Abstract
Background
A central challenge to precision medicine research efforts is the return of genetic research results in a manner that is effective, ethical, and efficient. Formal tests of alternate modalities are needed, particularly for racially marginalized populations that have historically been underserved in this context.
Methods
We are conducting a randomized controlled trial (RCT) to test scalable modalities for results return and to examine the clinical utility of returning genetic research results to a research cohort of Black women. The primary aim is to compare the efficacy of two communication modalities for results return: 1) a conventional modality that entails telephone disclosure by a Board-certified genetic counselor, and 2) an online self-guided modality that entails results return directly to participants, with optional genetic counselor follow-up via telephone. The trial is being conducted among participants in the Black Women’s Health Study (BWHS), where targeted sequencing of 4,000 participants was previously completed.
Results
Several ethical, legal, and social implications (ELSI) and challenges presented, which necessitated substantial revision of the original study protocol. Challenges included chain of custody, re-testing of research results in a CLIA lab, exclusion of VUS results, and digital literacy. Bioethical principles of autonomy, justice, non-maleficence, and beneficence were considered in the design of the study protocol.
Conclusion
This study is uniquely situated to provide critical evidence on the effectiveness of alternative models for genetic results return and provide further insight into the factors influencing access and uptake of genetic information among U.S. Black women.
Keywords: Return of results, hereditary cancer, genetic counseling, disclosure modality, eHealth, ELSI, Black women
1. Background
A central challenge to large-scale precision medicine research is the return of individual genetic research results to participants [1, 2]. With updates in federal regulations promoting transparency and greater access to clinical and research test results, research paradigms have been proposed for returning individual research results to participants [3]. A conceptual framework proposed in a 2018 report from the National Academics of Sciences, Engineering, and Medicine outlined two dimensions that guide decisions on returning individual research results: 1) the potential value of the result to the participant, and 2) the feasibility of the return [3]. Although studies have reported that research participants are interested in (and even expect) individual genetic research results [4–6], best practices for how to increase the feasibility of these efforts have yet to be elucidated. The scale of these endeavors has renewed concerns about the shortage of genetic counselors and has intensified calls to examine the utility of alternate modalities for communicating genetic results [7, 8] and expand research efforts to provide the much-needed evidence base [3].
Alternate modalities of providing genetic counseling and results disclosure are increasingly being proposed to address the growing demand for services, the shortage of genetic counselors, and the need to expand access to genetic information [7, 9, 10]. Some efforts have focused on the use of telephone counseling or videoconferencing as a means to expand access to genetic services [11–15]. Although these approaches address logistical barriers such as geography and distance from genetic service providers, they do not address the time challenges associated with providing pretest education, ensuring informed consent, and conducting results disclosure [16, 17].
The use of digital solutions has often been proposed as a supplement to counseling to increase efficiency and use of genetic counselors [7, 16–18]. Online modalities that provide direct-access to genetic results have been used in various contexts including research [8, 10, 19, 20] and industry [21], yet few studies have formally tested this approach in terms of its acceptability and effectiveness compared to conventional genetic counseling approaches to results disclosure [7, 10, 22]. An exception is a randomized non-inferiority trial, which compared a web-based platform to in-person genetic counseling for returning carrier results from exome sequencing [8]. Results from that study demonstrated that the web-based platform was non-inferior to the in-person counselor for knowledge, psychological distress, and decisional conflict at follow-up, suggesting that an online modality may be effective for subsets of test results.
In the context of clinical testing, prior studies have consistently shown that Black women are less likely to pursue BRCA testing and learn their genetic risk, even when offered [23–28]. In the context of genomic sequencing research, studies have similarly found that uptake of genomic research results is lower among nonwhite participants [29], raising concerns about disparities in access. There is some evidence that Black individuals may have a stronger preference to not involve health care providers in results disclosure, and prefer to review their results independently [30], suggesting that modalities that facilitate the latter may influence engagement and decisions to learn individual genetic research results. Our own preliminary survey research suggested that women in the Black Women’s Health Study (BWHS) cohort prefer self-guided modalities for results disclosure compared to results disclosed by a health care professional such as a genetic counselor.
The effective translation of precision medicine research will require a concerted effort to engage racial/ethnic minoritized participants in research and increase the generalizability of findings to help ensure disparities are not magnified [23, 31–33]. The present study will not only test alternate modalities to facilitate greater access to genetic information, but it will also provide much needed evidence on how alternate modalities for genetics education and results disclosure might influence test result uptake among a racially underrepresented population in the United States.
2. Methods
2.1. Study Objectives
This randomized controlled trial (RCT) was designed to test alternate communication modalities for cancer genetic research results disclosure. The primary aim of this study is to compare the efficacy of two communication modalities for returning hereditary cancer genetic research results to Black women: 1) a conventional modality that entails telephone disclosure by a Board-certified and licensed genetic counselor (control arm), and 2) an online self-guided modality that entails returning results directly to participants via a secure web platform, with optional genetic counselor follow-up via telephone (intervention arm). The primary outcomes of this study focus on psychosocial and decisional outcomes that have been examined in other noninferiority trials testing genetic communication interventions [12, 34]. These include the decision to learn genetic research results (test uptake), decision uncertainty, knowledge acquisition, and psychological reactions to learning genetic results (test-specific distress, anxiety, depression). Secondary aims of this study will examine 1) moderators of the intervention impact and 2) psychosocial, sociodemographic, and clinical predictors of result uptake.
2.2. Study Sample
The Black Women’s Health Study (BWHS) is an ongoing prospective cohort study of 59,000 self-identified Black women from across the U.S. who have been successfully followed since 1995 [35]. Germline DNA samples from 4,363 BWHS women (1,454 breast cancer cases, 2,909 unaffected controls) have previously undergone targeted sequencing of BRCA1/2 and other known or suspected high/moderate penetrance cancer susceptibility genes as part of a large collaborative study of breast cancer predisposition gene to investigate genetic etiology of breast cancer in Black women [36, 37].
2.3. Eligibility Criteria, Recruitment and Enrollment
Figure 1 depicts the study workflow. BWHS participants are eligible for the trial if their samples had been included in the previous sequencing, they are still living and not lost to follow-up, and have no known cognitive impairments (previously reported to BWHS). Notably, women with a prior cancer diagnosis were eligible for the trial due to the utility of genetic information for understanding risks for secondary and other cancers, as well as risks for family members who might benefit from cascade testing. Moreover, participants with only variant(s) of uncertain significance (VUS) identified are not eligible for the trial due to inconsistent classification across Clinical Laboratory Improvement Amendments (CLIA) certified labs and poor characterization among women of African ancestry [38, 39].
Figure 1:
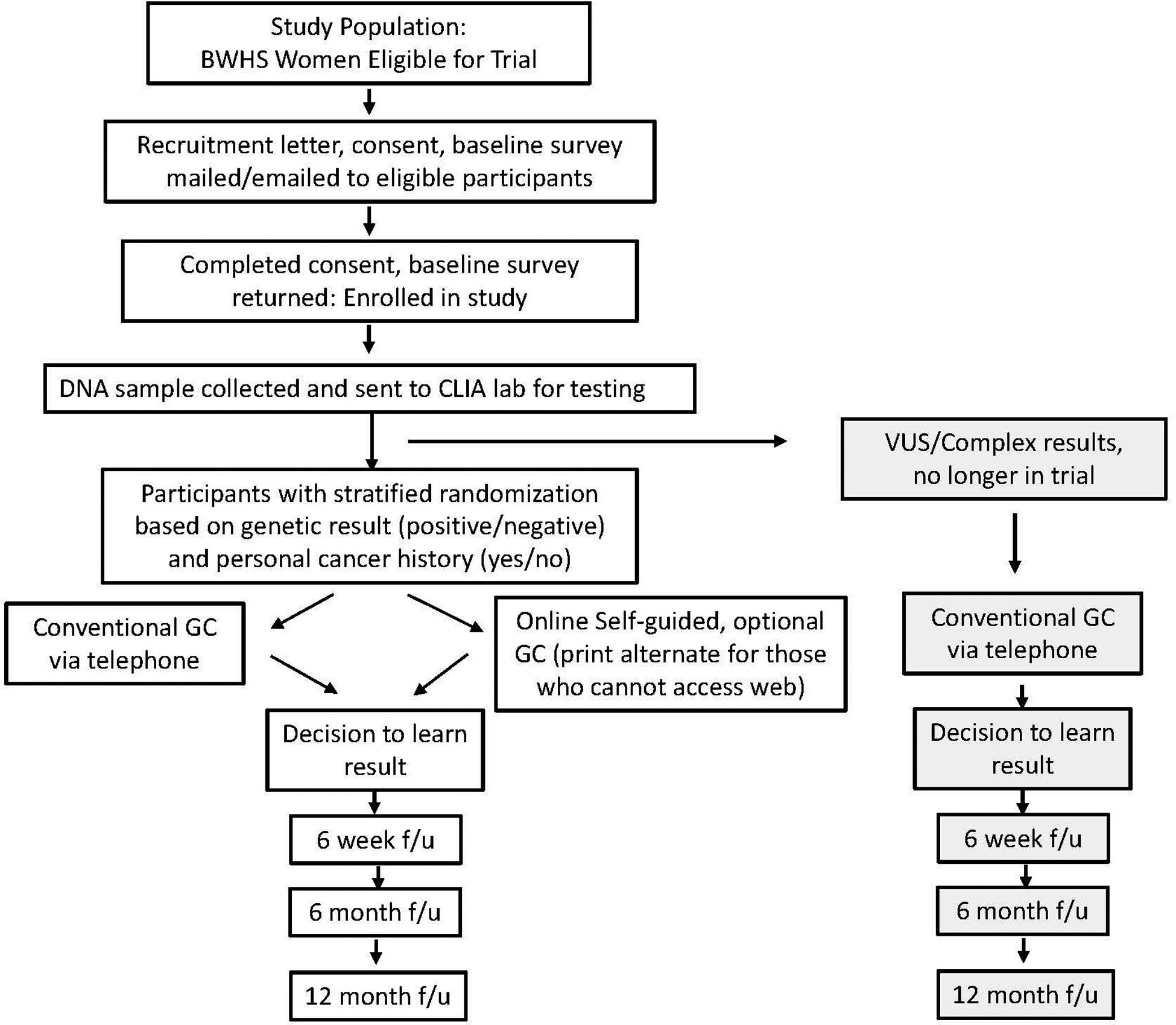
CONSORT diagram showing the study workflow
GC – genetic counselor
f/u – follow up
VUS – variant of uncertain significance
Eligible women will be approached by either email or postal mail, depending on prior engagement preferences with BWHS, with a study brochure, recruitment letter, informed consent document, and baseline survey. Both online and paper formats are offered to maximize accessibility to eligible participants and increase the likelihood of enrollment and survey completion.
2.4. Biospecimen Collection, CLIA lab testing and Randomization
Women who enroll in the trial by providing informed consent and completing the baseline survey are sent a DNA collection kit via U.S. Postal Service with detailed instructions to return a saliva sample for confirmation testing in a CLIA-certified laboratory of prior research sequencing results for 18 genes. Once samples are tested, participants are stratified by cancer status (affected/unaffected) and gene result status and then block randomized to one of two study arms. Participants with CLIA lab reports indicating VUS or other complex results (e.g., possibly mosaic, suspicion for clonal hematopoiesis of indeterminate potential (CHIP), reduced penetrance variants) are excluded from randomization and redirected to an option to learn their results from a genetic counselor via telephone. All discordant results (original research result versus CLIA lab result) are evaluated by the genetics team to determine eligibility for randomization.
2.4. Protocol for Returning Genetic Research Results
All participants enrolled in the trial receive cancer genetics education prior to the option to learn their genetic research results. Modality of education and disclosure of genetic research results is based on study arm (see below). Pre-disclosure education includes content areas recommended by the National Society of Genetic Counselors [40] and used by others in BRCA trials [13, 41] and contains coverage of cancer types associated with pathogenic variants in BRCA and other hereditary cancer genes, associated cancer risks, benefits/limitations of learning results, possible test results, implications of learning results for family members, and information related to genetic discrimination. Those who choose to learn their results following pre-disclosure education are provided with general management guidelines associated with the test result and additional resources. Additionally, participants receive a packet in the mail with a summary letter of their results, a gene details sheet (for any pathogenic/likely pathogenic results) and a copy of the CLIA lab report. Participants are also provided with an information packet to share with their health care provider, which includes a summary letter from the study team, a copy of the CLIA lab report, and National Comprehensive Cancer Network (NCCN) Guidelines® for any gene(s) with a pathogenic/likely pathogenic result.
2.4.1. Conventional (Control) arm
Women randomized to the conventional arm are contacted by study staff to schedule a call with a genetic counselor on the study team. Genetic counselors will provide the pre-disclosure education and then offer participants a choice to either learn their genetic research result or decline to learn. The genetic counselor will also track the length of session, questions/concerns voiced by participants, outcomes of the counseling, and counselor satisfaction with session. Reasons for choosing to decline results, if applicable, are noted by the genetic counselor. All telephone calls between study participants and the genetic counselor are audio recorded to assess fidelity to the counseling disclosure checklist and to provide additional data on participant questions, concerns, comprehension, and any misunderstandings. To ensure confidentiality, the genetic counselor will verify the participant’s name, date of birth, and state they are currently located in prior to starting the audio recording. The audio file produced from any phone call will be labeled only with the patient’s unique study ID. The audio files will be stored on a secure drive that is only accessible by BWHS research staff that are IRB approved for the study.
2.4.2. Online self-guided (Intervention) arm
Women randomized to the online arm are directed to view pre-disclosure education information via a secure web portal. Participants are required to enter their unique study ID and their date of birth, to access the portal. Once they are logged in, participants can review background pre-disclosure education sections at their own pace, after which they are presented with a choice to proceed within the website to learn their genetic research results or to stop and decline learning results. (Participants can return to the portal for up to 12 months if they change their mind and later wish to learn their results.) If there are women randomized to the online arm who cannot or prefer not to access the web portal, they are mailed the same pre-disclosure education information in print format and prompted to contact the study team via email or phone to request their genetic results in print. All participants in the online self-guided arm also have the option to schedule a call with a genetic counselor to discuss their results and/or address any concerns or questions they may have prior to or after learning their results. Calls occurring prior to learning results online will focus on addressing questions related to pre-disclosure education content only. Genetic counselors are blind to the genetic research result during these calls to prevent the unintentional disclosure of any results to participants in the online arm. All telephone calls with the genetic counselor are audio recorded. Individualized genetic counseling similar to a clinical encounter is not provided in this research setting.
2.6. Data Collection
In addition to the baseline survey completed as part of enrollment, study participants are asked to complete follow-up surveys at 6 weeks, 6 months, and 12 months following their decision to learn their genetic research results. The decision to learn genetic research results will be tracked by the genetic counselor (conventional arm) and via the web portal (online self-guided arm). Participants who decline to learn their results are asked additional survey items to assess reasons for declining to learn results. The selection of study measures and the timing of their administration was guided by our conceptual model (Figure 2, see Theoretical Framework below) and informed by prior studies examining the clinical utility of BRCA counseling and testing in a clinical context [11, 42, 43]. Table 1 lists the study constructs being measured and their measurement time points.
Figure 2.
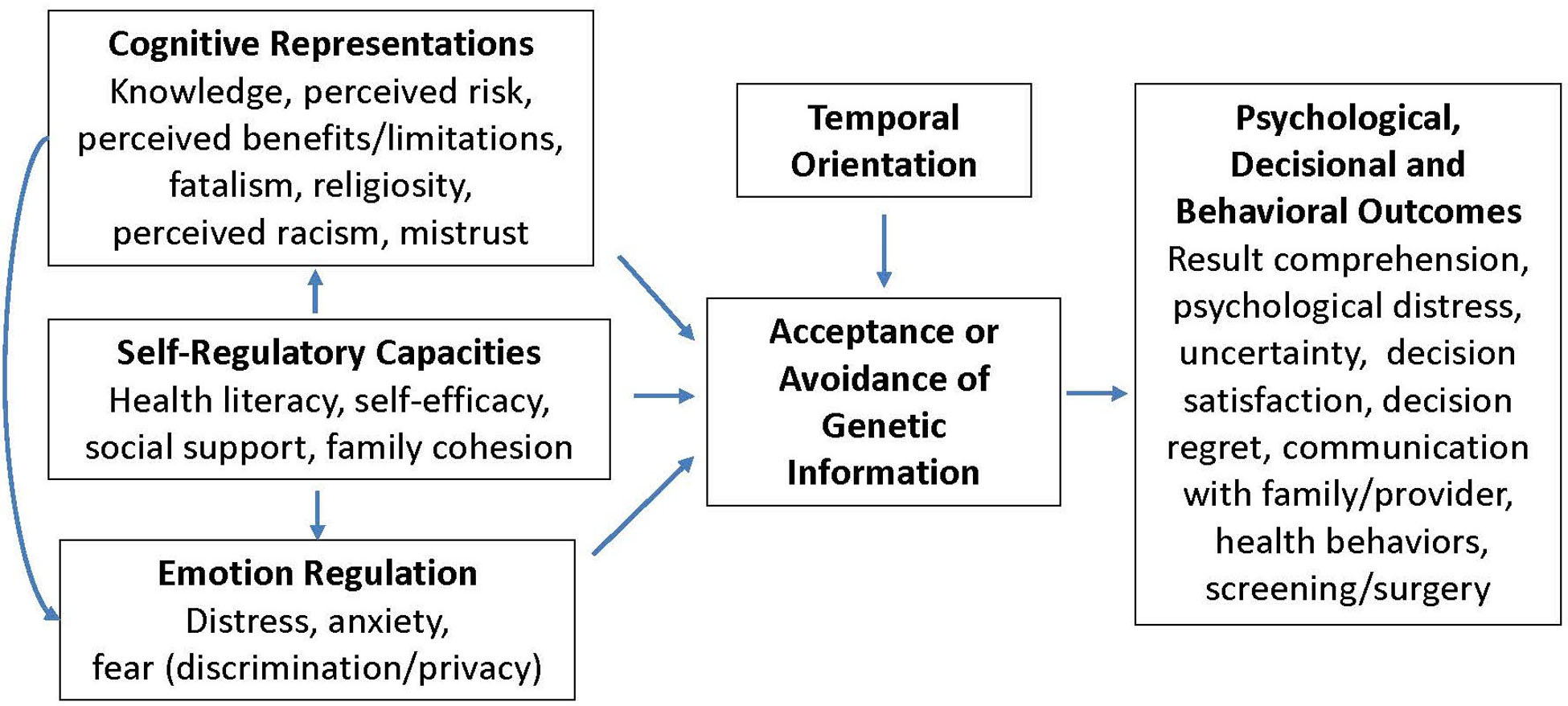
Conceptual Model
Table 1:
Study Constructs and Measurement Time Points
| Domain | Constructs | Time point* |
|---|---|---|
| Cognitive representations | Knowledge [47, 59–63] | T1, T2 |
| Perceived risk, benefits, limitations [46] | T1 | |
| Medical mistrust [64] | T1 | |
| Fear of discrimination [63] | T1 | |
| Fatalism [65] | T1 | |
| Religiosity | BWHS | |
| Temporal orientation | Temporal orientation [66] | T1 |
| Self-regulatory capacities | Health literacy [67] | T1 |
| Genetic self-efficacy [68, 69] | T1 | |
| Family cohesion [70] | T1 | |
| Financial toxicity/cost [71] | T1 | |
| Emotion regulation | Psychological distress (anxiety [72]/depression[73]) | T1, T2 |
| Test-specific distress [52] | T2, T3, T4 | |
| Decisional/behavioral outcomes | Decision to learn result (test uptake) | BWHS |
| Decision uncertainty [52] | T2, T3, T4 | |
| Decision satisfaction/regret [74, 75] | T2, T3, T4 | |
| Satisfaction with communication modality [8] | T2 | |
| Reasons for declining to learn results | T2, T4 | |
| Communication with family | T2, T3, T4 | |
| Communication with health care provider | T2, T3, T4 | |
| Reasons for not sharing results | T2, T4 | |
| Health behaviors (screening/surgery) | T1, T3, T4 |
T1 – baseline; T2 – 6 week follow up; T3 – 6 month follow up; T4 – 12 month follow up; BWHS (previously collected data from cohort or tracked in system)
2.5. Theoretical Framework
This study is guided by a framework of self-regulation, which has been extensively applied to inform understanding of decision-making processes in the context of genetic testing [15, 44–46]. In particular, this theoretical framework outlines the importance of both cognitive and affective processes, which together influence decisions to engage in genetic testing (i.e., learn results) and responses to genetic risk information. Recent conceptualizations have outlined important self-regulation principles that are relevant within the context of how individuals understand and respond to genetic information: cognitive representations (perceived risk/benefits/control, fatalistic beliefs), emotion regulation (anxiety, distress), defensive processes (avoidance, reactance), temporal orientation (value of future events), and self-regulatory capacities (e.g., literacy/numeracy, access, social norms, ability to engage)[44]. Constructs related to these self-regulation principles are being assessed in efforts to examine how they independently might explain acceptance/uptake of genetic information, and downstream responses to learning (or not learning) genetic risk (i.e., clinical utility).
2.7. Data Analysis
This study has one primary aim and two secondary aims. Hypothesis tests will employ a two-sided alpha level of 0.05. Tests of non-inferiority for primary outcomes will employ one-sided 99% confidence limits (97.5% confidence for secondary outcomes) and will compare these to non-inferiority margins used in the literature specific to the measures of interest [8, 11, 12, 34, 47]. For the primary aim comparing the efficacy of the two communication modalities for returning hereditary cancer predisposition genetic research results, we will examine the non-inferiority of the online self-guided modality as compared to the conventional modality using standard methods for tests of non-inferiority. As employed in the literature in similar studies of non-inferiority, particularly in the genetics context, each measure will require the establishment of its own non-inferiority margin (NIM) that is based on the consideration of a clinically meaningful difference between study arms in that outcome measure. These differences can then be computed in terms of a standardized effect size for means of a measure at a specific time point or a change from baseline for that measure. The standardized effect size allows for interpretation that incorporates the inherent variability in each measure. In the case of an outcome on a nominal scale, we employ non-inferiority margins defined as clinically meaningful differences in proportions established in the literature. First, for those eligible and enrolled in the study, we expect a 75% test result uptake rate [42, 48, 49]. For this outcome, we will employ a 10% difference in proportions between study arms as the NIM consistent with prior published work [47]. The remaining outcomes will be assessed among those who proceed and learn their test results. For breast cancer genetics knowledge, we will assume a NIM of 0.2 in standardized effect size that is equivalent to a 1 point difference in means [8, 11, 12, 47]. For psychological distress (anxiety/depression), the NIM margin will be set at a standardized effect size of 0.33, consistent with a 0.53 point difference on the GAD, and an effect size of 0.36 for a 0.28 point difference on the PHQ-2 [50, 51]. For test-specific distress, we will employ a NIM of 0.43 in standard effect size, or a 1 point in the MICRA [8]. Finally, for uncertainty, the NIM will be 0.88 in standardized effect size [52]. We will apply one-sided hypothesis tests at the 0.01 level in declaring non-inferiority to reflect our examination of multiple endpoints (applying a Bonferroni-Holm correction [53]). We will apply intention-to-treat (ITT) as our primary analytic principle. In supplemental per protocol analyses, we will additionally examine outcomes separately for online- and print-based self-guided modality vs. conventional modality.
We will also conduct similar non-inferiority analyses of a set of secondary outcomes employing the same methods as for our primary outcomes. These secondary outcomes will include decisional outcomes (decision satisfaction/regret, satisfaction with communication modality) and behavioral outcomes (communication with family, communication with health care provider, screening, surgery). Using prior studies as a guide [8, 11, 12, 47], we will set conservative non-inferiority margins for these secondary outcomes of 0.5 of a standard deviation in differences in means or 10% in differences in proportions between study arms. We will apply alpha levels of 0.05 for analyses of these secondary outcomes.
To examine potential moderators of intervention impact, we will examine potential effect modification for result uptake, knowledge, psychological/test-specific distress, and uncertainty following test result in the context of linear models and apply one-sided hypothesis tests at the 0.025 level. We will also examine for potential confounders, although confounding will be assessed in terms of changes in the estimates of group-level differences in outcome and not through hypothesis tests. Changes of greater than 10% in these differences will be judged to indicate confounding by individual confounders of groups of confounding variables (joint confounders).
To explore predictors of result uptake in addition to intervention effects, we will examine psychosocial predictors guided by our conceptual model. To identify the best set of predictor variables for result uptake, we will implement LASSO-based logistic regression analyses using PROC HPGENSELECT in SAS. Odds ratios, 95% confidence intervals, and p-values will be computed from these models.
2.8. Sample Size and Power
We estimate an overall enrolled sample of 916 women, with an estimated 686 (~75%) who will learn their genetic research results, based on prior estimates of uptake from studies disclosing BRCA results via telephone-based genetic counseling to Black women [75%, 48, 49] and to women participating in breast cancer genome sequencing studies [78%, 42]. Thus, the estimated sample size for analyses of our non-inferiority endpoints that are contingent on uptake is 686 overall, 343 per study arm. For all the primary outcomes examined, statistical power ranges from 80%−99%.
For logistic regression analyses exploring predictors of result uptake, we can estimate an odds ratio as small as 1.29 for a one standard deviation difference in the predictor for a generic Gaussian predictor from the set of those specified above with 80% power at a two-sided alpha of 0.05 with squared correlation with the other covariates in the model as large as 0.4. The smallest detectable odds ratio for a dichotomous predictor with equal distribution between groups is 1.72.
3. Results
Bioethical principles of autonomy and respect for persons, justice, non-maleficence, and beneficence were applied in the initial design of the online intervention, study protocol, and study workflow to address several ethical considerations, as well as when legal considerations and challenges emerged in subsequent study planning and implementation (see Figure 3).
Figure 3.
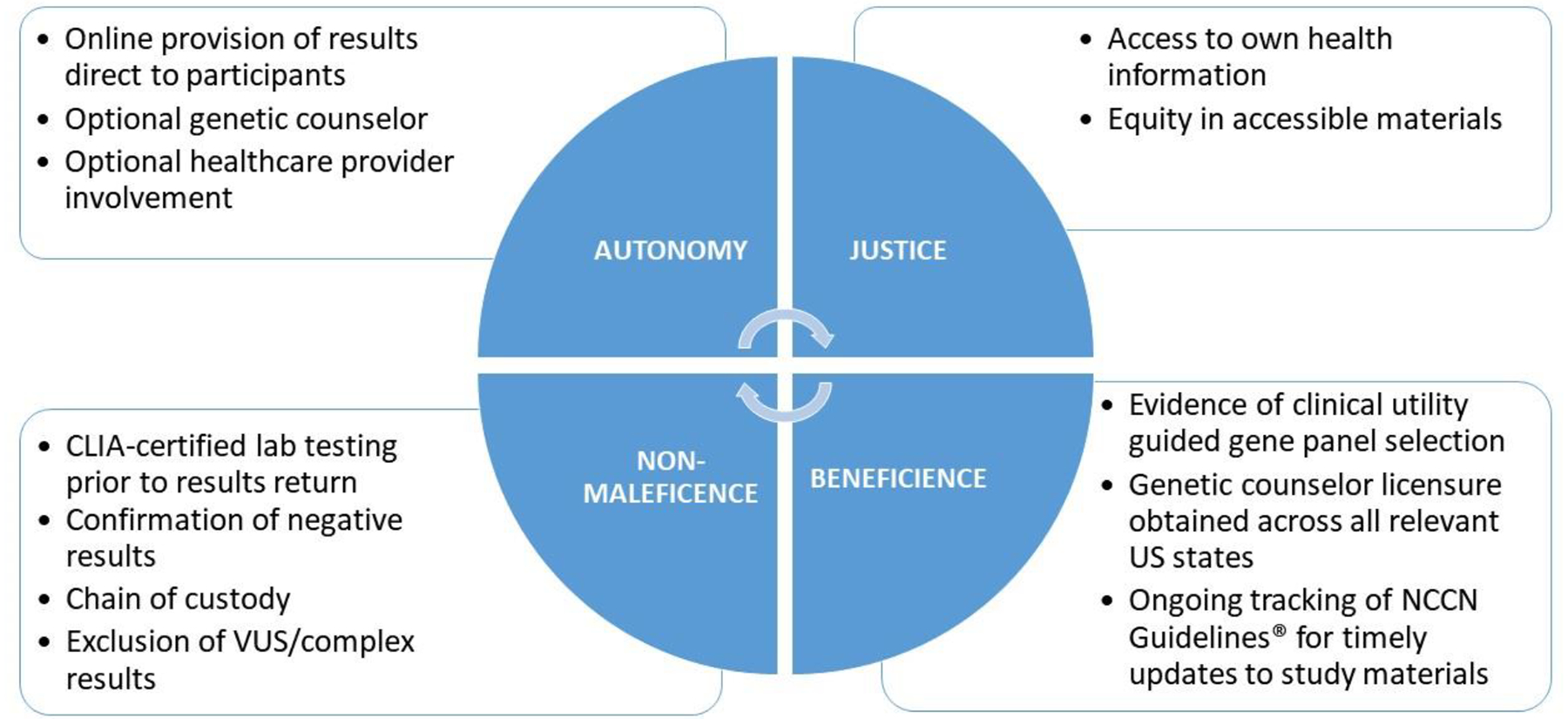
Application of Bioethical Principles
3.1. Autonomy and Respect for Persons: Respect a person’s right to make choices/take action based on personal values and beliefs
In efforts to ensure autonomy and respect for persons, this study set out to prioritize the online provision of genetic information directly to participants, to review at their own pace. Study participants have the option not to learn research results after reviewing the background (pretest) information about cancer risk and genetics. In addition, the involvement of a genetic counselor is optional for participants (in the online self-guided arm) to initiate. Finally, participants are encouraged to share their results with their health care provider and a provider information packet is provided to participants to support provider and patient decision-making. However, any engagement with health care providers is at the discretion of the participant.
3.2. Justice: Equitable treatment and distribution of benefits
To ensure access to ones’ own health information, this study focused on overcoming barriers to the return of personal health information obtained during research studies. When the genetic research study was initiated in the BWHS, informed consent for the original biospecimen collection indicated that the biosamples were for research purposes only and that no personal health results would be returned. For this trial of results return, a revised informed consent was necessary. A new research study and consent was created that would permit the return of hereditary cancer gene results that may have personal health implications. Efforts to ensure justice and equitable distribution of benefits also focused on accessibility of study materials. All online materials were designed following principles for plain language (plainlanguage.gov). Usability testing of online materials focused on literacy-related challenges. In addition, a print version of the website was created as an option within the online arm, to accommodate self-guided access for participants who may have lower digital literacy and/or are unable to access the information online.
3.3. Non-Maleficence: Do no harm, obligation not to inflict harm intentionally
Following the principles of non-maleficence, several protocol modifications were made as the study began. First, although our team had proposed to conduct CLIA confirmation testing of all positive results following results return, this approach was determined as unacceptable by the legal team at our institution. It was determined that genetic research results required confirmation at a CLIA-certified lab prior to return to participants. Moreover, unlike prior studies that have also returned genetic research results [42], our team was required to confirm both positive (pathogenic) and negative (benign) research results. Additional grant funding was necessary in order to accommodate the new requirement.
Second, chain of custody of the original sample provided to a research study biorepository was of concern to the study Data Safety and Monitoring Board (DSMB), and as such, the protocol was revised to obtain a new sample for CLIA-certified lab testing rather than send DNA stored at the BWHS biorepository. Although this decision resolved the most pertinent chain of custody concerns, this change further increased expenses and may inadvertently reduce study enrollment as it requires additional steps and saliva sample collection from participants.
Finally, to reduce the risk of any harm from online disclosure of complex results that require more nuanced discussion, all CLIA-certified lab results of variant of uncertain significance (VUS) or other complex result (e.g., possibly mosaic) will be returned by a genetic counselor. Participants with these results will not be randomized within the trial; however, they will be surveyed over time to determine the clinical utility of this information.
3.4. Beneficence: Do good, provides benefits to others
To ensure beneficence and benefit to participants, we include 18 high- and moderate-penetrance cancer susceptibility genes for results return. Evidence of clinical utility based on actionable clinical management guidelines from the National Comprehensive Cancer Network (NCCN)® (https://www.nccn.org/) informed the selection of a final 18 genes for inclusion, expanding the study beyond a focus on breast cancer to include other cancers with a hereditary component. In addition, genetic counselor licensure was obtained by a study genetic counselor across all U.S. states that require genetic counseling licensure where BWHS participants reside (24) to allay any concerns that return of results from a CLIA-certified laboratory in a research setting across state lines could violate state licensure law(s). Finally, our study team is continuously tracking NCCN Guidelines® to provide current gene-specific materials and resources to both study participants and their providers over the duration of the trial period.
4. Discussion
This study will formally test an innovative method for returning genetic research results to a unique cohort of Black women and determine its impact on result uptake as well as the clinical utility of testing, the latter of which has not been well documented for Black individuals. Study results will provide evidence on the efficacy of using online self-guided approaches to return genetic research results in comparison to conventional methods and the clinical utility of providing genetic testing results among a large sample of Black women. It will also generate evidence on the most efficacious means to return research results in large, geographically dispersed, cohort studies, and enhance our understanding of the factors that explain why Black women choose or decline to learn their genetic research results.
A strength of this study is the projected large sample size for the trial, which allows for a more extensive study of the clinical utility of cancer genetic results for Black women, compared to much smaller sample sizes (e.g., n=215 or less) observed among Black women followed either prospectively or in clinical trials [43, 48]. This study will also provide insight into reasons for any differential uptake of testing across different counseling modalities, observed previously for non-white study participants [11, 54], by examining moderators of intervention impact and predictors of learning test results, guided by a strong theoretical framework.
This work builds upon limitations of prior research efforts including lack of non-white representation (and thus generalizability) in genomic research [8] and lack of design rigor in prior evaluations of online modalities (i.e., prospective, non-RCT)[21, 55], with few exceptions [8, 10, 56]. Additionally, the present study is testing different modalities for returning negative results, which are often returned using a different modality (e.g., print/mailed) compared to positive results, under the assumption there is less risk for harm [57]. Yet, prior work has also reported that return of results via a mailed letter, often done for efficiency reasons, has not been without challenges [58], further demonstrating the need to evaluate the impact of different modalities for returning negative results.
5.0. Conclusion
This study is uniquely situated to provide critical empirical evidence on the efficacy of alternate modalities for genetic results return and provide further insight into the factors influencing uptake of genetic information related to cancer risk among Black women. Study findings will inform ongoing efforts to establish scalable approaches for effective return of genetic research results and increase access to personal health information.
Acknowledgements
This study is supported by the National Institutes of Health (R01MD014312, R01CA058420, U01CA164974, R01CA176785). Julie R. Palmer received support from the Karin Grunebaum Cancer Research Foundation and the Susan G. Komen Foundation.
The BWHS study protocol was approved by the Boston University Medical Campus Institutional Review Board (IRB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Department of Health and Human Services, the National Institutes of Health, the National Cancer Institute, or the state cancer registries.
We would like to thank Tuya Pal, Angela Bradbury, Brahim Aswald, Shelby Redfield, for their input and contributions to the work. We would also like to thank the staff and participants of the Black Women’s Health Study for their valuable contributions to this project.
In memoriam of Deborah J Bowen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Jarvik GP, et al. , Return of genomic results to research participants: the floor, the ceiling, and the choices in between. Am J Hum Genet, 2014. 94(6): p. 818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGuire AL, et al. , Can I be sued for that? Liability risk and the disclosure of clinically significant genetic research findings. Genome Res, 2014. 24(5): p. 719–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Academies of Sciences, E., and Medicine,, Returning individual research results to participants: Guidance for a new research paradigm. 2018, Washington, DC: The National Academies Press. . [PubMed] [Google Scholar]
- 4.Kaufman D, et al. , A survey of U.S. adults’ opinions about conduct of a nationwide Precision Medicine Initiative cohort study of genes and environment. PLoS One, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright MF, et al. , Preferences for results delivery from exome sequencing/genome sequencing. Genet Med, 2014. 16(6): p. 442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaphingst KA, et al. , Preferences for learning different types of genome sequencing results among young breast cancer patients: Role of psychological and clinical factors. Transl Behav Med, 2018. 8(1): p. 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchanan AH, Rahm AK, and Williams JL, Alternate Service Delivery Models in Cancer Genetic Counseling: A Mini-Review. Front Oncol, 2016. 6: p. 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biesecker BB, et al. , Web Platform vs In-Person Genetic Counselor for Return of Carrier Results From Exome Sequencing: A Randomized Clinical Trial. JAMA Intern Med, 2018. 178(3): p. 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu JH, et al. , Self-guided management of exome and whole-genome sequencing results: changing the results return model. Genet Med, 2013. 15(9): p. 684–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabor HK, et al. , My46: a Web-based tool for self-guided management of genomic test results in research and clinical settings. Genet Med, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz MD, et al. , Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol, 2014. 32(7): p. 618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinney AY, et al. , Randomized Noninferiority Trial of Telephone Delivery of BRCA1/2 Genetic Counseling Compared With In-Person Counseling: 1-Year Follow-Up. J Clin Oncol, 2016. 34(24): p. 2914–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinney AY, et al. , Expanding access to BRCA1/2 genetic counseling with telephone delivery: a cluster randomized trial. J Natl Cancer Inst, 2014. 106(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang Y, et al. , Economic Evaluation Alongside a Clinical Trial of Telephone Versus In-Person Genetic Counseling for BRCA1/2 Mutations in Geographically Underserved Areas. J Oncol Pract, 2016. 12(1): p. 59, e1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradbury A, et al. , Utilizing Remote Real-Time Videoconferencing to Expand Access to Cancer Genetic Services in Community Practices: A Multicenter Feasibility Study. J Med Internet Res, 2016. 18(2): p. e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trepanier AM, Cohen SA, and Allain DC, Thinking differently about genetic counseling service delivery. Curr Genet Med Rep, 2015. 3: p. 49–56. [Google Scholar]
- 17.Stoll K, Kubendran S, and Cohen SA, The past, present and future of service delivery in genetic counseling: Keeping up in the era of precision medicine. Am J Med Genet C Semin Med Genet, 2018. 178(1): p. 24–37. [DOI] [PubMed] [Google Scholar]
- 18.Hilgart J, Hayward JA, and Iredale R, E-genetics: exploring the acceptability and feasibility of using technology in cancer genetics services. Clin Genet, 2012. 81(6): p. 514–20. [DOI] [PubMed] [Google Scholar]
- 19.Wang C, et al. , A randomized trial examining the impact of communicating genetic and lifestyle risks for obesity. Obesity (Silver Spring), 2016. 24(12): p. 2481–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sweet K, et al. , Outcomes of a Randomized Controlled Trial of Genomic Counseling for Patients Receiving Personalized and Actionable Complex Disease Reports. J Genet Couns, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francke U, et al. , Dealing with the unexpected: consumer responses to direct-access BRCA mutation testing. PeerJ, 2013. 1: p. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts MC, et al. , Possible barriers for genetic counselors returning actionable genetic research results across state lines. Genet Med, 2017. 19(11): p. 1202–1204. [DOI] [PubMed] [Google Scholar]
- 23.Halbert CH, et al. , Low rates of acceptance of BRCA1 and BRCA2 test results among African American women at increased risk for hereditary breast-ovarian cancer. Genet Med, 2006. 8(9): p. 576–82. [DOI] [PubMed] [Google Scholar]
- 24.Halbert CH, et al. , Recruiting African American women to participate in hereditary breast cancer research. J Clin Oncol, 2005. 23(31): p. 7967–73. [DOI] [PubMed] [Google Scholar]
- 25.Cragun D, et al. , Factors associated with genetic counseling and BRCA testing in a population-based sample of young Black women with breast cancer. Breast Cancer Res Treat, 2015. 151(1): p. 169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong K, et al. , Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA, 2005. 293(14): p. 1729–36. [DOI] [PubMed] [Google Scholar]
- 27.Levy DE, et al. , Underutilization of BRCA1/2 testing to guide breast cancer treatment: black and Hispanic women particularly at risk. Genet Med, 2011. 13(4): p. 349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarthy AM, et al. , Health Care Segregation, Physician Recommendation, and Racial Disparities in BRCA1/2 Testing Among Women With Breast Cancer. J Clin Oncol, 2016. 34(22): p. 2610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiallos K, et al. , Choices for return of primary and secondary genomic research results of 790 members of families with Mendelian disease. Eur J Hum Genet, 2017. 25(5): p. 530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu JH, et al. , Attitudes of African Americans toward return of results from exome and whole genome sequencing. Am J Med Genet A, 2013. 161A(5): p. 1064–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith CE, et al. , Using Genetic Technologies To Reduce, Rather Than Widen, Health Disparities. Health Aff (Millwood), 2016. 35(8): p. 1367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrovski S and Goldstein DB, Unequal representation of genetic variation across ancestry groups creates healthcare inequality in the application of precision medicine. Genome Biol, 2016. 17(1): p. 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popejoy AB and Fullerton SM, Genomics is failing on diversity. Nature, 2016. 538(7624): p. 161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradbury AR, P.-M.L., Egleston BL, Hall MJ, Domchek SM, Daly MB, Ganschow P, Grana G, Olopade OI, Fetzer D, Brandt A, Chambers R, Clark DF, Forman A, Gaber R, Gulden C, Horte J, Long JM, Lucas T, Madaan S, Mattie K, McKenna D, Montgomery S, Nielsen S, Powers J, Rainey K, Rybak C, Savage M, Seelaus C, Stoll J, Stopfer JE, Yao XS., Randomized Noninferiority Trial of Telephone vs In-Person Disclosure of Germline Cancer Genetic Test Results. J Natl Cancer Inst, 2018. 110(9): p. 985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg L, Adams-Campbell L, and Palmer JR, The Black Women’s Health Study: a follow-up study for causes and preventions of illness. J Am Med Womens Assoc (1972), 1995. 50(2): p. 56–8. [PubMed] [Google Scholar]
- 36.Palmer JR, et al. , Contribution of Germline Predisposition Gene Mutations to Breast Cancer Risk in African American Women. J Natl Cancer Inst, 2020. 112(12): p. 1213–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu C, et al. , A Population-Based Study of Genes Previously Implicated in Breast Cancer. N Engl J Med, 2021. 384(5): p. 440–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balmana J, et al. , Conflicting Interpretation of Genetic Variants and Cancer Risk by Commercial Laboratories as Assessed by the Prospective Registry of Multiplex Testing. J Clin Oncol, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter NJ, et al. , Yeid of pathogenic/likely pathogenic variants in breast cancer patients undergoing an inherited cancer panel based upon ethnic background, in American Society of Medical Genetics. 2016: Tampa, FL. [Google Scholar]
- 40.Riley BD, et al. , Essential elements of genetic cancer risk assessment, counseling, and testing: updated recommendations of the National Society of Genetic Counselors. J Genet Couns, 2012. 21(2): p. 151–61. [DOI] [PubMed] [Google Scholar]
- 41.Peshkin BN, et al. , Telephone genetic counseling for high-risk women undergoing BRCA1 and BRCA2 testing: rationale and development of a randomized controlled trial. Genet Test, 2008. 12(1): p. 37–52. [DOI] [PubMed] [Google Scholar]
- 42.Bradbury AR, et al. , Returning individual genetic research results to research participants: Uptake and outcomes among patients with breast cancer. JCO Precision Oncology, 2018. 2: p. 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halbert CH, et al. , Effect of genetic counseling and testing for BRCA1 and BRCA2 mutations in African American women: a randomized trial. Public Health Genomics, 2010. 13(7–8): p. 440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cameron LD, et al. , Self-regulation principles underlying risk perception and decision making within the context of genomic testing. Social and Personality Psychology Compass, 2017. 11(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patrick-Miller LJ, et al. , Development of a communication protocol for telephone disclosure of genetic test results for cancer predisposition. JMIR Res Protoc, 2014. 3(4): p. e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang C, et al. , The role of cognitive appraisal and worry in BRCA1/2 testing decisions among a clinic population. Psychology and Health, 2007. 22(6): p. 719–736. [Google Scholar]
- 47.Manchanda R, et al. , Cluster-randomised non-inferiority trial comparing DVD-assisted and traditional genetic counselling in systematic population testing for BRCA1/2 mutations. J Med Genet, 2016. 53(7): p. 472–80. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez BD, et al. , Psychosocial Impact of BRCA Testing in Young Black Breast Cancer Survivors. Psychooncology, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vadaparampil ST, et al. , PgmNr 70: Psychosocial outcomes of genetic counseling in a population based sample of Black breast cancer survivors, in American Society of Human Genetics. 2017: Orlando, FL. [Google Scholar]
- 50.Lowe B, Kroenke K, and Grafe K, Detecting and monitoring depression with a two-item questionnaire (PHQ-2). J Psychosom Res, 2005. 58(2): p. 163–71. [DOI] [PubMed] [Google Scholar]
- 51.Staples LG, et al. , Psychometric properties and clinical utility of brief measures of depression, anxiety, and general distress: The PHQ-2, GAD-2, and K-6. Gen Hosp Psychiatry, 2019. 56: p. 13–18. [DOI] [PubMed] [Google Scholar]
- 52.Cella D, et al. , A brief assessment of concerns associated with genetic testing for cancer: The multidimensional impact of cancer risk assessment (MICRA) questionnaire. Health Psychology, 2002. 21(6): p. 564–572. [PubMed] [Google Scholar]
- 53.Holm S, A simple sequentially rejective Bonferroni test procedure. Scand J Statist, 1979. 6: p. 65–70. [Google Scholar]
- 54.Butrick M, et al. , Disparities in uptake of BRCA1/2 genetic testing in a randomized trial of telephone counseling. Genet Med, 2015. 17(6): p. 467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keller MA, et al. , Coriell Personalized Medicine Collaborative: A prospective study of the utility of personalized medicine. Personalized Medicine, 2010. 7(3). [DOI] [PubMed] [Google Scholar]
- 56.Green RC, G.K., Jarvik GP, Amendola LM, Appelbaum PS, Berg JS, Bernhardt BA, Biesecker LG, Biswas S, Blout CL, Bowling KM, Brothers KB, Burke W, Caga-Anan CF, Chinnaiyan AM, Chung WK, Clayton EW, Cooper GM, East K, Evans JP, Fullerton SM, Garraway LA, Garrett JR, Gray SW, Henderson GE, Hindorff LA, Holm IA, Lewis MH, Hutter CM, Janne PA, Joffe S, Kaufman D, Knoppers BM, Koenig BA, Krantz ID, Manolio TA, McCullough L, McEwen J, McGuire A, Muzny D, Myers RM, Nickerson DA, Ou J, Parsons DW, Petersen GM, Plon SE, Rehm HL, Roberts JS, Robinson D, Salama JS, Scollon S, Sharp RR, Shirts B, Spinner NB, Tabor HK, Tarczy-Hornoch P, Veenstra DL, Wagle N, Weck K, Wilfond BS, Wilhelmsen K, Wolf SM, Wynn J, Yu JH, CSER Consortium,, Clinical Sequencing Exploratory Research Consortium: Accelerating Evidence-Based Practice of Genomic Medicine. Am J Hum Genet, 2016. 98: p. 1051–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Porter KM, K.T., Koenig BA, Lewis KL, Rehm HL, Richards CS, Strande NT, Tabor HK, Wolf SM, Yang Y, Amendola LM, Azzariti DR, Berg JS, Bergstrom K, Biesecker LG, Biswas S, Bowling KM, Chung WK, Clayton EW, Conlin LK, Cooper GM, Dulik MC, Garraway LA, Ghazani AA, Green RC, Hiatt SM, Jamal SM, Jarvik GP, Goddard KAB, Wilfond BS, members of the CSER Actionability and Return of Results Working Group,, Approaches to carrier testing and results disclosure in translational genomics research: The clinical sequencing exploratory research consortium experience. Mol Genet Genomic Med, 2018. 6(6): p. 898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stuttgen K, et al. , Patient reactions to receiving negative genomic screening results by mail. Genet Med, 2020. 22(12): p. 1994–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lieberman S, et al. , Population screening for BRCA1/BRCA2 founder mutations in Ashkenazi Jews: proactive recruitment compared with self-referral. Genet Med, 2017. 19(7): p. 754–762. [DOI] [PubMed] [Google Scholar]
- 60.Lerman C, et al. , BRCA1 testing in families with hereditary breast-ovarian cancer: A prospective study of patient decision making and outcomes. Journal of the American Medical Association, 1996. 275(24): p. 1885–1892. [PubMed] [Google Scholar]
- 61.Cragun D, et al. , A Web-Based Tool to Automate Portions of Pretest Genetic Counseling for Inherited Cancer. J Natl Compr Canc Netw, 2020. 18(7): p. 841–847. [DOI] [PubMed] [Google Scholar]
- 62.Wang C, et al. , Genetic counseling for BRCA1/2: A randomized controlled trial of two strategies to facilitate the education and counseling process. American Journal of Medical Genetics, 2005. 134A: p. 66–73. [DOI] [PubMed] [Google Scholar]
- 63.Rose A, et al. , The association between knowledge and attitudes about genetic testing for cancer risk in the United States. J Health Commun, 2005. 10(4): p. 309–21. [DOI] [PubMed] [Google Scholar]
- 64.LaVeist TA, Isaac LA, and Williams KP, Mistrust of health care organizations is associated with underutilization of health services. Health Serv Res, 2009. 44(6): p. 2093–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen L, Condit CM, and Wright L, The psychometric property and validation of a fatalism scale. Psychol Health, 2009. 24(5): p. 597–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lukwago SN, et al. , Development and validation of brief scales to measure collectivism, religiosity, racial pride, and time orientation in urban African American women. Fam Community Health, 2001. 24(3): p. 63–71. [DOI] [PubMed] [Google Scholar]
- 67.Sarkar U, et al. , Validation of self-reported health literacy questions among diverse English and Spanish-speaking populations. J Gen Intern Med, 2011. 26(3): p. 265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaphingst KA, et al. , Patients’ understanding of and responses to multiplex genetic susceptibility test results. Genet Med, 2012. 14(7): p. 681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sheppard VB, et al. , Medical mistrust influences black women’s level of engagement in BRCA 1/2 genetic counseling and testing. J Natl Med Assoc, 2013. 105(1): p. 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boterhoven de Haan KL, et al. , Reliability and validity of a short version of the general functioning subscale of the McMaster Family Assessment Device. Fam Process, 2015. 54(1): p. 116–23. [DOI] [PubMed] [Google Scholar]
- 71.Bauer AG, et al. , Financial toxicity and strain among men receiving prostate cancer care in an equal access healthcare system. Cancer Med, 2020. 9(23): p. 8765–8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kroenke K, et al. , Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med, 2007. 146(5): p. 317–325. [DOI] [PubMed] [Google Scholar]
- 73.Kroenke K, Spitzer RL, and Williams JB, The Patient Health Questionnaire-2: Validity of a Two-Item Depression Screener. Medical Care, 2003. 41: p. 1284–92. [DOI] [PubMed] [Google Scholar]
- 74.Brehaut JC, et al. , Validation of a decision regret scale. Med Decis Making, 2003. 23(4): p. 281–92. [DOI] [PubMed] [Google Scholar]
- 75.Holmes-Rovner M, et al. , Patient satisfaction with health care decisions: the satisfaction with decision scale. Med Decis Making, 1996. 16(1): p. 58–64. [DOI] [PubMed] [Google Scholar]


