Abstract
Cardiovascular disease risk is known to be influenced by both the severity of a risk factor and the duration of exposure (e.g. LDL-C, tobacco smoke). However, this concept has been largely neglected within the obesity literature. While obesity severity has been closely linked with cardiometabolic diseases, the risk of developing these conditions among those with obesity may be augmented by greater obesity duration over the lifespan. Few longitudinal or contemporary studies have investigated the influence of both factors in combination – cumulative obesity exposure – instead generally focusing on obesity severity, often at a single time point, given ease of use and lack of established methods to encapsulate duration. Our review focuses on what is known about the influence of the duration of exposure to excess adiposity within the obesity-associated cardiometabolic disease risk equation by means of summarizing the hypothesized mechanisms for and evidence surrounding the relationships of obesity duration with diverse cardiovascular and metabolic disease. Through the synthesis of the currently available data, we aim to highlight the importance of a better understanding of the influence of obesity duration in cardiovascular and metabolic disease pathogenesis. We underscore the clinical importance of aggressive early attention to obesity identification and intervention to prevent the development of chronic diseases that arise from exposure to excess body weight.
Keywords: obesity, cardiometabolic, cardiovascular, duration
Graphical Abstract
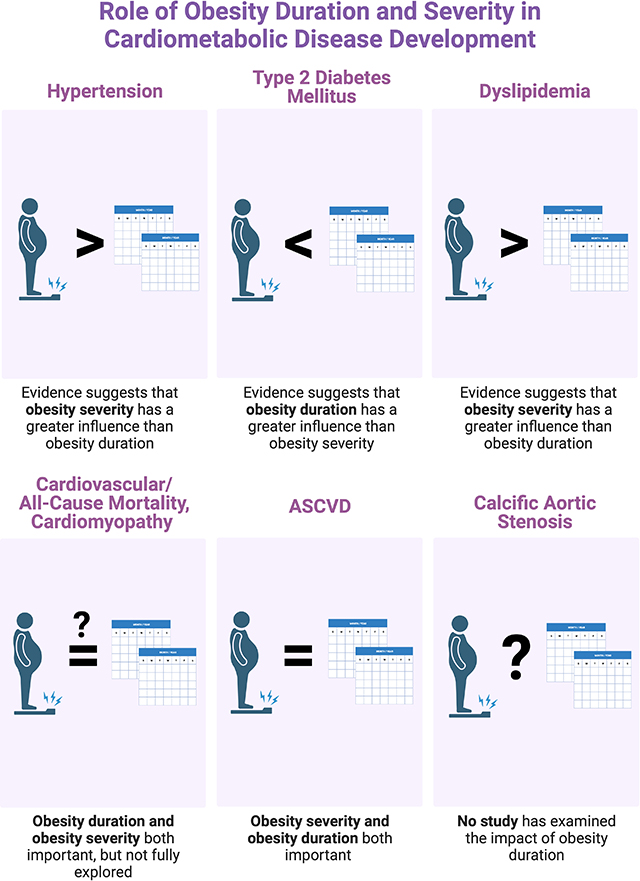
Introduction
The development of many cardiovascular diseases (CVD) is influenced by both risk factor severity or intensity and the duration of exposure (e.g. tobacco smoke, LDL-cholesterol, blood pressure, hyperglycemia).1–4 Obesity is an independent risk factor for atherosclerotic cardiovascular disease (ASCVD), heart failure, and aortic stenosis,5 and obesity severity drives the pathogenesis of myriad metabolic diseases, particularly diabetes, hypertension, and dyslipidemia, with greater BMI associated with a higher incidence.6 However, it is well recognized that individuals with equivalent degrees of adiposity often do not exhibit identical risk for cardiometabolic disease.7 While factors underlying these differences are still not well understood, one potentially mediating variable that has received surprisingly little attention is the duration of time an individual is exposed to excess adiposity. With increasing trends toward lifelong obesity,8 it is imperative that we better understand the implications of obesity duration.
For example, if we make the analogy of obesity to smoking and “pack-years,” it might stand to reason that determining somebody’s “obese-years” (a concept pioneered by Abdullah et al.9 as the product of the number of years lived with obesity and the degree of adiposity) would be more informative in determining their overall risk than just knowing the number of packs of cigarettes someone is currently smoking. Such a metric reflects cumulative exposure over a lifetime. While it may be reasonable to conclude that a similar association may exist between long-term obesity and metabolic risk factors as well as diagnosis with various CVD, this is an area that has received minimal research attention.
Though many long-term studies have assessed the specific effects of obesity severity or degree of adiposity on varied CVD incidence, few have tackled the question of cumulative obesity exposure – presumably, in large part, due to the difficulty of accurately determining duration of obesity. Even in the single prior review10 that suggested that obesity duration was worthy of investigation and emphasized that delaying obesity onset likely contributes to lower risk of developing CVD, the authors did not critique the existing data nor examine whether it was consistent across comorbid metabolic conditions.
Our aim is to review what is known about the role of obesity duration, as distinct from obesity severity, on CVD and metabolic risk factor development. We do so through a thorough discussion of the proposed pathophysiologic mechanisms and experimental evidence of relationships between obesity duration and conditions with which obesity has been consistently associated, including type 2 diabetes mellitus (T2DM), hypertension, dyslipidemia, ASCVD, structural heart disease, and overall mortality.
Adiposopathy, Inflammation, and Hyperinsulinemia
Once misconstrued as an indolent energy storage repository, adipose tissue is now recognized as a heterogeneous, complex, and dynamic organ.11 In the setting of caloric excess, white adipocytes increase in size and number. Both hypertrophy and hyperplasia, particularly within the visceral compartment and in tissues relatively devoid of adipose tissue, often lead to adipose dysfunction (adiposopathy)12 which contributes to the pathogenesis of numerous obesity-related cardiometabolic diseases (Figure 1).13 With increasing adipocyte size, several factors released by adipocytes increase, including free fatty acids (FFA), resistin and tumor necrosis factor α, whereas adiponectin secretion is reduced.14 Amounts of reactive oxygen and nitrogen species also increase.15 The presence of dysfunctional adipose tissue that characterizes adiposopathy in obesity is thought to mediate the pathogenesis of many of the conditions we will discuss.
Figure 1. Mechanistic pathways mediating the roles of both obesity/adiposity severity and duration in cardiovascular and metabolic disease pathogenesis.
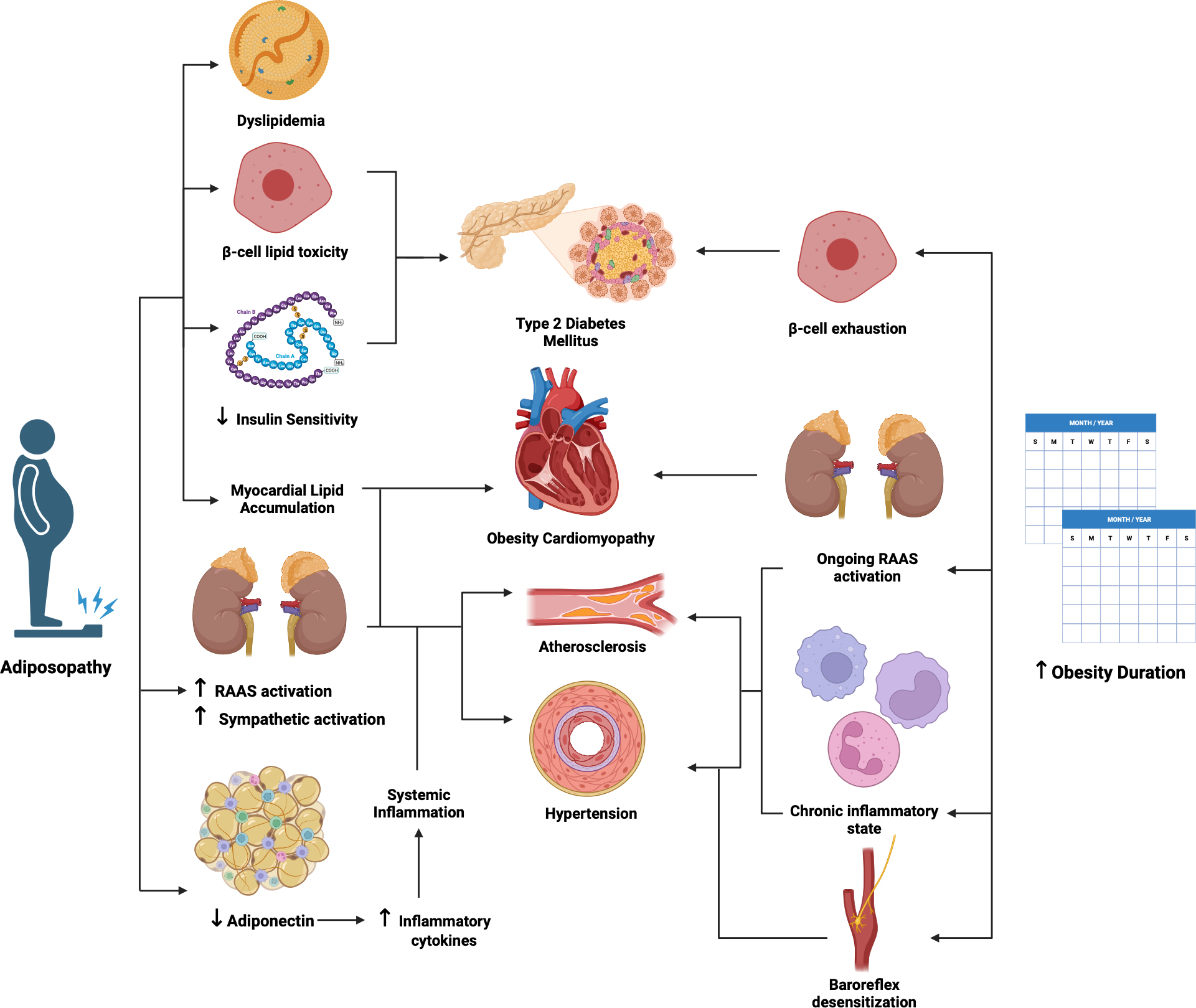
This figure summarizes the available evidence from human translational and animal studies supporting the diverse roles for obesity severity and duration in the pathophysiology of various cardiovascular diseases and associated conditions affecting cardiovascular risk. Dysfunctional adipose tissue mediates the pathogenesis through numerous mechanisms. Less is known about potentially unique means through which obesity duration impacts the development of these disorders, and the potential for their resolution/improvement with weight loss.
Created with BioRender.com. ASCVD, atherosclerotic cardiovascular disease; RAAS, renin-angiotensin- aldosterone system
The processes that mediate the transition of healthy to pathologic adipose tissue that stimulate chronic local and systemic inflammation in the setting of obesity remain incompletely characterized.16,17 A number of interrelated pathways are thought to contribute with hyperinsulinemia being prominent.18,19 Leptin normally suppresses insulin synthesis and secretion but in leptin deficiency (as demonstrated by work in ob/ob mice), hyperinsulinemia ensues and precedes the development of insulin resistance which together contribute to obesity.20 In fact, mice shielded from hyperinsulinemia on a high-fat diet are provided life-long protection against obesity.21–23 However, the signals that determine at what point adiposopathy, chronic inflammation, and hyperinsulinemia develop, and specifically how the extent and duration of obesity contribute to these processes and further contribute to metabolic dysfunction and cardiovascular pathology, are poorly understood and have not been well-studied in mouse models. Further, investigation of visceral adipose tissue of aging mice has suggested that a pro-inflammatory immune cell phenotypic profile develops as the tissue ages, regardless of the presence of obesity.24,25 However, it remains unclear whether this plays a mechanistic role in the evolving cardiometabolic disease risk seen with chronic obesity exposure – highlighting the importance of dedicated study in this complicated area.
Cardiovascular and All-Cause Mortality
Obesity is associated with increased morbidity and early mortality, particularly from CVD. In a pooled analysis of 20 prospective studies from the United States, Sweden, and Australia, individuals with severe (class III) obesity had greater sex- and age-adjusted total mortality compared to normal weight controls, with cardiovascular cause-specific mortality largely accounting for the excess deaths seen in this group.26 However, this study, in addition to many others, seems to assume all individuals with the same BMI severity have similar CVD risk, ignoring differences in obesity phenotype, duration, and trajectory.
An analysis examining the influence of obesity severity and duration on mortality in the Atherosclerosis Risk in Communities (ARIC) cohort suggested a lack of influence of obesity duration on this outcome. With the intent to assess dynamic obesity trends, in this report, subjects were subdivided into four classes (decline, stable/slow decline, moderate increase, and rapid increase) for several different measures of adiposity (BMI, waist circumference (WC), self-reported weight at age 25, tricep skinfold, and calf circumference) across up to four triennial visits.27 The stable/slow decline group represented the reference and had, on average, a slow decline for 3 of the 4 obesity measures during follow-up (BMI, tricep skin fold, and calf measurement) but a slight increase in WC over this period. There was no difference in mortality between the stable/slow decline group and the rapid increase group despite adjustment for cardiovascular risk profile and baseline BMI. Notably, the moderate increase group exhibited the lowest overall mortality risk and the decline subclass demonstrated an elevated mortality risk compared to the reference group. The authors performed several sensitivity analyses in an attempt to explain these perplexing results, but despite removal of individuals with incident heart failure, incident cancer, and diabetes at baseline, the results remained unchanged. While excluding overtly frail individuals did not alter the results, a subgroup analysis of “pre-frail” individuals strengthened the associations seen between the classes and mortality risk, versus analysis of “robust” individuals which eliminated the positive findings. They recognized the inherent confounding factor present in observational studies, stating that the inability to distinguish intentional from unintentional weight loss was a major limitation of their results. Overall, while individuals with declines in adiposity metrics exhibited the greatest risk of mortality, the mechanisms explaining this observation are not at all clear and likely are at least partly related to varied processes mediating unintentional weight loss and frailty.
In contrast to ARIC, within the Framingham Heart Study (FHS), years lived with obesity was positively associated with risk for all-cause [adjusted HR 1.07 (95% CI 1.05–1.08)] and cardiovascular mortality [adjusted HR 1.06 (95% CI 1.05–1.07] even after adjustment for BMI severity at last follow-up, age, diabetes, smoking status, and incident CVD.28 Obesity duration exhibited a dose-response relationship with these outcomes with a doubling of risk in association with 5 – 15 years of obesity and tripling with ≥15 years of obesity compared to those who were never obese. Despite its rigor, a limitation of this analysis is that obesity duration could not be definitively calculated in subjects with obesity at enrollment, though sensitivity analyses suggested that the outcomes were not greatly impacted. Nonetheless, the findings support a significant independent (and possibly complementary) association of duration of obesity with all-cause and cardiovascular mortality.
Type 2 Diabetes Mellitus
Over 90% of patients with T2DM are either overweight or obese29 and the preponderance of the literature suggests that obesity severity and duration are both important in the pathogenesis. Adipocyte hypertrophy leads to increased FFA secretion where excess lipids such as ceramides and saturated fatty acids are toxic to pancreatic islet cells, leading to β-cell dysfunction and apoptosis.30, 31 High levels of FFA also facilitate infiltration of immune cells into dysfunctional adipose tissue further impairing insulin sensitivity.32 These processes occur gradually, with cumulative damage progressing from insulin resistance to a diagnosis of diabetes, especially as the duration of obesity increases.33 Long-term obesity may also result in permanent elevations in circulating FFA that further worsens insulin resistance despite increased insulin secretion, especially if β-cell exhaustion occurs.34 Early abdominal obesity impacts the development of insulin resistance and longer duration leads to progressive deterioration of β-cell function, leading to overt diabetes.35
Among a sample of over sixty-thousand men from Denmark with longitudinal weight data, those who were persistently overweight from childhood to early adulthood had greater risk of developing diabetes during mid-adulthood than those who were overweight during only one of the time points, though they did not control for current obesity severity.36 Similarly, the U.S. National Longitudinal Study of Adolescent Health found that persistent obesity starting in young adulthood (vs. adult onset) was associated with increased likelihood of T2DM in both men and women, with a two-fold higher risk of diabetes in early adulthood in women with obesity at <16 years vs women who became obese at ≥18 years of age.37 This association held after adjustment for current BMI and WC, age, race/ethnicity, education, and parental history of diabetes. Men, however, had a comparable risk of developing diabetes in early adulthood regardless of whether their BMI increased above 30 kg/m2 before or after 18 years of age. The authors suggested that the impact of obesity during pubertal development differs between men and women which may explain the differences in outcomes. Obesity onset in females during this period may be particularly harmful for insulin sensitivity that, in conjunction with the metabolic changes that occur with longer obesity duration, may increase the risk of developing diabetes in the future.
Araújo et al.38 evaluated participants at four time points during the transition from ages 13 to 24 years and used area under the curve to represent the duration and severity of BMI over the 11-year period (BMIAUC). The BMIAUC represented an individual’s trajectory with higher BMIAUC denoting greater cumulative exposure to excess adiposity while accounting for fluctuations, although the study was limited by the lack of BMI information prior to the study period. While individuals with similar BMIAUC had vastly different BMI trajectories, the study accounted for this by way of analyzing the effect of BMIAUC independent of the final BMI at 24 years and found BMIAUC to be strongly predictive of insulin resistance. The CARDIA Study, which used repeat measurements of BMI and WC (a commonly used measure of abdominal obesity and surrogate index of visceral adiposity) from 2 to 25 years after baseline, looked specifically at abdominal obesity in young adulthood and concluded that longer duration of abdominal obesity conferred additional risk of developing diabetes independent of abdominal obesity severity, family history of diabetes, and physical activity.35
Several studies isolated to adults have also shown that greater obesity duration increases the likelihood of developing T2DM. Norris et al.,39 using three British birth cohorts, found that individuals with <5 years of obesity had a 5% higher HbA1c, compared to those who were never obese. This was increased to 20% among those with obesity for 20 to 30 years. While the association was attenuated after adjustment for mean excess BMI, a significant relationship remained. A secondary analysis of the FHS found a strong relationship between obesity duration and risk for incident diabetes despite adjustments for BMI at the time of diagnosis.40 When stratified by sex and age of obesity onset, there was 11% and 22% increased risk for diabetes for each additional 2-years of obesity duration among men with an age of obesity onset <50 years and ≥50 years, respectively. The exposure-disease relationship was similar in women with an age of obesity onset <50 years (10%) compared to their male counterparts, but far less strong for women with an of obesity onset ≥50 years (8%) than in men of similar age. These findings diverge from evidence that supports a stronger obesity-associated risk of diabetes risk in young women, as men were diagnosed with T2DM at lower BMIs.41 The authors speculated that estrogen in premenopausal women may attenuate insulin resistance, as mouse models have identified that impairing estrogen receptor-mediated signaling results in hepatic insulin resistance and elevated hepatic glucose production resulting in hyperglycemia.42 They proposed that in addition to their predisposition to visceral adipose deposition and hypertrophy, low estrogen levels leave men more prone to developing insulin resistance.
The relationship between obesity duration and diabetes risk in the FHS cohort described above was at least three times stronger than in two other cohorts. Everhart et al.43 enrolled Native American participants and determined that the association between obesity duration and T2DM incidence was highly significant after adjusting for age, sex, and current BMI. Despite this, the current presence of excess adiposity also appears important, as there was not excess age-sex-adjusted risk of diabetes for those in this study who developed obesity and then subsequently lost weight to a BMI <30kg/m2. Further, a prospective study of men 40–59 years without diabetes at enrollment found that age-adjusted risk of diabetes increased progressively with duration of overweight or obesity.44 Men who had been overweight, obese, or severely obese for ≥5 years consistently had greater age-adjusted risk of diabetes than men who had been in the corresponding category for <5 years (i.e. RR 8.04 vs 4.36 in BMI ≥30kg/m2 group). Several other groups have found a positive relationship of obese-years with diabetes incidence, with some data suggesting, perhaps not surprisingly, that both variables contribute to risk of developing diabetes.9, 45
Overall, studies demonstrate a robust relationship of obesity duration with incident diabetes despite adjustment for severity of obesity,37–39, 43–44, 46–47 although some have shown a slightly muted relationship after adjustment.39, 48–49 The available evidence seems to suggest that persistent obesity, especially when beginning in childhood or adolescence, poses a substantial risk for diabetes development in adulthood irrespective of obesity severity. Some data suggest sex differences, but these findings are inconsistent and without strong evidence underlying any mechanism. Finally, despite the relative homogeneity of findings in these studies, there are very limited data available on the role of race in the relationship between obesity duration and diabetes incidence. While we have evidence that certain racial groups are at an increased risk of developing type 2 diabetes at lower BMI severity (e.g. South Asian ethnicities), for example, we lack data to determine if this is consistent for obesity duration exposure.
Hypertension
Obesity is a key modifiable factor in the development and persistence of hypertension.50 The impact of obesity on blood pressure is multifactorial and encompasses neurohormonal alterations, increased renal sodium uptake, physical renal compression by adipose tissue, and systemic inflammation.51 Obesity-mediated activation of the renin-angiotensin-aldosterone system (RAAS) and angiotensinogen production and release by adipose tissue, in combination with elevated leptin levels, promote sodium retention and sympathetic nervous system activation.52–53 Paradoxically, however, leptin receptor-deficient db/db and leptin-deficient ob/ob mice exhibited elevated blood pressures, baroreflex dysfunction, and sympathetic activation that was reversed with pharmacologic RAAS blockade, suggesting that dysregulated angiotensin II may be the culprit of autonomic dysfunction regardless of leptin deficiency,54–55 but also raising doubt on the relevance of these models to human obesity.56 Interestingly, overfeeding humans and animals leads to rapid activation of the sympathetic nervous system and RAAS even before significant weight gain is achieved.57 Ongoing sympathetic and RAAS activation that occur in long-standing obesity ultimately lead to small artery remodeling and vasoconstriction, which increases wall stress and contributes to worsening hypertension.58 Further, arterial baroreceptor responses that modulate elevations in blood pressure through parasympathetic activation and sympathetic inhibition becomes less effective as chronic stimulation in an obese state leads to baroreflex desensitization.59
Available evidence suggests that while both obesity degree and duration are important in the pathogenesis of hypertension, obesity severity is likely the more important component of the equation. The effect of excess body weight on blood pressure is evident prior to adulthood. The National Longitudinal Study of Adolescent Health examined changes in weight from adolescence to adulthood, finding that chronically overweight or obese subjects were at greater risk of hypertension than those who remained at normal weight, became obese in adulthood only, or were overweight/obese in adolescence and became non-obese in adulthood, with OR from 2.7 to 6.5 depending upon race/ethnicity.60 However, weight was only measured at two time points and no adjustment was made for current BMI. Except for African-American men and to some extent Hispanic men, those who were overweight or obese in adolescence but not in adulthood, did not exhibit increased risk of hypertension compared to those who maintained normal weight throughout, suggesting that any effect of obesity duration on blood pressure may be reversible with weight loss. In the study by Araújo et al.38 discussed above in the context of diabetes, there was a positive association between BMIAUC and systolic blood pressure, although this was largely attenuated after adjustment for BMI at 24 years of age, arguing that the mechanisms underlying obesity’s influence on blood pressure are not affected by chronicity. Compared to analyses of diabetes, the relationship with hypertension was much more influenced by subjects’ current BMI.
Blood pressure and weight were observed biennially among adults 30 to 62 years without hypertension in FHS. The investigators found a positive relationship between obesity duration and incident hypertension, but similar to studies of adolescents, this relationship was diminished after adjustment for most recent BMI.61 These findings are paralleled by those using 1999–2010 U.S. National Health and Nutrition Examination Survey (NHANES) data showing that while individuals who were obese both at 25 years of age and at the time of the survey had greater odds of elevated blood pressure, this association was eliminated after adjustment for current BMI.62 Finally, while obesity duration was associated with higher blood pressure in an analysis of three British birth cohorts, the relationship that remained after adjustment for BMI severity was weak.39
Overall, evidence suggests that obesity duration has a modest effect at most on blood pressure when separated from obesity severity. Additional support for this conclusion comes from studies that generally demonstrate that weight loss even in the setting of long-term obesity can lead to blood pressure improvement.63 However, the issue is far from settled. As mentioned above, similar to diabetes, limited data suggest potential influences of sex and race, but influences of these variables are underinvestigated. Further, some data suggest that while blood pressure improves with weight loss, not all mechanisms mediating obesity-associated hypertension may be reversed, but whether obesity duration plays a role in these responses is uncertain.64
Dyslipidemia
In approximately 2/3 of individuals with obesity, adipocyte hyperplasia and hypertrophy that lead to adiposopathy result in dyslipidemia characterized by elevated circulating triglycerides, VLDL, and apolipoprotein B in conjunction with low HDL-C.65–66 Adipose tissue location also influences obesity-related effects of lipid metabolism, with visceral adiposity particularly associated with higher triglycerides and lower HDL-C.67
The available data suggest that obesity severity is more integral to dyslipidemia pathogenesis than obesity duration, consistent with the literature that demonstrates individuals who have undergone weight loss will experience improvement in lipid profiles despite a background of chronic obesity.68 That said, studies exploring the effect of obesity duration on dyslipidemia incidence and severity are few relative to those investigating hypertension or diabetes.
In a longitudinal study of participants in their early twenties, groups were identified based on interpolated BMI trajectories from birth to 23 years: never obese, former obesity (childhood only), transient obesity (preadolescence only), recent onset obesity (adolescence to early adulthood), and persistent obesity (childhood to adulthood).69 Persistent and recent-onset obesity were associated with worsened cardiometabolic risk profiles, specifically lower HDL-C. Nonetheless, individuals with early childhood or preadolescence obesity onset that transitioned to non-obesity status by early adulthood exhibited largely normal lipid profiles that were comparable to never obese participants. In a study of three British birth cohorts described above,39 obesity durations of <5 years and 20–30 years were associated with 12.4% and 24.8% lower HDL-C, respectively, compared to those of never obese individuals. While this trend was attenuated after adjustment for obesity severity, a statistically significant association nonetheless remained.
These findings align with those from a pooled analysis of prospective cohort studies regarding how duration of high adiposity from childhood to adulthood influences cardiovascular risk.70 High adiposity status was defined by age and sex-specific thresholds for children and BMI >30kg/m2 for adults. Over an average of 23 years, individuals with consistently high adiposity had greater risk of elevated LDL-C and triglycerides, as well as low HDL-C, versus those with normal BMI status throughout this timeframe (control group). Subjects who were overweight or obese during childhood but reached normal BMI status by adulthood had profiles similar to that of the control group as all trends were largely attenuated and became nonsignificant following adjustment for adult obesity – minimizing a potential role of duration.
The CARDIA study, which calculated excess BMI- and WC-years to represent the product of duration and severity of overall and abdominal adiposity, respectively, found these metrics were associated with greater triglycerides, total cholesterol, and lipid-lowering mediation use, and lower HDL-C.71 When obesity duration was investigated independently among Black and White CARDIA participants between 26 and 30 years at entry, every additional year of overweight or obese status increased the odds of developing dyslipidemia (HDL-C <50mg/dL in women, <40mg/dL in men; LDL-C ≥130mg/dL; or triglycerides ≥200mg/dL) by 10–15%.72 However, while the model included baseline BMI, it did not adjust for BMI at the time dyslipidemia incidence was determined. To our knowledge, a single analysis of NHANES data is the only one to demonstrate a significant association between overweight duration and dyslipidemia despite adjustment for concomitant BMI.73 There was a graded increase in odds of hypertriglyceridemia (men and women) and low HDL-C (women only) when moving from the normal weight to overweight <10 years to overweight ≥10 years groups, independent of current BMI. A major limitation of these results, however, was that duration was determined by recalled weights from 10 years prior to the time of survey.
The evidence remains limited but overall mirrors our discussion of hypertension – with the scale tipped in favor of BMI severity having a primary role in influencing lipid profiles. Further, although adiposity distribution has an important and well-established influence on lipid profiles, this relationship may not pertain to obesity duration, as both excess BMI and WC-years demonstrate similar associations with obesogenic dyslipidemia.
Atherosclerotic Cardiovascular Disease
While obesity contributes to the development of atherosclerosis through its mediation of atherogenic dyslipidemia, hypertension, and T2DM as described above, the condition is also an independent risk factor for ASCVD.5 Much of the independent risk is felt to arise from the systemic and vascular inflammation elicited and perpetuated by excess adiposity. Adiponectin has a protective effect on the development of ASCVD through several mechanisms, including increased nitric oxide production, downregulation of pro-atherogenic mediators, coronary plaque stabilization, and arterial vasodilation.11 When adiponectin becomes dysregulated in obesity, these processes are overwhelmed by inflammatory cytokines and other mediators.74–75 Excess adipose tissue in perivascular areas has also been suggested to contribute to vascular damage by means of local inflammation.76 The inflammatory effects seen in obesity are also driven by a complex coalition of inflammatory signals that include neutrophil, monocyte, macrophage, eosinophil, and mast cell proliferation and activation within both the circulation and adipose tissue77 ultimately perpetuating the systemic inflammation driving ASCVD pathogenesis.78–79 While recent data suggest that weight loss in obese hypercholesteremic mice can promote atherosclerotic plaque regression through induction of a unique subset of macrophages and inhibition of immune progenitor reprogramming, the impact of prolonged obesity on the capacity for resolution remains uncertain.80
Several studies suggest that obesity duration is particularly associated with increased risk of ASCVD, independent of the presence of obesity-associated risk factors. However, whether this is driven primarily by obesity severity is unclear given limitations in the analyses. Several prospective studies employing obese-years as a proxy for cumulative exposure found this metric to be a better predictor of incident ASCVD than either measure alone. In CARDIA, for each additional 50 excess BMI-years (defined as the subtracted difference between the actual BMI and reference BMI [25 kg/m2] multiplied by the duration in years), there was an increase in the incidence of coronary heart disease (CHD; defined as myocardial infarction, hospitalization for angina/acute coronary syndrome, or coronary artery disease-related death) after adjustment for age, race, sex, study center, education, smoking, alcohol use, physical activity, and energy intake [adjusted HR 1.25 (95% CI 1.07–1.46)], that was attenuated, but persisted, with inclusion of all obesity-associated CHD risk factors [adjusted HR 1.06 (95% CI 0.86 – 1.32)].71 When obese-years were calculated for FHS participants with 50 years of follow-up data, for every 10 unit increase in obese-years (BMI units >29kg/m2 multiplied by number of years with that BMI), the HR for CHD increased by 4% for males and 3% for females after adjustment for age, smoking status and physical activity.81 However, a major limitation of this metric is that it only used BMI at a single time point, not reflecting any possible changes in weight.
Obesity duration has been found to influence objective measures of ASCVD severity in several cohorts. There was a strong positive association between duration of overall and abdominal obesity and both the presence and extent of coronary artery calcium (CAC) even after adjustment for current BMI and WC in CARDIA subjects without obesity at enrollment.82 Obesity duration was further associated with 10-year progression of CAC in these middle-aged subjects. The Bogalusa Heart Study examined the relationship between obesity identified in youth and carotid intima-media thickness (CIMT) in adulthood and found greater CIMT only among those who were consistently obese from childhood to adulthood.83 The Cardiovascular Risk in Young Finns Study subsequently found that consistent obesity from youth to adulthood was associated with greater age- and sex-adjusted CIMT compared to those who remained non-obese or who had been obese in their youth but became non-obese in adulthood.84 The relationship remained significant even after adjustment for obesity-related risk factors. However, as opposed to the findings within Bogalusa, CIMT of those who were obese beginning in childhood was comparable to those who were obese in adulthood only, which may suggest that this association is explained more by severity of adult obesity rather than total obesity duration, as the relationship between adult BMI and CIMT was highly significant despite adjustments for metabolic risk factors. The argument that BMI severity may be more important in ASCVD is further supported by data from the Atherosclerosis Risk in Young Adults study where a positive relationship between adolescent BMI and adult CIMT was attenuated after adjustment for adult BMI.85
At this time, obesity duration and severity both appear important in the pathogenesis of ASCVD separate from their contributions to ASCVD risk factors, though the distinct effect of each has not been confidently demonstrated.
Structural Heart Disease
Obesity-associated inflammation and oxidative stress contribute not only to arterial pathology, but also myocardial dysfunction. Chronic RAAS activation alters cardiac fibroblast production leading to excess collagen deposition, myocardial stiffening, hypertrophy, and ultimately diastolic dysfunction.86 Animal models suggest that myocardial lipid accumulation and lipotoxicity further contribute to altered cardiac hemodynamics and cellular damage that mediate obesity cardiomyopathy.87
While obesity duration has always been considered important to the associated cardiomyopathy, separate mediating effects of severity and duration have only been cursorily explored. Obesity duration has repeatedly been associated with left ventricular (LV) diastolic impairment,88–89 increased LV mass,89–91 and LV systolic function.89, 91 For example, one study found that LV dimension, wall thickness, and stroke volume were significantly positively correlated with obesity duration when subjects with <15 years of obesity were compared to those with ≥15 years of obesity, despite no difference in degree of obesity between the two groups.88 Alpert et al.89 found that greater duration of obesity was associated with higher LV mass, worse LV systolic function, and greater impairment of LV diastolic filling in patients undergoing bariatric surgery. In a separate cohort of candidates for bariatric surgery (average BMI 49 kg/m2) LV wall thickness strongly correlated with obesity duration.90 However, neither of these small analyses accounted for obesity severity. The CARDIA study illustrated that even after adjusting for hypertension, T2DM, demographics, smoking, and physical activity, each additional five years of overall obesity were associated with a 2.5g increase in LV mass and 0.3% decrease in LV ejection fraction on echocardiograms completed 25 years after enrollment. However, obesity severity at year 25 was also strongly associated with nearly all measures of LV structure, possibly suggesting that cardiac remodeling is due to both excess adiposity severity and the cumulative impact of longer obesity duration (predominantly by increasing LV mass), though an interaction term was not included in their models.91
For every 10 additional obese-years experienced by FHS participants, there was 6% increased adjusted risk of developing congestive heart failure (CHF) for males and 4% for females.81 A comparison of predictors found obese-years to provide more precise estimation of the risk of CHF than severity or duration of obesity alone. Among CARDIA subjects, each additional 50 excess-BMI years was associated with increased risk of CHF that persisted after inclusion of all obesity-associated CHF risk factors [adjusted HR 1.32 (95% CI 1.05–1.66)] and was a better predictor of CHF than either time-varying BMI or WC.71
A single study investigated the impact of weight history on markers of subclinical myocardial damage through a secondary analysis of the ARIC cohort.92 The authors identified subjects who did not have CVD at the time of collection of blood for high sensitivity cardiac troponin-T (hs-cTnT; Visit 4) and determined obesity duration during two different periods: 1) Visits 1 through 4 (representing a 9-year span in middle age) and 2) age 25 (self-reported and collected at Visit 1) through Visit 4 (representing young adulthood to late middle age). Every 10 years of obesity were associated with hs-cTnT [OR 1.26 (95% CI, 1.17–1.35)] after adjustment for diabetes, systolic blood pressure, anti-hypertensive medication use, LDL-C, HDL-C, triglycerides, eGFR, and NT-proBNP. Each 100 additional excess BMI-years showed a similar association with hs-cTnT [OR 1.21 (95% CI, 1.14–1.27)].
Calcific aortic valve stenosis (CAVS) is the most common valvular abnormality and shares many risk factors with ASCVD, including hypertension, T2DM and dyslipidemia. However, medical therapies, such as lipid-lowering agents, do not slow progression of CAVS or associated events (e.g., need for replacement, CHF).93 In an analysis of 71,817 men and women without CVD at enrollment, overall and abdominal obesity were both associated with CAVS after adjustment for age, diabetes, hypertension, and dyslipidemia. Obese individuals with substantially increased WC were at highest risk of CAVS among the participants.94 A subsequent Mendelian randomization study corroborated the relationship between BMI and CAVS concluding that among 14 CVD outcomes including CAD, CHD, and atrial fibrillation, genetically-predicted BMI was most strongly associated with CAVS.95 However, no study to date has examined the impact of obesity duration on CAVS development, despite this condition developing over decades of exposure to pathologic stimuli and no demonstrable change in disease trajectory with weight loss.96
Conclusions
While the pathophysiology underlying the influence of obesity on the development of CVD and metabolic risk factors has been explored extensively, much less is known about how these mechanisms are influenced by long-term exposure to excess adiposity. The current evidence suggests that obesity duration’s influence in cardiometabolic disease is complex and heterogenous. While its impact appears to outweigh that of obesity severity in diabetes mellitus, obesity duration’s influence on other metabolic risk factors, such as hypertension and dyslipidemia, appears less pronounced. We cannot be certain that duration of obesity doesn’t play a role in the reversibility of these obesity-related risk factors, as the question has received inadequate research attention. Chronic exposure to excess adiposity is clearly important to the pathogenesis of ASCVD and cardiomyopathy, and increases mortality, though the best attempts to delineate its roles are still fraught with limitations. We have yet to do more than scrape the surface in investigating the influence of obesity duration on CAVS – the condition with the strongest association with BMI. Longer duration of abdominal or visceral obesity augments the risk for developing diabetes, dyslipidemia, and ASCVD, though it remains unclear if it poses additional risk compared to subcutaneous adipose tissue accumulation of the same degree.
Despite improving treatments for obesity, millions of individuals experience obesity for extended durations, often beginning in child- or young adulthood, before losing weight and appreciating and treating residual risk following successful weight loss will remain a prevalent concern. There is thus urgent need for focused effort to understand the consequences of cumulative obesity exposure. It is imperative that studies analyze contemporary cohorts to better characterize the impact of obesity duration (in addition to severity) on cardiometabolic disease. Furthermore, within this context, efforts to additionally define the influence of additional variables, including adipose distribution, overall body composition, and lifestyle factors (e.g. dietary composition, physical activity/cardiovascular fitness) within the equation. There remains a need for data from appropriate animal models in the area of obesity duration to provide critical mechanistic insights critical to deciphering the heterogeneous impact of obesity exposure on disease development.
As limited data suggest that greater durations of obesity are associated with reduced adherence to obesity treatments,97 and may also alter the pharmacokinetics of weight loss medications98, any influence of obesity duration on interventions and therapeutics for obesity and related conditions also requires exploration. Within the context of obesity, variations in physical activity and diet can influence the pathophysiologic processes driving cardiovascular disease,99 adding complexity to the relationship between chronic obesity exposure and cardiovascular outcomes. Nonetheless, these variables were not adjusted for in any of the reports contained within the present review and thus are important considerations for future analyses. With insight into the importance of obesity duration, we may improve our treatments to counteract obesity and its related diseases – potentially impacting hundreds of millions of people worldwide who suffer from chronic obesity and are at high risk for diseases that decrease life expectancy and increase disability.
Highlights.
Despite decades of attention to the contribution of obesity to cardiometabolic disease, few studies have assessed the specific contribution of cumulative obesity exposure
We find that among diverse cardiovascular diseases and metabolic risk factors often associated with obesity, some exhibit clear associations with obesity duration (e.g. diabetes, atherosclerosis), others are uncertain (e.g. hypertension, dyslipidemia), and some are uninvestigated (e.g. calcific aortic stenosis).
Our observations support the urgent need for clinical and pre-clinical research into the impact of obesity duration on the 1) pathogenesis of cardiometabolic disease, 2) potential residual risk associated with prolonged obesity exposure despite weight loss, and 3) impact of obesity duration on the efficacy of present and developing therapies for obesity.
Non-standard Abbreviations and Acronyms
- ARIC
Atherosclerosis Risk in Communities
- ASCVD
atherosclerotic cardiovascular disease
- CAC
coronary artery calcium
- CAVS
calcific aortic valve stensosis
- CIMT
carotid intima-media thickness
- CHD
coronary heart disease
- CHF
congestive heart failure
- CVD
cardiovascular diseases
- FFA
free fatty acids
- FHS
Framingham Heart Study
- hs-cTnT
high sensitivity cardiac troponin-T
- LV
left ventricle
- NHANES
National Health and Nutrition Examination Survey
- RAAS
renin-angiotensin-aldosterone system
- T2DM
type 2 diabetes mellitus
- WC
waist circumfrence
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose.
Disclosures: JOA has acted as a consultant/advisor for Novo-Nordisk and Intellihealth. SPH has acted as a consultant for Novo-Nordisk.
References
- 1.Lubin JH, Couper D, Lutsey PL, et al. Risk of Cardiovascular Disease from Cumulative Cigarette Use and the Impact of Smoking Intensity. Epidemiology. 2016;27:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domanski MJ, Tian X, Wu CO, et al. Time Course of LDL Cholesterol Exposure and Cardiovascular Disease Event Risk. J Am Coll Cardiol. 2020;76:1507–1516. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs FD, Whelton PK. High Blood Pressure and Cardiovascular Disease. Hypertension. 2020;75:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leon BM, Maddox TM. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6:1246–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piché ME, Tchernof A, Després JP. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ Res. 2020;126:1477–1500. [DOI] [PubMed] [Google Scholar]
- 6.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med. 2008;168:1617–24. [DOI] [PubMed] [Google Scholar]
- 7.Powell-Wiley TM, Poirier P, Burke LE, et al. ; American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2021;143:e984–e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels SR, Jacobson MS, McCrindle BW, et al. American Heart Association Childhood Obesity Research Summit Report. Circulation. 2009;119:e489–517. [DOI] [PubMed] [Google Scholar]
- 9.Abdullah A, Wolfe R, Mannan H, et al. Epidemiologic merit of obese-years, the combination of degree and duration of obesity. Am J Epidemiol. 2012;176:99–107. [DOI] [PubMed] [Google Scholar]
- 10.Ortega FB, Lavie CJ, Blair SN. Obesity and Cardiovascular Disease. Circ Res. 2016;118:1752–70. [DOI] [PubMed] [Google Scholar]
- 11.Cercato C, Fonseca FA. Cardiovascular risk and obesity. Diabetol Metab Syndr. 2019;11:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bays H Central obesity as a clinical marker of adiposopathy; increased visceral adiposity as a surrogate marker for global fat dysfunction. Curr Opin Endocrinol Diabetes Obes. 2014;21:345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol. 2011;57:2461–73. [DOI] [PubMed] [Google Scholar]
- 14.Blaschke F, Takata Y, Caglayan E, et al. Obesity, peroxisome proliferator-activated receptor, and atherosclerosis in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2006;26:28–40. [DOI] [PubMed] [Google Scholar]
- 15.Keane KN, Cruzat VF, Carlessi R, et al. Molecular Events Linking Oxidative Stress and Inflammation to Insulin Resistance and β-Cell Dysfunction. Oxid Med Cell Longev. 2015;2015:181643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuster JJ, Ouchi N, Gokce N, et al. Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ Res. 2016;118:1786–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu H, Ballantyne CM. Metabolic Inflammation and Insulin Resistance in Obesity. Circ Res. 2020;126:1549–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erion KA, Corkey BE. Hyperinsulinemia: a Cause of Obesity? Curr Obes Rep. 2017;6:178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams KJ, Wu X. Imbalanced insulin action in chronic over nutrition: Clinical harm, molecular mechanisms, and a way forward. Atherosclerosis. 2016;247:225–82. [DOI] [PubMed] [Google Scholar]
- 20.Gray SL, Donald C, Jetha A, et al. Hyperinsulinemia precedes insulin resistance in mice lacking pancreatic beta-cell leptin signaling. Endocrinology. 2010;151:4178–86. [DOI] [PubMed] [Google Scholar]
- 21.Mehran AE, Templeman NM, Brigidi GS, et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012;16:723–37. [DOI] [PubMed] [Google Scholar]
- 22.Templeman NM, Clee SM, Johnson JD. Suppression of hyperinsulinaemia in growing female mice provides long-term protection against obesity. Diabetologia. 2015;58:2392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’souza AM, Johnson JD, Clee SM, et al. Suppressing hyperinsulinemia prevents obesity but causes rapid onset of diabetes in leptin-deficient Lepob/ob mice. Mol Metab. 2016;5:1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miard S, Picard F. Obesity and aging have divergent genomic fingerprints. Int J Obes (Lond). 2008;32:1873–4. [DOI] [PubMed] [Google Scholar]
- 25.Lumeng CN, Liu J, Geletka L, et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol. 2011;187:6208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitahara CM, Flint AJ, Berrington de Gonzalez A, et al. Association between class III obesity (BMI of 40–59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Med. 2014;11:e1001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raffield LM, Howard AG, Graff M, et al. Obesity Duration, Severity, and Distribution Trajectories and Cardiovascular Disease Risk in the Atherosclerosis Risk in Communities Study. J Am Heart Assoc. 2021;10:e019946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdullah A, Wolfe R, Stoelwinder JU, et al. The number of years lived with obesity and the risk of all-cause and cause-specific mortality. Int J Epidemiol. 2011;40:985–96. [DOI] [PubMed] [Google Scholar]
- 29.Bramante CT, Lee CJ, Gudzune KA. Treatment of Obesity in Patients With Diabetes. Diabetes Spectr. 2017;30:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44:863–70. [DOI] [PubMed] [Google Scholar]
- 31.Guzik TJ, Skiba DS, Touyz RM, et al. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res. 2017;113:1009–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. [DOI] [PubMed] [Google Scholar]
- 33.Felber JP, Golay A, Jéquier E, et al. The metabolic consequences of long-term human obesity. Int J Obes. 1988;12:377–89. [PubMed] [Google Scholar]
- 34.Felber JP, Golay A. Pathways from obesity to diabetes. Int J Obes Relat Metab Disord. 2002;26 Suppl 2:S39–45. [DOI] [PubMed] [Google Scholar]
- 35.Reis JP, Hankinson AL, Loria CM, et al. Duration of abdominal obesity beginning in young adulthood and incident diabetes through middle age: the CARDIA study. Diabetes Care. 2013;36:1241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bjerregaard LG, Jensen BW, Ängquist L, et al. Change in Overweight from Childhood to Early Adulthood and Risk of Type 2 Diabetes. N Engl J Med. 2018;378:1302–1312. [DOI] [PubMed] [Google Scholar]
- 37.The NS, Richardson AS, Gordon-Larsen P. Timing and duration of obesity in relation to diabetes: findings from an ethnically diverse, nationally representative sample. Diabetes Care. 2013;36:865–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Araújo J, Severo M, Barros H, et al. Duration and degree of adiposity: effect on cardiovascular risk factors at early adulthood. Int J Obes (Lond). 2017;41:1526–1530. [DOI] [PubMed] [Google Scholar]
- 39.Norris T, Cole TJ, Bann D, et al. Duration of obesity exposure between ages 10 and 40 years and its relationship with cardiometabolic disease risk factors: a cohort study. PLoS Med. 2020;17:e1003387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdullah A, Stoelwinder J, Shortreed S, et al. The duration of obesity and the risk of type 2 diabetes. Public Health Nutr. 2011;14:119–26. [DOI] [PubMed] [Google Scholar]
- 41.Logue J, Walker JJ, Colhoun HM, et al. ; Scottish Diabetes Research Network Epidemiology Group. Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia. 2011;54:3003–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan H, Yang W, Zhou F, et al. Estrogen Improves Insulin Sensitivity and Suppresses Gluconeogenesis via the Transcription Factor Foxo1. Diabetes. 2019;68:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Everhart JE, Pettitt DJ, Bennett PH, et al. Duration of obesity increases the incidence of NIDDM. Diabetes. 1992;41:235–40. [DOI] [PubMed] [Google Scholar]
- 44.Wannamethee SG, Shaper AG. Weight change and duration of overweight and obesity in the incidence of type 2 diabetes. Diabetes Care. 1999;22:1266–72. [DOI] [PubMed] [Google Scholar]
- 45.Abdullah A, Amin FA, Hanum F, et al. Estimating the risk of type-2 diabetes using obese-years in a contemporary population of the Framingham Study. Glob Health Action. 2016;9:30421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pontiroli AE, Galli L. Duration of obesity is a risk factor for non-insulin-dependent diabetes mellitus, not for arterial hypertension or for hyperlipidaemia. Acta Diabetol. 1998;35:130–6. [DOI] [PubMed] [Google Scholar]
- 47.Sakurai Y, Teruya K, Shimada N, et al. Association between duration of obesity and risk of non-insulin-dependent diabetes mellitus. The Sotetsu Study. Am J Epidemiol. 1999;149:256–60. [DOI] [PubMed] [Google Scholar]
- 48.Hu Y, Bhupathiraju SN, de Koning L, et al. Duration of obesity and overweight and risk of type 2 diabetes among US women. Obesity (Silver Spring). 2014;22:2267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Power C, Thomas C. Changes in BMI, duration of overweight and obesity, and glucose metabolism: 45 years of follow-up of a birth cohort. Diabetes Care. 2011;34:1986–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall JE, do Carmo JM, da Silva AA, et al. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruiz HH, López Díez R, Arivazahagan L, et al. Metabolism, Obesity, and Diabetes Mellitus. Arterioscler Thromb Vasc Biol. 2019;39:e166–e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aizawa-Abe M, Ogawa Y, Masuzaki H, et al. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 2000;105:1243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ailhaud G, Fukamizu A, Massiera F, et al. Angiotensinogen, angiotensin II and adipose tissue development. Int J Obes Relat Metab Disord. 2000;24 Suppl 4:S33–5. [DOI] [PubMed] [Google Scholar]
- 54.Goncalves AC, Tank J, Diedrich, et al. Diabetic hypertensive leptin receptor-deficient db/db mice develop cardioregulatory autonomic dysfunction. Hypertension. 2009;53:387–92. [DOI] [PubMed] [Google Scholar]
- 55.Hilzendeger AM, Goncalves AC, Plehm R, et al. Autonomic dysregulation in ob/ob mice is improved by inhibition of angiotensin-converting enzyme. J Mol Med (Berl). 2010;88:383–90. [DOI] [PubMed] [Google Scholar]
- 56.Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev. 2014;10:131–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hall JE, do Carmo JM, da Silva AA, et al. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol. 2019;15:367–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kotsis V, Stabouli S, Papakatsika S, et al. Mechanisms of obesity-induced hypertension. Hypertens Res. 2010;33:386–93. [DOI] [PubMed] [Google Scholar]
- 59.Grassi G, Seravalle G, Cattaneo BM, et al. Sympathetic activation in obese normotensive subjects. Hypertension. 1995;25:560–3. [DOI] [PubMed] [Google Scholar]
- 60.Suglia SF, Clark CJ, Gary-Webb TL. Adolescent obesity, change in weight status, and hypertension: racial/ethnic variations. Hypertension. 2013;61:290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanamas SK, Wong E, Backholer K, Abdullah A, et al. Duration of obesity and incident hypertension in adults from the Framingham Heart Study. J Hypertens. 2015;33:542–5; discussion 545. [DOI] [PubMed] [Google Scholar]
- 62.Dowd JB, Zajacova A. Long-term obesity and cardiovascular, inflammatory, and metabolic risk in U.S. adults. Am J Prev Med. 2014;46:578–84. [DOI] [PubMed] [Google Scholar]
- 63.Hall ME, Cohen JB, Ard JD, et al. ; American Heart Association Council on Hypertension; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; and Stroke Council. Weight-Loss Strategies for Prevention and Treatment of Hypertension: A Scientific Statement From the American Heart Association. Hypertension. 2021;78:e38–e50. [DOI] [PubMed] [Google Scholar]
- 64.Straznicky NE, Grima MT, Eikelis N, et al. The effects of weight loss versus weight loss maintenance on sympathetic nervous system activity and metabolic syndrome components. J Clin Endocrinol Metab. 2011;96:E503–8. [DOI] [PubMed] [Google Scholar]
- 65.Vekic J, Zeljkovic A, Stefanovic A, et al. Obesity and dyslipidemia. Metabolism. 2019;92:71–81. [DOI] [PubMed] [Google Scholar]
- 66.Bays HE, Toth PP, Kris-Etherton PM, et al. Obesity, adiposity, and dyslipidemia: a consensus statement from the National Lipid Association. J Clin Lipidol. 2013;7:304–83. [DOI] [PubMed] [Google Scholar]
- 67.Després JP, Moorjani S, Lupien PJ, et al. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis. 1990;10:497–511. [DOI] [PubMed] [Google Scholar]
- 68.Poobalan A, Aucott L, Smith WC, et al. Effects of weight loss in overweight/obese individuals and long-term lipid outcomes--a systematic review. Obes Rev. 2004;5:43–50. [DOI] [PubMed] [Google Scholar]
- 69.Correa-Burrows P, Rogan J, Blanco E, et al. Resolving early obesity leads to a cardiometabolic profile within normal ranges at 23 years old in a two-decade prospective follow-up study. Sci Rep. 2021;11:18927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–85. [DOI] [PubMed] [Google Scholar]
- 71.Reis JP, Allen N, Gunderson EP, et al. Excess body mass index- and waist circumference-years and incident cardiovascular disease: the CARDIA study. Obesity (Silver Spring). 2015;23:879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hankinson A, Daviglus ML, Loria C, et al. Longer duration of overweight/obesity associated with higher odds of developing hypertension or dyslipidemia: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Circulation. 2018. Mar 23. [Google Scholar]
- 73.Janssen I, Katzmarzyk PT, Ross R. Duration of overweight and metabolic health risk in American men and women. Ann Epidemiol. 2004;14:585–91. [DOI] [PubMed] [Google Scholar]
- 74.Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev. 2003;14:447–55. [DOI] [PubMed] [Google Scholar]
- 75.Cigolini M, Tonoli M, Borgato L, et al. Expression of plasminogen activator inhibitor-1 in human adipose tissue: a role for TNF-alpha? Atherosclerosis. 1999;143:81–90. [DOI] [PubMed] [Google Scholar]
- 76.Owen MK, Noblet JN, Sassoon DJ, et al. Perivascular adipose tissue and coronary vascular disease. Arterioscler Thromb Vasc Biol. 2014;34:1643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lumeng CN. Innate immune activation in obesity. Mol Aspects Med. 2013;34:12–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uribe-Querol E, Rosales C. Neutrophils Actively Contribute to Obesity-Associated Inflammation and Pathological Complications. Cells. 2022;11:1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ridker PM. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ Res. 2016;118:145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scolaro B, Brown EJ, Krautter F, et al. Short-term Caloric Restriction in Mice Promotes Resolution of Atherosclerosis, While Weight Regain Accelerates its Progression. BioRxiv. 2023;Preprint. [Google Scholar]
- 81.Abdullah A, Amin FA, Stoelwinder J, et al. Estimating the risk of cardiovascular disease using an obese-years metric. BMJ Open. 2014;4:e005629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reis JP, Loria CM, Lewis CE, et al. Association between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. JAMA. 2013;310:280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Freedman DS, Dietz WH, Tang R, et al. The relation of obesity throughout life to carotid intima-media thickness in adulthood: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2004;28:159–66. [DOI] [PubMed] [Google Scholar]
- 84.Juonala M, Raitakari M, S A Viikari J, et al. Obesity in youth is not an independent predictor of carotid IMT in adulthood. The Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2006;185:388–93. [DOI] [PubMed] [Google Scholar]
- 85.Oren A, Vos LE, Uiterwaal CS, et al. Change in body mass index from adolescence to young adulthood and increased carotid intima-media thickness at 28 years of age: the Atherosclerosis Risk in Young Adults study. Int J Obes Relat Metab Disord. 2003;27:1383–90. [DOI] [PubMed] [Google Scholar]
- 86.Gutiérrez-Cuevas J, Sandoval-Rodriguez A, Meza-Rios A, et al. Molecular Mechanisms of Obesity-Linked Cardiac Dysfunction: An Up-Date on on Current Knowledge. Cells. 2021;10:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ebong IA, Goff DC Jr, Rodriguez CJ, et al. Mechanisms of heart failure in obesity. Obes Res Clin Pract. 2014;8:e540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakajima T, Fujioka S, Tokunaga K, et al. Noninvasive study of left ventricular performance in obese patients: influence of duration of obesity. Circulation. 1985;71:481–6. [DOI] [PubMed] [Google Scholar]
- 89.Alpert MA, Lambert CR, Panayiotou H, et al. Relation of duration of morbid obesity to left ventricular mass, systolic function, and diastolic filling, and effect of weight loss. Am J Cardiol. 19951;76:1194–7. [DOI] [PubMed] [Google Scholar]
- 90.Rocha IE, Victor EG, Braga MC, et al. Echocardiography evaluation for asymptomatic patients with severe obesity. Arq Bras Cardiol. 2007;88:52–8. English, Portuguese. [DOI] [PubMed] [Google Scholar]
- 91.Reis JP, Allen N, Gibbs BB, et al. Association of the degree of adiposity and duration of obesity with measures of cardiac structure and function: the CARDIA study. Obesity (Silver Spring). 2014;22:2434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ndumele CE, Cobb L, Lazo M, et al. Weight History and Subclinical Myocardial Damage. Clin Chem. 2018;64:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rossebø AB, Pedersen TR, Boman K, et al. ; SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–56. [DOI] [PubMed] [Google Scholar]
- 94.Larsson SC, Wolk A, Håkansson N, et al. Overall and abdominal obesity and incident aortic valve stenosis: two prospective cohort studies. Eur Heart J. 2017;38:2192–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Larsson SC, Bäck M, Rees JMB, et al. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: a Mendelian randomization study. Eur Heart J. 2020;41:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sarajlic P, Wolk A, Bäck M, et al. Physical Activity Does Not Reduce Aortic Valve Stenosis Incidence. Circ J. 2018;82:2372–2374. [DOI] [PubMed] [Google Scholar]
- 97.Hoffmann K, Kopciuch D, Michalak M, et al. Adherence of Obese Patients from Poland and Germany and Its Impact on the Effectiveness of Morbid Obesity Treatment. Nutrients. 2022;14:3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smit C, De Hoogd S, Brüggemann RJM, et al. Obesity and drug pharmacology: a review of the influence of obesity on pharmacokinetic and pharmacodynamic parameters. Expert Opin Drug Metab Toxicol. 2018;14:275–285. [DOI] [PubMed] [Google Scholar]
- 99.Mozaffarian D, Wilson PW, Kannel WB. Beyond established and novel risk factors: lifestyle risk factors for cardiovascular disease. Circulation. 2008;117:3031–8. [DOI] [PubMed] [Google Scholar]


