes when they believe they are competent to make the change (“competence”), their decision to change is volitional (“autonomy”), and they find themselves in an autonomy-supportive environment that promotes human connectedness (“relatedness.”)18 A motivational interviewing based counselling approach fulfills all of these conditions and encourages its clients to identify their own reasons and decisions for change. It accomplishes this goal by providing clients a sense of connectedness and competence created through active listening, empathy, and affirmation.
Based on these findings, the Support, Educate, Empower (SEE) pilot study program was conceived as an in-person, individualized, motivational interviewing based counselling program which simultaneously utilizes medication reminders to improve medication adherence among people with glaucoma. In a previous publication, the authors of this study have found that the SEE program was successful in improving its participants’ medication adherence rate by an average of 21 percentage points (60% to 81%).23 Using the framework of SDT, we hypothesize that the SEE program resulted in positive changes to several patient-centered outcome measures and that these positive changes were associated with improvement in medication adherence rates. Furthermore, we also postulated that the SEE program resulted in decreased amount of glaucoma related distress. To explore these hypotheses, we assessed how different patient-centered outcome measures changed after the intervention and how predictive these respective changes were in estimating changes to medication adherence rates and glaucoma related distress scores.
Methods:
The methods used in the SEE program have previously been described.24, 23 Data for this study originated from subjects recruited for participation in the Support, Educate, Empower (SEE) Program pilot study (Clinical-Trials.gov Identifier #NCT03159247), a prospective, non-controlled study that examined the effect of personalized glaucoma education and coaching on medication adherence in glaucoma patients who were ≤80% adherent to their medications as assessed by electronic medication monitoring over three months. Written informed consent was obtained and this study was approved by the University of Michigan Institutional Review Board and adhered to all the tenets of the Declaration of Helsinki. The outline and summary of the methodology used in the study is described below.
Participants and Sample Selection
Patients who received ophthalmic care at a single institution (University of Michigan), ≥ 40 years old, took ≥ 1 glaucoma medication, and had a diagnosis of any type of glaucoma, glaucoma suspect or ocular hypertension were identified. Those who had severe mental illness (defined as schizophrenia, history of a major depressive episode with psychosis, or bipolar disorder), cognitive impairment, did not speak English or did not instill their own eye drops were excluded. Potential participants were contacted by phone and their self-reported adherence was assessed using two validated scales, the Boland adherence measure and the Morisky Medication Adherence Scale.25,26 In order to be considered for inclusion, patients had to self-report poor medication adherence by scoring <95% adherence on the Boland measure and ≤6 on the Morisky scale, the thresholds for poor adherence indicated by each scale.
Participants who met eligibility and screening criteria were invited to an initial study visit with a coordinator to give written informed consent, and collect survey data on participant demographics, clinical information, and patient-centered outcomes. The participants then underwent a 3-month baseline electronic medication adherence monitoring period, and those who had ≤80% electronically-monitored medication adherence were enrolled into the 7-month intervention phase (SEE personalized glaucoma coaching program). During the intervention, participants could elect to have automated text, phone call, sound and/or visual reminders for each scheduled medication dose. Participants also received 3 in-person motivational-interviewing based coaching sessions and 5 phone calls for between visit support from the same glaucoma coach. The coaching sessions were supported by an e-Health tool that generated personalized education and motivational-interviewing based coaching content personalized to the participant’s glaucoma diagnosis, test results, recommended treatment and barriers to medication adherence. At the end of the intervention, or 10 months after the initial study visit, the participants completed the same surveys of patient-centered outcomes that they had completed at the baseline visit prior to the intervention.
Electronically monitored medication adherence
Medication adherence was monitored electronically (AdhereTech, New York, USA) during the 3-month baseline eligibility period and throughout the 7-month SEE Program. Participants placed each of their glaucoma medications in a separate marked monitoring bottle. Whenever a monitoring bottle is opened, a time-date stamp was sent to our database through the cellular data network and recorded as a medication taking event. An adherent event was defined as opening the adherence monitor within a specified time window of the dose on the previous day,27 specifically 24±4 hours for medications dosed once per day, 24±2 hours for medications dosed twice per day, and 24±1.3 hours for medications dosed 3 times per day. Adherence was measured during specified time periods as a continuous variable on a scale from 0 to 100, representing the percentage of prescribed glaucoma medications doses that were taken as scheduled. For further details please refer to the methodology previously published in Newman-Casey et al, 202023.
Patient-centered outcome measures
Eight scales were used to measure the impact of the SEE personalized glaucoma coaching program on different patient-centered domains. The program’s impact on the three key domains of SDT were measured using the Treatment Self-Regulation Questionnaire (TSRQ), the Health Care Climate Questionnaire (HCCQ), and the Perceived Competence scale adapted for glaucoma medication adherence as the health behavior. The TSRQ has two subscales: the TSRQ-Autonomous (TSRQ-A, range = 1 – 7, Cronbach’s α = 0.85 – 0.93) that assesses the degree of autonomous motivation and the TSRQ-Controlled (TSRQ-C, range = 1 – 7, α = 0.73 – 0.91) that assesses the degree of extrinsic or controlled motivation. Higher scores in TSRQ-A and lower scores in TSRQ-C means an individual is more “autonomous” and less “extrinsically” motivated.28 The HCCQ (range = 1 – 7, α = 0.95) measured the degree to which people feel that their autonomy was supported through their relationship with their healthcare provider. Higher scores represented greater feeling of relatedness and autonomy support.29 At the baseline visit prior to the SEE coaching intervention, the HCCQ was phrased to assess participants’ perceived level of relatedness and autonomy support from their eyecare providers. However, after the SEE coaching intervention, HCCQ assessed participants’ perceived level of relatedness and autonomy support from their glaucoma coaches. Lastly, the Perceived Competence scale (range = 1 – 7) measured how competent participants felt in managing their glaucoma with higher scores indicating greater levels of perceived competence.30 This scale was validated for internal consistency on patients with diabetes (α = 0.80 – 0.94), a chronic disease that has many parallels to glaucoma in its need for continuous self-management.30 Competence was also in part assessed using the Glaucoma Knowledge scale (range = 0 – 100%) with higher scores indicating more glaucoma knowledge.31,32
Other patient-centered outcomes that were not part of the three SDT domains were also assessed. Participants’ perceived distress from their glaucoma was measured using the Glaucoma Distress scale (range 1 – 3, α = 0.74) where higher scores indicated greater levels of distress.33,34 Participants’ self-efficacy, or their personal beliefs about their capabilities to manage their glaucoma, was assessed using Glaucoma Medication Self-Efficacy (GMSE, range = 1 – 3, α = 0.91) with higher scores indicating greater self-efficacy.
Participants’ perception of the benefits of their glaucoma treatment was assessed using the Perceived Benefits scale (range = 1 – 6) scale where higher scores indicated a greater perception of benefit from adhering to glaucoma treatment.35 The Perceived Benefits scale was validated for internal consistency with people with diabetes (α = 0.74).35 Finally, the Confidence Asking Question scale was used to assess participants’ confidence in knowing what questions to ask their ophthalmologist (CAQ-1, range = 0 – 10) and in obtaining answers from their ophthalmologist (CAQ-2, range = 0 – 10) with higher scores indicating greater levels of confidence.36 CAQ-1 and CAQ-2 were not tested for internal consistency.
Statistical analysis
Surveys were scored according to official documentation from scale developers. Pre-intervention, post-intervention, and change in self-reported survey scores were summarized using descriptive statistics including mean, standard deviation (SD), minimum, maximum, and median. Pre- to post-intervention scores were tested for differences using paired t-tests and visualized with scatterplots.
The two main outcomes of interest were change in medication adherence and change in glaucoma-related distress. The relationship between these outcomes and change in self-reported survey measures was assessed with Pearson correlation coefficients (r) and visualized with scatterplots. Linear regression was used to investigate the association between each outcome and change in self-reported survey measures. Models were adjusted for income and number of glaucoma medications, both of which were found to be associated with change in medication adherence after SEE Program participation in previous work, but not adjusted for patient demographics (age, gender, race) which did not show significant associations with change in medication adherence after SEE Program participation.37 P-values were adjusted for multiple comparisons using the Holm-Bonferroni method. Statistical analysis was conducted using RStudio version 1.4.1106 (RStudio Team, Boston, MA, 2021) including the ggplot2 package (Wickham, 2009),38 and SAS version 9.4 (SAS Institute, Cary, NC).
Results:
Forty-six participants were eligible for SEE program intervention inclusion, out of which 39 participants finished the 7-month SEE coaching intervention and the post-intervention surveys. These participants were on average 63.4 years old (SD = 10.7) at enrollment, and were 56% male, 44% White, 49% Black, and 3% Latinx. In terms of income, 23% reported income <$25,000, 40% reported $25–50,000, 23% reported $51-$100,000, and 14% reported >$100, 000 in income. Patients were on between 1 and 4 medications to treat their glaucoma (49% on a single medication, 26% on 2 medications, 20% on three medications, and 5% of four medications). Medication adherence at baseline was on average 59.9% (SD = 18.5, range = 13.3 to 80.0) and significantly improved during the SEE glaucoma counseling to 81.3% (SD = 17.6, range = 19.8 to 99.6), for an average change of 21.4 percentage points (SD=16.6, range = −3.2 to 74.4; p<0.0001). Adherence during the last 2 months of intervention (after the last glaucoma coaching session) was on average 83.6% (SD = 17.5, range = 20.0 to 100.0).
Self-Determination Theory Metrics
Pre- and post-intervention SDT metrics are summarized in Table 1. Autonomous motivation (TSRQ-A) significantly improved from a pre-intervention mean score of 5.5 (SD = 0.8) to post-intervention mean score of 6.0 (SD = 0.6, p-value = 0.002, adjusted p-value = 0.04) (Figure 1a). Controlled motivation (TSRQ-C) did not significantly change, with a pre-intervention mean score of 3.6 (SD = 1.5) and a post-intervention mean score of 3.4 (SD = 1.2, p = 0.4, adjusted p = 1.0) (Figure 1b). The post-intervention mean healthcare climate score (HCCQ) assessing relatedness and autonomy support from the glaucoma coaches was 6.7 (SD = 0.5) which was not directly comparable, but significantly better than the pre-intervention mean healthcare climate score assessing relatedness and autonomy support from participants’ eye care providers (5.6, SD = 1.3, p = 0.00006, adjusted p = 0.002). Perceived competence significantly improved from a pre-intervention mean score of 5.3 (SD = 1.2) to a post-intervention mean score of 6.2 (SD = 1.0, p-value = 0.00004, adjusted p-value = 0.0002) (Figure 1c). Glaucoma knowledge did not significantly change with a pre-intervention mean score of 81% (SD = 17) to post-intervention mean score of 82% (SD = 12, p = 0.91, adjusted p = 0.97) (Figure 1d).
Table 1.
Comparison of pre-intervention and post-intervention mean scores for patient-centered outcome measures.
| N | Pre-intervention Mean Score (± SD), median | Post-intervention Mean Score (± SD), median | Mean Change in Score (± SD), median | P-value* | Holm-Bonferroni Adjusted p-value | |
|---|---|---|---|---|---|---|
| Treatment Self-Regulation Autonomous (TSRQ-A) | 38 | 5.5 (± 0.8), 5.6 | 6.0 (± 0.6), 6.0 | 0.5 (± 0.9), 0.4 | 0.002 | 0.044 |
| Treatment Self-Regulation Controlled (TSRQ-C) | 38 | 3.6 (± 1.5), 3.2 | 3.4 (± 1.2), 3.8 | − 0.2 (± 1.2), − 0.2 | 0.400 | 1.0 |
| Healthcare Climate Questionnaire (HCCQ) Ɨ | 38 | 5.6 (± 1.3), 6.0 | 6.7 (± 0.5), 6.8 | - | 0.00006 | 0.002 |
| Perceived Competence | 36 | 5.3 (± 1.2), 5.8 | 6.2 (± 1.0), 6.5 | 0.9 (± 1.2), 0.9 | 0.00004 | 0.0002 |
| Glaucoma Knowledge (%) | 38 | 81 (± 17), 89 | 82 (± 12), 78 | 1 (± 16), 0 | 0.906** | 0.969 |
| Glaucoma Distress | 38 | 2.2 (± 1.0), 2.0 | 1.5 (± 0.8), 1.3 | − 0.6 (± 1.1), − 0.3 | 0.0003 | 0.004 |
| Glaucoma Medication Self-Efficacy (GMSE) | 38 | 2.5 (± 0.4), 2.5 | 2.7 (± 0.4), 2.8 | 0.2 (± 0.4), 0.1 | 0.037 | 0.372 |
| Perceived Benefits | 38 | 5.2 (± 0.7), 5.3 | 5.4 (± 0.5), 5.7 | 0.2 (± 0.7), 0.3 | 0.065 | 0.847 |
| Confidence Getting Questions Answered (CAQ-1) | 38 | 7.8 (± 2.3), 9.0 | 8.8 (± 1.4), 9.0 | 1.0 (± 2.0), 0 | 0.005 | 0.009 |
| Confidence Knowing What to Ask (CAQ-2) | 38 | 7.1 (± 2.6), 8.0 | 8.2 (± 2.1), 9.0 | 1.1 (± 2.0), 1.0 | 0.002 | 0.008 |
SD = Standard Deviation
p-values are paired t-test p-values comparing the pre-intervention mean score to the post-intervention mean score, unless otherwise noted
p-value is obtained using Wilcoxon Signed Rank test
HCCQ mean change in score not available as pre-intervention HCCQ assessed patient’s level of support from ophthalmologist while post-intervention HCCQ assessed patient’s level of support from glaucoma coach and are thus not directly comparable
Figure 1.
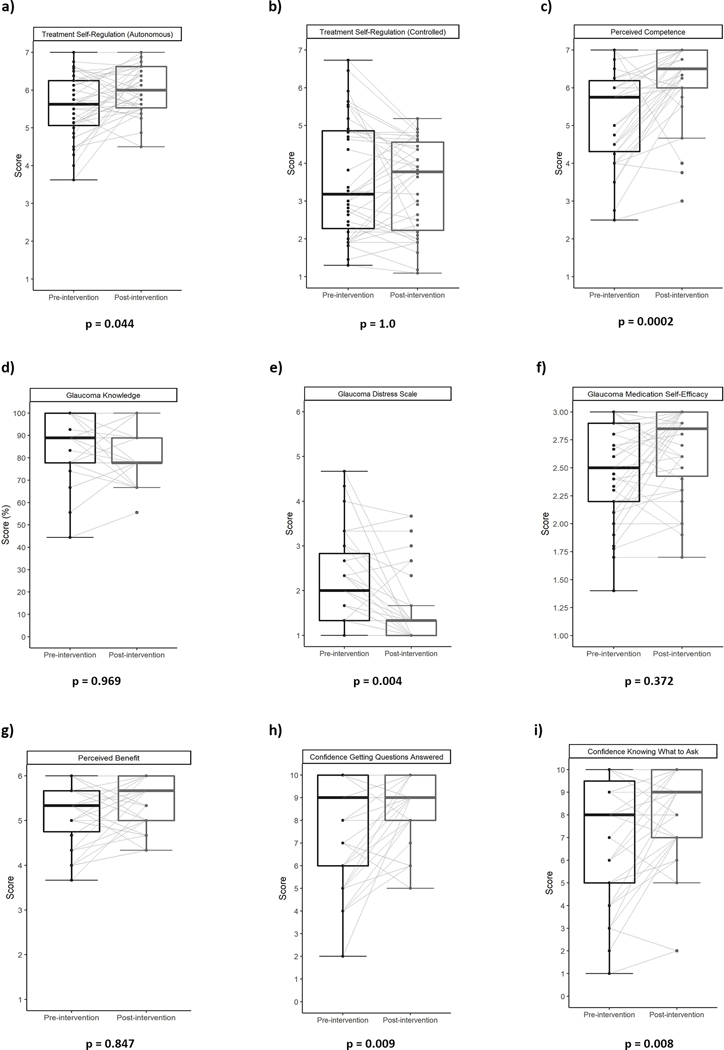
Pre- and post-intervention patient-centered metric scores visualized using paired box plots with each line representing an individual participants change in scores presented for a) Treatment Self-Regulation (Autonomous); b) Treatment Self-Regulation (Controlled); c) Perceived Competence; d) Glaucoma Knowledge; e) Glaucoma Distress Scale; f) Glaucoma Medication Self-Efficacy; g) Perceived Benefit; h) Confidence Getting Questions Answered (CAQ-1); and i) Confidence Knowing what to Ask (CAQ-2).
Other patient-centered outcome measures
The Glaucoma Distress score decreased significantly by 32% from its pre-intervention mean of 2.2 (SD = 1.0) to a post-intervention mean score of 1.5 (SD = 0.8, p = 0.0003, adjusted p = 0.004) indicating participants experienced lower levels of glaucoma-related distress after the intervention (Figure 1e). GMSE increased from a pre-intervention mean score of 2.5 (SD = 0.4) to post-intervention mean score of 2.7 (SD = 0.4, p = 0.04, adjusted p = 0.4) indicating that participants’ self-efficacy improved after the intervention but that it was not a significant improvement after adjusting for multiple comparisons (Figure 1f). The Perceived Benefits scale did not show a significant change with a pre-intervention mean score of 5.2 (SD = 0.7) and post-intervention mean score of 5.4 (SD = 0.5, p-value = 0.07, adjusted p = 0.85) (Figure 1g). Participants showed a statistically significant increase in their confidence knowing what questions to ask (CAQ-1) from pre-intervention mean score of 7.1 (SD = 2.6) to post-intervention mean score of 8.2 (SD = 2.1, p = 0.002, adjusted p = 0.008) (Figure 1h). Participants’ confidence in obtaining answers from their ophthalmologists (CAQ-2) also significantly increased from pre-intervention score of 7.8 (SD = 2.3) to post-intervention score of 8.8 (SD = 1.4, p = 0.005, adjusted p = 0.009) (Figure 1i).
Associations with change in medication adherence and change in glaucoma-related distress
Change in self-reported survey measures showed some preliminary associations with change in adherence and change in glaucoma-related distress (Table 2). Specifically, change in glaucoma distress was negatively correlated with change in medication adherence (r=−0.34, p=0.0367), such that a decrease/improvement in distress was associated with increased medication adherence. Additionally, change in GMSE was positively correlated with change in adherence where improved self-efficacy was associated with improved adherence (r=0.39, p=0.0142). However, none of these correlations were significant after Holm adjustment for multiple comparisons (adjusted p=0.55 for glaucoma distress, adjusted p=0.23 for GMSE). When it was analyzed as the outcome, increased glaucoma-related distress was positively correlated with increased controlled motivation (TSRQ-C) (r=0.34, p=0.036). In contrast, decreased glaucoma-related distress was correlated with increased perceived competence (r=−0.56, p=0.0003). Only the correlation with perceived competence remained significant after Holm adjustment for multiple comparisons (adjusted p=0.47 for TSRQ-C and adjusted p=0.0047 for perceived competence) (Figure 2).
Table 2.
Pearson correlation coefficients between change in medication adherence and change in self-reported survey measures
| Correlation with Change in Adherence | Correlation with Change in Glaucoma-Related Distress | |||||
|---|---|---|---|---|---|---|
| Change Variable | Correlation | P-value | Adjusted P-value | Correlation | P-value | Adjusted P-value |
| Treatment Self-Regulation Autonomous (TSRQ-A) | 0.11 | 0.5055 | 1.0000 | −0.02 | 0.9093 | 1.0000 |
| Treatment Self-Regulation Controlled (TSRQ-C) | 0.00 | 0.9918 | 1.0000 | 0.34 | 0.0363 | 0.4718 |
| Healthcare Climate Questionnaire (HCCQ) Ɨ | 0.09 | 0.6065 | 1.0000 | −0.10 | 0.5587 | 1.0000 |
| Perceived Competence | 0.24 | 0.1444 | 1.0000 | −0.56 | 0.0003 | 0.0047 |
| Glaucoma Knowledge | 0.27 | 0.1056 | 1.0000 | 0.25 | 0.1241 | 1.0000 |
| Glaucoma Distress | −0.34 | 0.0367 | 0.5511 | |||
| Glaucoma Medication Self-Efficacy (GMSE) | 0.39 | 0.0142 | 0.2268 | −0.15 | 0.3718 | 1.0000 |
| Perceived Benefit | −0.10 | 0.5517 | 1.0000 | 0.00 | 0.9973 | 1.0000 |
| Confidence Getting Questions Answered (CAQ-1) | −0.23 | 0.1716 | 1.0000 | 0.04 | 0.8312 | 1.0000 |
| Confidence Knowing What to Ask (CAQ-2) | 0.11 | 0.5220 | 1.0000 | −0.19 | 0.2484 | 1.0000 |
HCCQ pre-intervention score, or how supported participants felt from their ophthalmologist, was used to find its correlation with change in adherence and glaucoma-related distress. Unlike other measures, changes in HCCQ score between pre-intervention and post-intervention was not available for this correlation analysis as post-intervention HCCQ measured how supported participants felt from their glaucoma coaches.
Figure 2.
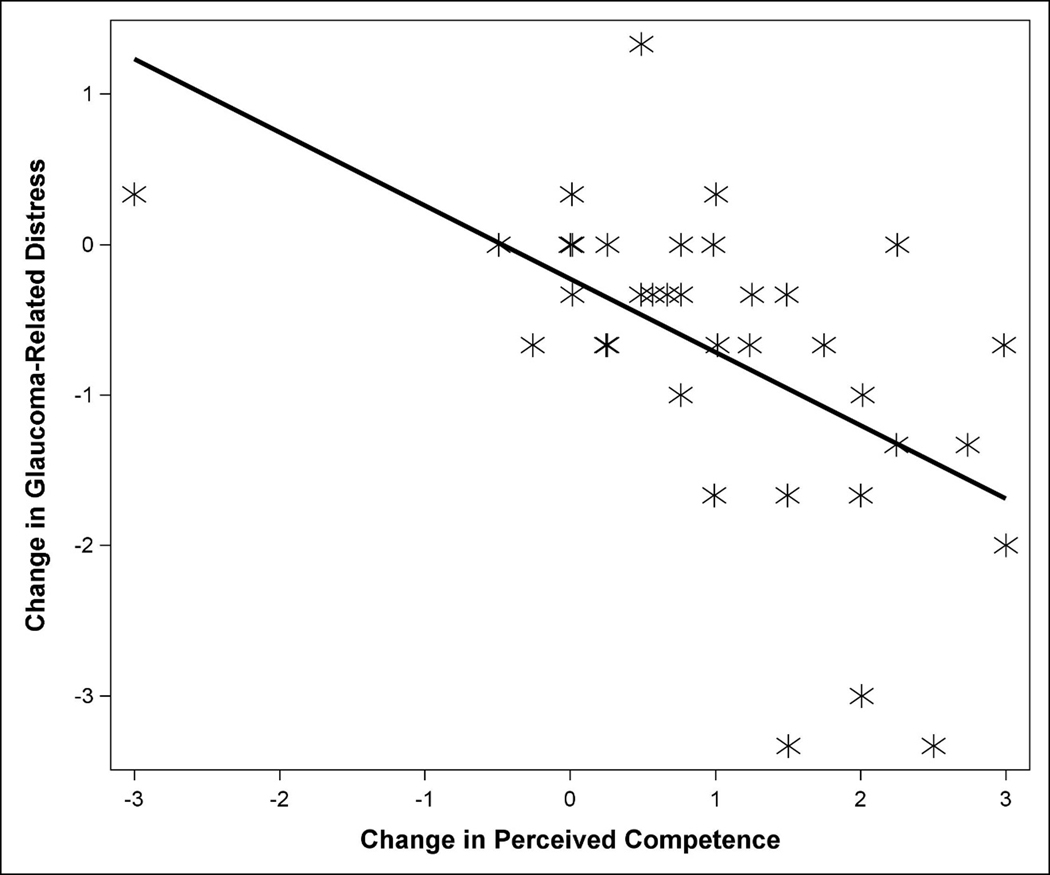
Scatterplot showing the relationship between change in glaucoma-related distress and change in perceived competence. Solid line represents the best fit regression line.
Linear regression model results for the effect of change in self-reported measures on change in adherence or change in glaucoma-related distress, after adjusting for income and number of glaucoma medications, are displayed in Table 3. No significant associations between change in self-reported measures and change in medication adherence were found (all p>0.05). However, perceived competence was independently associated with change in glaucoma-related distress even after Holm adjustment for multiple comparisons. Specifically, a 1-unit improvement in perceived competence was associated with a 0.43-unit decrease (improvement) in glaucoma-related distress (95% CI, −0.67 to −0.20); adjusted p=0.007). Increased controlled motivation (p=0.052) and improved glaucoma knowledge (p=0.029) were marginally associated with worse glaucoma-related distress. However, neither remained significant after Holm adjustment for multiple comparisons (adjusted p=0.41 and 0.26, respectively).
Table 3.
Linear regression models for predicting change in medication adherence or change in glaucoma-related distress. Change in adherence is defined as the difference from baseline adherence to adherence during the last 2 months after the final SEE coaching session. Change in glaucoma-related distress is defined as the difference from baseline glaucoma related distress to post-intervention glaucoma-related distress. All models are adjusted for household income and number of glaucoma medications. Each row is a separate model, for each outcome.
| Change in Adherence | Change in Glaucoma-Related Distress | |||||
|---|---|---|---|---|---|---|
| Change Variable | Estimate (95% CI) | P-value | Adjusted P-value | Estimate (95% CI) | P-value | Adjusted P-value |
| Perceived Competence | 2.1 (−1.9, 6.2) | 0.2925 | 1.0000 | −0.43 (−0.67, −0.20) | 0.0007 | 0.0071 |
| Perceived Benefit | −1.6 (−8.2, 5.0) | 0.6289 | 1.0000 | −0.11 (−0.57, 0.34) | 0.6158 | 1.0000 |
| HCCQ Ɨ | 0.6 (−2.8, 4.0) | 0.7137 | 1.0000 | −0.12 (−0.35, 0.11) | 0.3026 | 1.0000 |
| TSRQ - Autonomous | −0.2 (−5.6, 5.3) | 0.9528 | 1.0000 | −0.13 (−0.50, 0.25) | 0.4867 | 1.0000 |
| TSRQ - Controlled | 1.0 (−2.7, 4.7) | 0.5800 | 1.0000 | 0.24 (0.00, 0.48) | 0.0515 | 0.4119 |
| Glaucoma Distress | −1.8 (−7.0, 3.4) | 0.4864 | 1.0000 | |||
| GMSE | 9.7 (−1.6, 21.0) | 0.0913 | 1.0000 | −0.15 (−0.98, 0.67) | 0.7060 | 1.0000 |
| Glaucoma Knowledge | 0.1 (−0.2, 0.4) | 0.5141 | 1.0000 | 0.02 (0.00, 0.05) | 0.0287 | 0.2584 |
| Confidence Getting Questions Answered (CAQ-1) | −1.7 (−3.8, 0.5) | 0.1277 | 1.0000 | −0.02 (−0.17, 0.14) | 0.8379 | 1.0000 |
| Confidence Knowing What to Ask (CAQ-2) | 0.6 (−1.7, 2.9) | 0.6223 | 1.0000 | −0.11 (−0.26, 0.05) | 0.1720 | 1.0000 |
HCCQ, healthcare climate questionnaire; TSRQ, treatment self-regulation questionnaire; CAQ, confidence asking questions; SEE, support, educate, empower; CI, confidence interval; Int, intervention
HCCQ pre-intervention score, or how supported participants felt from their ophthalmologist, was used to predict change in adherence and glaucoma-related distress. Unlike other measures, changes in HCCQ score between pre-intervention and post-intervention was not available for this linear regression analysis as post-intervention HCCQ measured how supported participants felt from their glaucoma coaches
Discussion:
SEE program participants experienced significant positive changes after the personalized counselling intervention including an increase in their perceived competence, autonomous motivation to self-manage their glaucoma, and confidence in asking and getting answers to their questions from their ophthalmologist. SEE program participants felt that their glaucoma coaches were significantly more supportive than their ophthalmologists in helping them self-manage their glaucoma. Additionally, they also experienced a 32% decrease in their glaucoma-related distress after participating in the program. Our multivariate analysis demonstrated that improvements in perceived competence score predicted improvements in glaucoma-related distress. However, contrary to our hypothesis that positive changes in self-determination theory metrics would predict changes in our participants’ medication adherence rates, none of the positive changes in Self-Determination Theory metrics correctly predicted the 21% change in medication adherence that our participants experienced23, This may be due to this pilot study’s limited sample size and lack of control group.
The SEE Program was built using principles of SDT with an aim to improve participants’ glaucoma medication adherence by supporting their autonomy, competence, and relatedness. SDT stands in contrast to the other theories of human motivation and behavior change (i.e. B.F. Skinner’s operant theory) that focuses on extrinsic ways to control behaviors. SDT from its very conception stresses the importance of autonomy in explaining human behavior and considers the relationship between the individual, society, and others (“relatedness”) as a key component of its philosophy.18 It thus postulates that the dynamic interplay between autonomy, competence, and relatedness guides how people behave. We found that using this SDT framework has helped SEE participants feel more strongly supported, more autonomously motivated, and more competent in managing their glaucoma.
For instance, SEE program participants related better and felt significantly more supported by their glaucoma coaches in helping them self-manage their condition than they did from their ophthalmologists. Our glaucoma coaches, unlike most ophthalmologists, were extensively trained in motivational interviewing techniques. Previous studies on parenting have shown that utilizing the concept of unconditional positive regard within the framework of motivational interviewing, as our glaucoma coaches did, promotes both autonomy and relatedness.39,40 Furthermore, research in learning and task completion have shown that providing positive feedback, again like our glaucoma coaches did, enhances both autonomous motivation and perceived competence. 41 These are all findings we saw in our SEE program participants. These results are especially significant in a healthcare setting, where many patients neither feel competent nor related to, and often are told to behave in certain ways (e.g. doctor’s orders, prescription instructions). It also demonstrates the distinct and important supplementary role glaucoma coaches can play in improving patient-centered outcomes. Ophthalmologists already shoulder important responsibilities such as making medical decisions or providing surgical care, which are only set to get heavier as our populations grow and age. 42,43 Tasking them to provide each patient with at least 120 minutes of motivational interviewing guided coaching seems like a heavy ask. Utilizing glaucoma coaches can free ophthalmologist from this additional responsibility.
We found it particularly encouraging that our participants experienced an increase in their autonomous motivation without an increase in controlled motivation. This finding reinforces the notion that the SEE program is acting to guide people to identify their own reasons to improve their glaucoma self-management as opposed to merely serving as an authoritative source of external motivation. These results are promising, especially since autonomous motivation is much more likely to lead to longer term behavior change compared to extrinsic motivation.44
In addition to the improvements in the key tenets of SDT, disease-related distress was decreased. Glaucoma-related distress was measured using a scale adapted for glaucoma patients from the Diabetes Distress scale.33 In a previous study, we found that increased glaucoma-related distress adversely affected participants medication adherence rates.34 This finding mirrors the conclusion of several studies in diabetes research, which found associations between higher diabetes-related distress, higher hemoglobin A1C values, and poorer medication adherence.45,46 In fact, the awareness of the importance of disease-related distress has grown significantly in the field of diabetes, so much so that the Department of Health and Human Services made a Funding Opportunity Announcement in 2020 for studies aiming to improve glycemic control by way of decreasing diabetes-related distress (FOA Number: RFA-DK-19–021). While the evidence for lowering disease-related distress as the primary target for improving patients’ glaucoma related biological outcomes is less robust, we believe that glaucoma-related distress is an important patient-centered outcome measure and it is encouraging that the SEE program was successful in significantly reducing (32%) the amount of distress its participants felt from living with glaucoma as a chronic disease.
In addition, changes in participants’ glaucoma-related distress scores were both significantly correlated with (coefficient = −0.56, adjusted p-value = 0.005) and predicted by (estimate = −0.43, adjusted p-value = 0.007) changes in perceived competence scale score. This result closely mirrors the findings that improved perceived competence was associated with improved diabetes distress in adults with type 1 diabetes.47 Several causal mechanisms have been proposed for this association, including the hypotheses that low perceived competence may be a consequence of poor ability to cope with daily stressors caused by diabetes or that the very act of perceiving one’s competence as low can be distressing in and of itself.48 While more work is needed to elucidate the mechanism of the association behind glaucoma-related distress and perceived competence, our result suggests that the SEE program was successful in improving its participants’ perceived competence which in turn decreased their glaucoma related distress.
There were several limitations to this study. First, the SEE program is a pilot study program with a small sample size of 39 participants. It was powered to detect meaningful differences in medication adherence rates before and after the counselling intervention and was not powered to detect differences in these exploratory outcomes. Therefore, results from the analyses presented in this manuscript should be interpreted as hypothesis generating, rather than hypothesis testing, and should be further explored in the context of a larger randomized controlled trial. Second, the SEE Program is an uncontrolled prospective cohort study, meaning no control or untreated group was present. Third, electronic medication monitoring may have some limitations in assessing the true medication adherence of our participants, especially due to the novel nature of electronic medication monitors as well as the observer effect (Hawthorne) it brings. We made deliberate study design choices to minimize this bias, for example measuring adherence over three months, the time period over which the Hawthorne effect is thought to wane, which is described in greater detail elsewhere. 49,50 Lastly, we measured our participants’ patient-centered outcome measure over the course of a 7-month period while they were participating in the SEE program pilot study. Further study is required to assess if the improvements noted in this study lasts beyond the 7-month period without further behavioral counselling, or if there is a decrease in medication adherence after the 7 months. Despite these limitations, we found it important to publish our results as they offer us insight into the potential benefits of motivational-interviewing guided approaches. Next steps include assessing whether these patient-centered outcome measure improvements among SEE Program participants hold true in a randomized controlled clinical trial which is currently underway (NCT04735653). The larger RCT will enable a deeper exploration of the relationships between the changes in these patient centered outcome measures and how they may mediate the relationship between glaucoma related distress and glaucoma medication adherence.
In conclusion, using motivational-interviewing based health coaching consistent with the theoretical framework of SDT can be a powerful tool for promoting autonomous motivation, perceived competence, and feeling supported to make health-promoting self-management choices among glaucoma patients. Health coaches trained in glaucoma-specific motivational interviewing techniques could help empower people to improve their glaucoma self-management. The successful utilization of health coaches in the Medicare Diabetes Prevention Program shows us that starting insurance reimbursement and training programs would allow wide-spread uptake of glaucoma health coaches in ophthalmology clinics across the US.51
Financial Support:
National Eye Institute (Bethesda, MD, K23EY025320, PANC), National Center for Advancing Translational Sciences (Bethesda, MD, TL1TR002242, JC, MS), and Research to Prevent Blindness Career Development Award (New York, NY, PANC). The funding organizations had no role in the design or conduct of this research.
Footnotes
No conflicting relationship exists for any author.
References
- 1.Olthoff CMG, Schouten JSAG, van de Borne BW, Webers CAB. Noncompliance with Ocular Hypotensive Treatment in Patients with Glaucoma or Ocular Hypertension: An Evidence-Based Review. Ophthalmology. 2005;112(6):953–961.e957. [DOI] [PubMed] [Google Scholar]
- 2.Newman-Casey PA, Blachley T, Lee PP, Heisler M, Farris KB, Stein JD. Patterns of Glaucoma Medication Adherence over Four Years of Follow-Up. Ophthalmology. 2015;122(10):2010–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman-Casey PA, Robin AL, Blachley T, et al. The Most Common Barriers to Glaucoma Medication Adherence: A Cross-Sectional Survey. Ophthalmology. 2015;122(7):1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sleath B, Blalock S, Covert D, et al. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology. 2011;118(12):2398–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossi GCM, Pasinetti GM, Scudeller L, Radaelli R, Bianchi PE. Do Adherence Rates and Glaucomatous Visual Field Progression Correlate? Eur J Ophthalmol. 2010;21(4):410–414. [DOI] [PubMed] [Google Scholar]
- 6.Newman-Casey PA, Niziol LM, Gillespie BW, Janz NK, Lichter PR, Musch DC. The Association between Medication Adherence and Visual Field Progression in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2020;127(4):477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman DS, Hahn SR, Gelb L, et al. Doctor-patient communication, health-related beliefs, and adherence in glaucoma results from the Glaucoma Adherence and Persistency Study. Ophthalmology. 2008;115(8):1320–1327, 1327.e1321–1323. [DOI] [PubMed] [Google Scholar]
- 8.Muir KW, Santiago-Turla C, Stinnett SS, et al. Health literacy and adherence to glaucoma therapy. Am J Ophthalmol. 2006;142(2):223–226. [DOI] [PubMed] [Google Scholar]
- 9.Thompson AC, Woolson S, Olsen MK, Danus S, Bosworth HB, Muir KW. Relationship between electronically measured medication adherence and vision-related quality of life in a cohort of patients with open-angle glaucoma. BMJ Open Ophthalmol. 2018;3(1):e000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newman-Casey PA, Weizer JS, Heisler M, Lee PP, Stein JD. Systematic review of educational interventions to improve glaucoma medication adherence. Semin Ophthalmol. 2013;28(3):191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray TA, Orton LC, Henson D, Harper R, Waterman H. Interventions for improving adherence to ocular hypotensive therapy. Cochrane Database Syst Rev. 2009(2):Cd006132. [DOI] [PubMed]
- 12.Okeke CO, Quigley HA, Jampel HD, et al. Interventions Improve Poor Adherence with Once Daily Glaucoma Medications in Electronically Monitored Patients. Ophthalmology. 2009;116(12):2286–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norell SE. Improving medication compliance: a randomised clinical trial. Br Med J. 1979;2(6197):1031–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook PF, Bremer RW, Ayala AJ, Kahook MY. Feasibility of motivational interviewing delivered by a glaucoma educator to improve medication adherence. Clin Ophthalmol. 2010;4:1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rendell J. Effect of health education on patients’ beliefs about glaucoma and compliance. Insight. 2000;25(4):112–118. [DOI] [PubMed] [Google Scholar]
- 16.Gray TA, Fenerty C, Harper R, et al. Individualised patient care as an adjunct to standard care for promoting adherence to ocular hypotensive therapy: an exploratory randomised controlled trial. Eye (Lond). 2012;26(3):407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dreer LE, Owsley C, Campbell L, Gao L, Wood A, Girkin CA. Feasibility, Patient Acceptability, and Preliminary Efficacy of a Culturally Informed, Health Promotion Program to Improve Glaucoma Medication Adherence Among African Americans: “Glaucoma Management Optimism for African Americans Living with Glaucoma” (GOAL). Curr Eye Res. 2016;41(1):50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan RM, Deci EL. Self-Determination Theory : Basic Psychological Needs in Motivation, Development, and Wellness. New York, UNITED STATES: Guilford Publications; 2017. [Google Scholar]
- 19.Silva MN, Markland D, Carraça EV, et al. Exercise autonomous motivation predicts 3-yr weight loss in women. Med Sci Sports Exerc. 2011;43(4):728–737. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy S, Goggin K, Nollen N. Adherence to HIV medications: Utility of the theory of self-determination. Cognit Ther Res. 2004;28(5):611–628. [Google Scholar]
- 21.Hollenhorst CN, Elliott V, Heisler M, Schneider K, Resnicow K, Newman-Casey PA. Patient Experience during the Support, Educate, Empower Glaucoma Coaching Program to Improve Medication Adherence: A Pilot Study. Ophthalmol Glaucoma. 2020;3(4):238–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moral RR, Torres LAPd, Ortega LP, et al. Effectiveness of motivational interviewing to improve therapeutic adherence in patients over 65 years old with chronic diseases: A cluster randomized clinical trial in primary care. Patient Educ Couns. 2015;98(8):977–983. [DOI] [PubMed] [Google Scholar]
- 23.Newman-Casey PA, Niziol LM, Lee PP, Musch DC, Resnicow K, Heisler M. The Impact of the Support, Educate, Empower Personalized Glaucoma Coaching Pilot Study on Glaucoma Medication Adherence. Ophthalmology Glaucoma. 2020;3(4):228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman-Casey PA, Niziol LM, Mackenzie CK, et al. Personalized behavior change program for glaucoma patients with poor adherence: a pilot interventional cohort study with a pre-post design. Pilot and feasibility studies. 2018;4:128–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang DS, Friedman DS, Frazier T, Plyler R, Boland MV. Development and Validation of a Predictive Model for Nonadherence with Once-Daily Glaucoma Medications. Ophthalmology. 2013;120(7):1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. [DOI] [PubMed] [Google Scholar]
- 27.Newman-Casey PA, Killeen O, Miller S, et al. A Glaucoma-Specific Brief Motivational Interviewing Training Program for Ophthalmology Para-professionals: Assessment of Feasibility and Initial Patient Impact. Health Commun. 2020;35(2):233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levesque CS, Williams GC, Elliot D, Pickering MA, Bodenhamer B, Finley PJ. Validating the theoretical structure of the Treatment Self-Regulation Questionnaire (TSRQ) across three different health behaviors. Health Educ Res. 2007;22(5):691–702. [DOI] [PubMed] [Google Scholar]
- 29.Williams GC, Grow VM, Freedman ZR, Ryan RM, Deci EL. Motivational predictors of weight loss and weight-loss maintenance. J Pers Soc Psychol. 1996;70(1):115–126. [DOI] [PubMed] [Google Scholar]
- 30.Williams GC, Deci EL. Internalization of biopsychosocial values by medical students: A test of self-determination theory. J Pers Soc Psychol. 1996;70(4):767–779. [DOI] [PubMed] [Google Scholar]
- 31.Rao VS, Peralta EA, Rosdahl JA. Validation of a glaucoma knowledge assessment in glaucoma patients. Clin Ophthalmol. 2016;10:1913–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Eye Health Education Program Eye Q Test. https://www.nei.nih.gov/sites/default/files/nehep-pdfs/EyeQTest_for_Toolkit.pdf. Accessed January 24, 2022.
- 33.Polonsky WH, Fisher L, Earles J, et al. Assessing Psychosocial Distress in Diabetes: Development of the Diabetes Distress Scale. Diabetes Care. 2005;28(3):626–631. [DOI] [PubMed] [Google Scholar]
- 34.Salman M, Andrews C, Heisler M, Darnley-Fisch D, Newman-Casey PA. Psychosocial Predictors of Glaucoma Medication Adherence Among the Support, Educate, Empower (SEE) Personalized Glaucoma Coaching Pilot Study Participants. Am J Ophthalmol. 2020;216:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chao J, Nau DP, Aikens JE, Taylor SD. The mediating role of health beliefs in the relationship between depressive symptoms and medication adherence in persons with diabetes. Research in Social and Administrative Pharmacy. 2005;1(4):508–525. [DOI] [PubMed] [Google Scholar]
- 36.Heisler M, Spencer M, Forman J, et al. Participants’ assessments of the effects of a community health worker intervention on their diabetes self-management and interactions with healthcare providers. Am J Prev Med. 2009;37(6 Suppl 1):S270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller DJ, Niziol LM, Elam AR, et al. Demographic, Clinical, and Psychosocial Predictors of Change in Medication Adherence in the Support, Educate, Empower Program. Ophthalmol Glaucoma. 2022;5(1):47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hadley W. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2016. [Google Scholar]
- 39.Assor A, Roth G, Deci EL. The emotional costs of parents’ conditional regard: a self-determination theory analysis. J Pers. 2004;72(1):47–88. [DOI] [PubMed] [Google Scholar]
- 40.Kanat-Maymon Y, Roth G, Assor A, Raizer A. Controlled by Love: The Harmful Relational Consequences of Perceived Conditional Positive Regard. J Pers. 2016;84(4):446–460. [DOI] [PubMed] [Google Scholar]
- 41.Deci EL, Ryan RM. The Empirical Exploration of Intrinsic Motivational Processes11Preparation of this chapter was facilitated by Research Grant MH 28600 from the National Institute of Mental Health to the first author. In: Berkowitz L, ed. Advances in Experimental Social Psychology. Vol 13. Academic Press; 1980:39–80. [Google Scholar]
- 42.Lee PP, Hoskins HD, Jr, Parke DW, III. Access to Care: Eye Care Provider Workforce Considerations in 2020. Arch Ophthalmol. 2007;125(3):406–410. [DOI] [PubMed] [Google Scholar]
- 43.Vajaranant TS, Wu S, Torres M, Varma R. A 40-Year Forecast of the Demographic Shift in Primary Open-Angle Glaucoma in the United States. Invest Ophthalmol Vis Sci. 2012;53(5):2464–2466. [DOI] [PubMed] [Google Scholar]
- 44.Vansteenkiste M, Simons J, Soenens B, Lens W. How to become a persevering exerciser? Providing a clear, future intrinsic goal in an autonomy-supportive way. J Sport Exerc Psychol. 2004;26(2):232–249. [Google Scholar]
- 45.Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33(1):23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez JS, Shreck E, Psaros C, Safren SA. Distress and type 2 diabetes-treatment adherence: A mediating role for perceived control. Health Psychol. 2015;34(5):505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohn J, Graue M, Assmus J, et al. Self-reported diabetes self-management competence and support from healthcare providers in achieving autonomy are negatively associated with diabetes distress in adults with Type 1 diabetes. Diabet Med. 2015;32(11):1513–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duangdao KM, Roesch SC. Coping with diabetes in adulthood: a meta-analysis. J Behav Med. 2008;31(4):291–300. [DOI] [PubMed] [Google Scholar]
- 49.Ajit RR, Fenerty CH, Henson DB. Patterns and rate of adherence to glaucoma therapy using an electronic dosing aid. Eye. 2010;24(8):1338–1343. [DOI] [PubMed] [Google Scholar]
- 50.Cho J, Niziol LM, Heisler M, Newman-Casey PA. Increased Near Activities Function Associated With Increased Glaucoma Medication Adherence Among Support, Educate, Empower (SEE) Participants. J Glaucoma. 2021;30(8):744–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Medicare Diabetes Prevention Program Expanded Model. In. Medicare Learning Network: Centers for Medicare and Medicaid Services 2018.


