Abstract
Slow wave sleep (SWS), characterized by large electroencephalographic oscillations, facilitates crucial physiologic processes that maintain synaptic plasticity and overall brain health. Deficiency in older adults is associated with depression and cognitive dysfunction, such that enhancing sleep slow waves has emerged as a promising target for novel therapies. Enhancement of SWS has been noted after infusions of propofol, a commonly used anesthetic that induces electroencephalographic patterns resembling non-rapid eye movement sleep. This paper 1) reviews the scientific premise underlying the hypothesis that sleep slow waves are a novel therapeutic target for improving cognitive and psychiatric outcomes in older adults, and 2) presents a case series of two patients with late-life depression who each received two propofol infusions. One participant, a 71-year-old woman, had a mean of 2.8 minutes of evening SWS prior to infusions (0.7% of total sleep time). SWS increased on the night after each infusion, to 12.5 minutes (5.3% of total sleep time) and 24 minutes (10.6% of total sleep time), respectively. Her depression symptoms improved, reflected by a reduction in her Montgomery-Asberg Depression Rating Scale (MADRS) score from 26 to 7. In contrast, the other participant, a 77-year-old man, exhibited no SWS at baseline and only modest enhancement after the second infusion (3 minutes, 1.3% of total sleep time). His MADRS score increased from 13 to 19, indicating a lack of improvement in his depression. These cases Provide proof-of-concept that propofol can enhance SWS and improve depression for some individuals, motivating an ongoing clinical trial (ClinicalTrials.gov NCT04680910).
Keywords: Older adults, slow wave sleep, depression, cognition, anesthesia, propofol, sleep, electroencephalography
BRIEF SUMMARY
Deficiency of slow wave sleep (SWS) in older adults is putatively associated with depression and cognitive dysfunction. Sleep slow waves may serve as a target for novel strategies to improve both depression and cognitive function. This work presents two older adults with treatment-resistant depression who received propofol infusions as a therapeutic probe of the relationships between SWS, depression, and cognitive dysfunction. These cases provide proof-of-concept that propofol can enhance SWS and improve depression for some individuals.
INTRODUCTION
SWS Deficiency at the Nexus of Cognitive Impairment and Late-Life Treatment-Resistant Depression
Treatment-resistant depression (TRD) in older adults is a leading cause of disability,1 excess mortality from suicide,2,3 and dementia.4–6 The number of patients with late-life TRD (LL-TRD) is likely to increase over time7,8 as growing proportions of the population reach old age. Using failure of two oral antidepressant classes as a defining characteristic of TRD, it has been shown that additional trials of traditional antidepressants, such as venlafaxine, are unlikely to achieve remission.9 Moreover, long-term outcomes are dismal, with high recurrence rates.10 While aripiprazole,11 electroconvulsive therapy12 and transcranial magnetic stimulation13 are alternatives for some, novel treatments are desperately needed.
Cognitive problems are common in LL-TRD. Executive function impairment often coexists with LL-TRD,14 potentially due to microvascular lesions affecting the white matter tracts connecting prefrontal and subcortical structures.15 Impaired executive function is associated with poor responses to oral antidepressant treatment, including nonresponse to aripiprazole augmentation.16,17 Cognitive skills related to executive function, such as alertness and processing speed, are commonly reduced in those with depression and sleep disturbances.18,19 Cognitive disturbances are associated with functional impairment, progression to Alzheimer’s disease and related dementias, and reduced survival. New treatments should target core pathophysiology. Sleep disruption is an important candidate,20 as it contributes not only to difficult-to-treat depression21 but also to deficits in alertness and executive function.22
SWS Deficiency as a Mechanistic Pathway for Cognitive Dysfunction in Older Adults
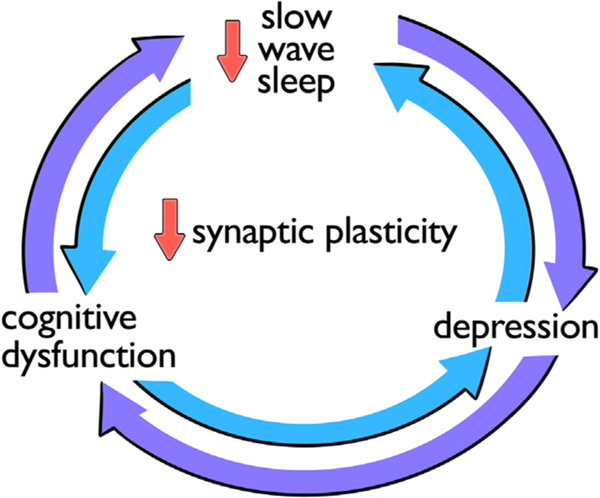
Sleep is a daily critical period for restoring physiologic and brain functions that promote good mental and cognitive health. It is divided into rapid eye movement (REM) and non-rapid eye movement (NREM) sleep. NREM Stage 3 (N3),23 synonymous with slow wave sleep (SWS), is characterized by oscillating cortical electrical activity that generates large-amplitude, low-frequency electroencephalographic (EEG) waves. SWS is linked to subjective feelings of restorative sleep,24 reduced neurohumoral stress response,25 and memory consolidation.26 Synaptic remodeling critical for learning and memory is thought to occur during SWS,27 such that sleep slow waves may serve as markers of synaptic plasticity.28 In addition, memories are putatively replayed during SWS through hippocampal-dependent pathways.29 Emerging evidence suggests that enhancing sleep slow waves improves declarative memory.30 More recently, sleep slow waves have been shown to regulate the biochemical milieu within the brain through the glymphatic system31 by stimulating cerebrospinal fluid waves.32 The physiologic processes that link SWS to cognitive performance have important implications for aging patients, who tend to have less SWS relative to younger individuals.33 Healthy glymphatic flow during SWS may reduce deposition of dementia-associated proteins, such as amyloid-beta31 and tau.34 Slow wave activity (SWA), a measure of power in sleep slow waves, is inversely correlated with tau and amyloid-beta deposition in older adults, suggesting that measures of SWS have utility as mechanistic markers for aging-related pathways to cognitive impairment.35 Pharmacologic SWS enhancement may have a beneficial effect on both cognitive function and mood in LL-TRD (Figure 1).
FIGURE 1.
Bidirectional relationships are thought to exist between disrupted slow wave sleep, cognitive dysfunction, and depression. Abnormalities in slow wave sleep, a crucial period for synaptic plasticity, is a potential mechanistic link underlying these relationships.
SWS as a Novel Antidepressant Target
SWS abnormalities have long been associated with depression. In nondepressed individuals, SWS dominates early sleep cycles, with replacement by REM later in the sleep period. SWA peaks in the first cycle and declines with each successive SWS cycle, reflecting decreasing sleep pressure and restoration of synaptic homeostasis.36 In contrast, patients with depression typically exhibit maximum SWA during later N3 cycles, supporting the hypothesis that depressed patients have impaired homeostasis of synaptic plasticity.37 Reduced SWA, particularly in the first cycle,38 has been considered part of the core pathophysiology of depression, even when accounting for age.39 This characteristic shift in peak SWA can be quantified through the delta sleep ratio (DSR). The DSR is the ratio of SWA for the first and second N3 cycles,38 with a ratio greater than 1.5 observed in those without depression. The DSR has been used as a predictor for treatment response40 and vulnerability to recurrence.38 Reduced SWS is a known risk factor for depression,41 with coincident REM intrusion into early sleep cycles and increased REM duration. Many antidepressants augment SWS, including lithium,42 trazodone,43 nefazodone, mirtazapine,44 sertraline,45 and clomipramine.46 Furthermore, newer antidepressants, such as ketamine47 and agomelatine,48 increase SWS duration or SWA. It has been proposed that addressing SWS deficiency is crucial for sustaining an antidepressant response.49 Restoring SWS to the early cycles of overnight sleep facilitates normalization of REM sleep architecture; subsequent REM delay and reduced REM duration is observed with antidepressant response. Overall, objective metrics based on EEG sleep slow waves are associated with depression, treatment response, and cognitive functioning. Despite these observations, no studies have specifically targeted SWS to treat LL-TRD.
Propofol as a Therapeutic Probe of Sleep, Depression, and Cognitive Dysfunction
Propofol is a commonly used sedative in anesthetic practice. Low doses induce a shift toward lower EEG frequencies, with patterns resembling NREM Stage 2 (N2) sleep. Higher doses induce EEG slow waves resembling those of N3/SWS.50 Even greater doses induce burst suppression, a pattern in the EEG waveform characterized by episodes of zero-voltage EEG (signifying suppression of cortical activity) interspersed with bursts of mixed-frequency activity. With this escalation of dose, response to noxious stimulation decreases.
Sleep-like states achieved during propofol infusions51 may satisfy some homeostatic sleep needs. Other functions of sleep slow waves, such as the ability to entrain sleep spindles, may not be satisfied by propofol.50 Studies in sleep-deprived rodents have shown that propofol facilitates recovery from SWS deficiency,52 suggesting that propofol may engage intrinsic mechanisms for regulating SWS. These results mirror anecdotes of patients feeling rested and restored after propofol sedation. The similarities between sleep and propofol sedation have motivated a trial of propofol to treat refractory insomnia.53 In these patients (28–68 years of age), propofol infusions from 10 P.M. to midnight on five consecutive days had a durable effect on subsequent sleep architecture, enhancing nighttime SWS for up to 6 months after the last infusion.53 Effects on cognition and comorbid psychiatric disorders were not assessed. The potential for SWS enhancement distinguishes propofol from dexmedetomidine, another sedative that can generate EEG states that mimic sleep. In addition, evidence for antidepressant effects exists for propofol but not dexmedetomidine.
A recent clinical trial has demonstrated propofol’s antidepressant potential in patients 18–45 years of age.54 The administration of 10 propofol infusions targeting burst suppression yielded antidepressant responses, with some sustained for up to 3 months. Associated changes in SWS and cognition remain unknown. Burst suppression is often avoided intraoperatively, as a sign of excessive anesthetic exposure; the degree of intraoperative burst suppression has been associated with postoperative cognitive dysfunction.55 Targeting EEG slow waves, rather than burst suppression, may address SWS deficits and enhance subsequent overnight SWS. In addition, avoiding burst suppression may minimize risk of potentially unfavorable cognitive outcomes. Overall, prior studies have utilized five or more infusions,53,54 targeted a different EEG marker,54 and have not specifically recruited geriatric patients.53,54 In contrast, we are repurposing propofol as a therapeutic probe of the relationships between SWS, depression, and cognitive dysfunction in the aged. We present two cases showing varied responses to this approach, demonstrating proof-of-concept and potential limitations.
CASE DESCRIPTIONS
Two older adults with longstanding LL-TRD were referred after failing to improve in a clinical trial.56 They were recruited as pilot participants in the Slow Wave Induction by Propofol to Eliminate Depression (SWIPED) investigation (ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04680910).
After informed consent was obtained, both participants underwent a history and physical examination to rule out any contraindications for two sessions of intravenous propofol general anesthesia. Baseline and postinfusion sleep recordings were acquired using the first-generation version of the Dreem, a wireless wearable headband equipped with dry EEG electrodes.57 Sleep EEG recordings were manually scored in 30-second epochs by certified sleep technologists, using modified American Academy of Sleep Medicine (AASM) criteria.57,58 EEG data were imported into EEGLAB,59 using custom-written MATLAB scripts. After temporal downsampling to 250 Hz, a 0.1 −0.6 Hz band-stop filter was applied to reduce respiratory artifact. Spectral analysis was performed using the Chronux toolbox60 (5-second nonoverlapping time windows, time-bandwidth of 3, and 5 tapers). Five-second epochs with amplitude greater than 250 mcV were excluded. SWA was calculated as 0.5–4 Hz power in the F7-F8 EEG per 30-second N2/N3 epoch during first and second cycles of sleep. Average SWA was calculated across the first 30 minutes of N2/N3 for the first and second cycles of the sleep recordings, with the DSR as the ratio of the first and second cycle SWA. Prior to each propofol infusion, both participants fasted overnight to minimize aspiration risk. Sedation sessions were directly supervised by a board-certified anesthesiologist. They began with placement of an intravenous catheter, standard monitors in accordance with American Society of Anesthesiologist guidelines, and a 64-channel high-density EEG cap. EEG signals were monitored in real time using a Net Amps and Net Station software to optimize safe induction of slow waves while avoiding burst suppression. After infusions, participants recovered under nursing supervision and were discharged home.
Case 1
The patient was a 71-year-old Caucasian non-Hispanic female who had suffered from depression since approximately 15 years of age. She reported previous trials of fluoxetine, citalopram, venlafaxine, paroxetine, escitalopram, bupropion, and transcranial magnetic stimulation. She presented in an episode characterized by depressed mood, anhedonia, disturbed sleep, fatigue/low energy, feelings of worthlessness/guilt, and difficulty concentrating that had persisted for several years. The patient did not achieve lasting remission despite several adequate treatment trials, including: 1) 90 mg duloxetine augmented by aripiprazole 7.5 mg, 2) monotherapy with nortriptyline, and 3) citalopram 40 mg and bupropion 150 mg. She continued citalopram and bupropion for the duration of the study.
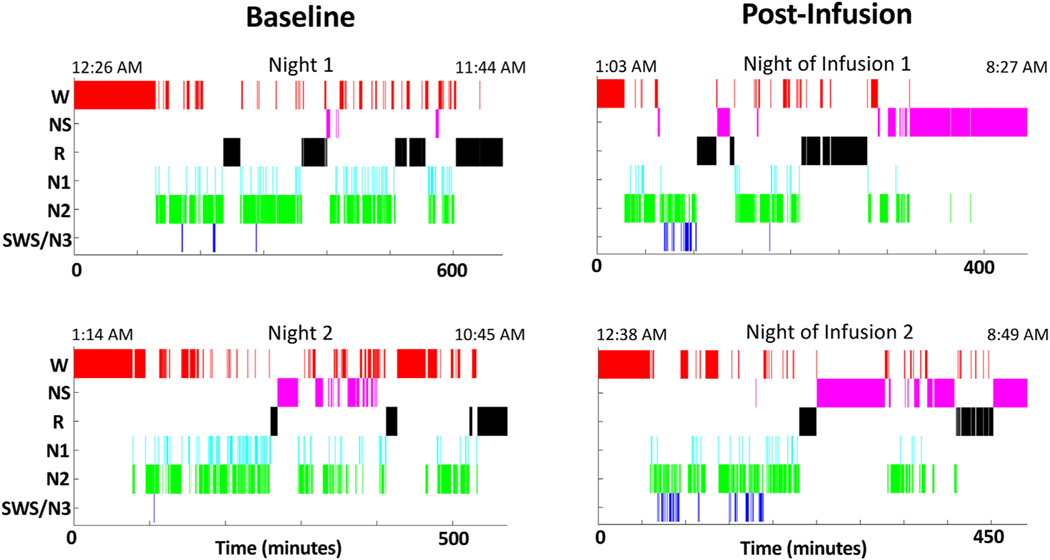
Two baseline overnight sleep EEG recordings demonstrated a paucity of SWS sleep (average 2.8 minutes). SWS composed only 0.7% of her total sleep time, compared to a normative 7%–9% for those 70–80 years of age33 (Table 1). The patient tolerated intravenous propofol infusions without any adverse events. On the first infusion, a total of 1,006 mg of propofol were administered over 1 hour and 42 minutes. On the second infusion, a total of 1,035 mg of propofol were administered over 1 hour and 34 minutes. EEG slow waves were observed during both infusions. Using an automated detection algorithm,61 2 seconds and 96 seconds of burst suppression were detected during the first and second infusions, respectively. Postinfusion overnight at-home sleep studies showed SWS enhancement on the night after each infusion, to 12.5 minutes (5.3% of total sleep time) and 24 minutes (10.6% of total sleep time, a nearly 10fold increase), respectively (Table 1, Figure 2). This participant also demonstrated a reduction in REM sleep duration and total sleep time. She also showed an earlier sleep onset and offset compared to baseline (Figure 2). The average preinfusion DSR was 0.92 while the average DSR postinfusion was 1.64. These measures mirrored changes in her depression assessments. Prior to the first infusion, her Montgomery-Åsberg Depression Rating Scale (MADRS) score was 26, which improved to 7 after the first infusion and remained at 7 nine months later.
TABLE 1.
Two Older Adults With Treatment-Resistant Depression Recorded at-Home Sleep Electroencephalograms (EEG) Before and After Receiving Morning Propofol Infusions
| Average Baseline Minutes (% Total Sleep Time) | Night of Infusion 1 Minutes (% Total Sleep Time) | Night of Infusion 2 Minutes (% Total Sleep Time) | ||
|---|---|---|---|---|
|
| ||||
| Case 1 | Total sleep time | 378.5 | 235 | 226 |
| N3/SWS | 2.8 (0.7) | 12.5 (5.3) | 24 (10.6) | |
| REM | 122.5 (32.4) | 88.5 (37.7) | 57 (25.2) | |
| Case 2 | Total sleep time | 171.8 | 314.5 | 230.5 |
| N3/SWS | 0 (0) | 0 (0) | 3 (1.3) | |
| REM | 52.8 (30.7) | 92.5 (29.4) | 22.5 (9.8) | |
Case 1 was a 71-year-old woman. Case 2 was a 77-year-old man. Metrics from two nights of sleep (recorded prior to Infusion 1) were averaged to generate baseline sleep measures. Both participants exhibited slow wave sleep (SWS) deficiency at baseline. Participants conducted additional sleep EEG recordings at home on the nights after each infusion. Case 1 demonstrated SWS enhancement after each propofol infusion. Case 2 demonstrated minimal SWS enhancement after the second propofol infusion. Both participants generally exhibited reduced REM duration after infusions. TST = total sleep time, REM = rapid eye movement sleep, N3 = non-REM stage 3 sleep, SWS = slow wave sleep.
FIGURE 2.
Hypnograms were generated from sleep electroencephalographic (EEG) data recorded by a 71-year-old woman with treatment-resistant depression. The patient provided EEG recordings before and after receiving propofol infusions. Both baseline sleep recordings were conducted prior to Infusion 1. Sleep onset occurred at 2:35 A.M. and 2:48 A.M., respectively, for the first and second preinfusion nights. Postinfusion EEG recordings were conducted on the night following each morning propofol infusion. Sleep onset occurred at 1:31 A.M. and 1:37 A.M., respectively, for the first and second postinfusion nights. After each infusion, the patient exhibited slow wave sleep enhancement and earlier sleep onset compared to baseline. W = awake, NS = nonscorable epochs, R = rapid eye movement (REM) sleep, N1 = non-REM stage 1 sleep, N2 = non-REM stage 2 sleep, N3 = non-REM stage 3 sleep, SWS = slow wave sleep.
Case 2
The patient was a 77-year-old Caucasian non-Hispanic male with unipolar depression and untreated obstructive sleep apnea. He presented with reduced interest/pleasure, changes in appetite, disturbed sleep, fatigue/low energy, feelings of worthlessness/guilt, and difficulty concentrating. He noted that his depression symptoms started at approximately 15 years of age. Over the past two years, his therapy had included unsuccessful trials of bupropion (up to 300 mg) and escitalopram (10 mg). More recently, the patient had failed to remit after trials of 1) bupropion up to 450 mg, 2) nortriptyline, 3) venlafaxine 150 mg augmented with aripiprazole up to 15 mg, and 4) sertraline up to 200 mg then augmented with lithium carbonate up to 450 mg. He remained on lithium and sertraline for the duration of the SWIPED trial.
Baseline sleep recordings showed a short total sleep time and absent SWS (Table 1). During the first propofol infusion, the patient had moderate airway obstruction that required chin lift maneuvers but otherwise tolerated the session well. Due to intermittent airway obstruction, he received 779 mg of propofol over 1 hour and 4 minutes. For the second infusion, he received 652 mg propofol over 1 hour and 33 minutes. These doses were comparatively less than that administered to the patient in Case 1. EEG slow waves were not readily identified during either infusion. Burst suppression was only detected during the second infusion (3 seconds total). Postinfusion sleep recordings showed only modest SWS enhancement after the second infusion (3 minutes, 1.3% of total sleep time). He had an increased total sleep time (Table 1) and earlier sleep onset (Supplementary Figure 1). Due to his fragmented sleep, corresponding cycles of sleep were challenging to discern, with DSR showing wide variability. DSR for preinfusion Night 1 was 1.4 and 2.0 for Night 2. The postinfusion DSR values were 1.6 (Night 1) and 0.41 (Night 2). Before the first propofol infusion, his MADRS score was 13, which increased to 19 after the first infusion. Six months later, his MADRS score was 20.
DISCUSSION
Disrupted SWS is at the nexus of depression and cognitive dysfunction in older adults, making it a promising treatment target for LL-TRD. Targeting SWS may correct core depression pathophysiology. Rather than intervening upon a particular neurotransmitter system, we aim to promote SWS to facilitate optimal cognitive and mental health. Using propofol as a therapeutic probe to engage endogenous sleep circuitry may yield new pharmacologic and nonpharmacologic avenues for rapidly alleviating TRD. This strategy mirrors that of the neurosteroid brexanolone62 to correct pathophysiology associated with postpartum depression.
The above case series offers mixed support and proof-of-concept that propofol can enhance SWS and improve depression. Of the two patients, one exhibited induction of EEG slow waves during propofol infusions, enhancement of SWS on postinfusion nights, and a sustained improvement in depression lasting at least several months. While it is possible that burst suppression contributed to her antidepressant response, the duration of burst suppression observed here was less than 20% of the target duration that improved depression in a prior study that utilized ten propofol infusions.54 The second patient did not demonstrate EEG slow waves during infusions, SWS enhancement on postinfusion nights, nor improvement in depression. He received a lower total propofol dose. Both participants showed a potential cumulative effect of serial infusions, as the duration of SWS increased more after the second infusion than the first. A corresponding shift of the DSR from low values into the range expected for nondepressed individuals (> 1.5) was observed for Case 1, while Case 2 showed limitations in evaluating this metric. Future study will be needed to assess whether patients with low preinfusion DSR values will be most responsive to any propofol-induced SWS enhancement. These data provide evidence that targeting slow waves during infusions may have a different effect on sleep homeostasis than targeting other EEG patterns. The case series supports two distinct but related hypotheses, 1) that targeting EEG slow waves during propofol infusions may facilitate subsequent SWS enhancement, and 2) that SWS enhancement may improve depression. The latter can also be expressed as a precision medicine hypothesis, that patients with LL-TRD and concomitant SWS deficiency will show an antidepressant effect from propofol only if their SWS improves. This hypothesis is consistent with the experimental therapeutics agenda of the National Institute of Mental Health, which proposes that novel treatments should both engage a target (in this case SWS enhancement) and improve an outcome (depression).
We acknowledge limitations and alternative explanations for improvement. The improvements for Case 1 could have been related to a placebo effect, but this is somewhat unlikely given the duration of the response.
There are several explanations for the lack of SWS enhancement in Case 2. This patient did not show EEG slow waves during propofol infusions, which may be necessary for subsequent SWS enhancement. He did not show SWS before infusions, but baseline SWS may be required for enhancement. This patient’s OSA may be a contributing factor, as well. The many abnormalities in sleep architecture that are associated with OSA may hinder SWS enhancement. These data demonstrate that this approach may lead to clinical improvements in only a subset of patients, depending on their baseline characteristics (e.g., the presence of SWS at baseline or lack of OSA).
We acknowledge additional changes in sleep architecture following propofol infusions. Both participants had earlier sleep onset after infusions compared to preinfusion times. The earlier times of sleep onset and offset after infusions could reflect phase advance in circadian rhythms. Total sleep time decreased after infusions for Case 1 but increased for Case 2, such that sleep restriction could have contributed to the effects (Table 1). However, sleep restriction typically has a short duration of effect, which is inconsistent with the persistence of the response in Case 1 seven weeks after the intervention. REM duration also generally decreased after propofol infusions. These changes will require future consideration.
Our next step in this research is an ongoing Phase I trial of 15 patients with LL-TRD. This trial expands evaluation of psychiatric outcomes to include anhedonia and cognitive function and includes a more extensive follow-up period. Prior to enrollment, participants will be screened for current depression symptoms with the Patient Health Questionnaire-9 (PHQ-9). At enrollment, the study team will administer the Columbia Suicide Severity Rating Scale (C-SSRS), MADRS, Montreal Cognitive Assessment (MoCA), and Snaith-Hamilton Pleasure Scale (SHAPS) to assess baseline suicidality, depression severity, cognitive ability, and anhedonia, respectively. In addition, participants will record two nights of baseline sleep using the Dreem headband. Participants will then undergo high-density EEG monitoring during two propofol infusions administered 2−6 days apart. Propofol will be individually dosed to maximize induction of EEG slow waves during infusions. The Feeling Scale will be employed to gauge participants’ affect before and after infusions. The C-SSRS, MADRS, and SHAPS will be repeated approximately seven days after the second infusion. On the night of each infusion, participants will record overnight sleep EEG. They will record up to five additional nights over three weeks following the second infusion. We will assess for SWS enhancement and changes in DSR. Postintervention psychiatric assessments, including the C-SSRS, MADRS, MoCA, and SHAPS, will be performed approximately three weeks after the second infusion. The study visit will also entail a high-density EEG recording without propofol sedation. Finally, outcomes will be assessed approximately ten weeks after the second infusion. This Phase I trial will lay the groundwork for a subsequent randomized controlled trial elucidating the relationships between disrupted SWS, depression, and cognitive dysfunction in those with LL-TRD.
Supplementary Material
HIGHLIGHTS.
-
What is the primary question addressed by this study?
Can propofol enhance slow wave sleep in geriatric patients to address core pathophysiology of depression and associated cognitive dysfunction?
-
What is the main finding of this study?
Two older adults with treatment-resistant depression received two morning infusions of propofol. One of the two demonstrated slow wave sleep enhancement and a sustained improvement of depression.
-
What is the meaning of the finding?
The above case series lays the groundwork for an ongoing open-label Phase I clinical trial by providing proof-of-concept of propofol-induced slow wave sleep enhancement and antidepressant response.
DISCLOSURE
The authors appreciate the efforts of Thomas Nguyen, Alyssa Labonte, and Tiffany Yatsko in collecting these data. We appreciate feedback from Charles F. Reynolds III and Jordan F. Karp on manuscript drafts. Research reported in this publication was supported by the Washington University Center for Perioperative Mental Health grant number P50 MH122351 from the National Institute of Mental Health (NIMH) of the National Institutes of Health (NIH), NIMH grant U01 MH128483, NIMH grant K01 MH128663, and NIH grant UL1TR002345. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
BJAP has a patent pending on control of anesthetic state modulation and an agreement with Elemind on the use of nonpharmacologic potentiation of EEG slow waves. EJL reports consulting fees from Merck, Boehringer-Ingelheim, Pritikin ICR, IngenioRx, and Prodeo, grant funding from Janssen, the COVID Early Treatment Fund, and Fast-Grants, and a patent pending on sigma-1 receptor agonists for COVID-19. BPL reports consulting fees from Merck and Eli Lilly. He is also on the Scientific Advisory Board for Beacon Biosignals. RLR, OH, and EL have no conflicts to disclose concerning any product mentioned or concept discussed in this article. MK, BPL, EJL, and BJAP have received funding from the National Institutes of Health.
Footnotes
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.jagp.2023.03.009.
DATA STATEMENT
These data were presented by poster at the Center on Biological Rhythms and Sleep Research Symposium in St. Louis, MO, May 2022 and during the Foundation for Anesthesia Education and Research poster session at the American Society of Anesthesiologists Annual Meeting in New Orleans, LA, October 2022.
References
- 1.Lenze EJ, Rogers JC, Martire LM, et al. : The association of late-life depression and anxiety with physical disability: a review of the literature and prospectus for future research. Am J Geriatr Psychiatry 2001; 9(2):113–135 [PubMed] [Google Scholar]
- 2.Wolkowitz OM, Reus VI, Mellon SH: Of sound mind and body: depression, disease, and accelerated aging. Dialogues Clin Neurosci 2011; 13(1):25–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szanto K, Lenze EJ, Waern M, et al. : Research to reduce the suicide rate among older adults: methodology roadblocks and promising paradigms. Psychiatr Serv 2013; 64(6):586–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ownby RL, Crocco E, Acevedo A, et al. : Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry 2006; 63(5):530–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byers AL, Yaffe K: Depression and risk of developing dementia. Nat Rev Neurol 2011; 7(6):323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diniz BS, Butters MA, Albert SM, et al. : Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry 2013; 202(5):329–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas L, Mulsant BH, Solano FX, et al. : Response speed and rate of remission in primary and specialty care of elderly patients with depression. Am J Geriatr Psychiatry 2002; 10(5):583–591 [PubMed] [Google Scholar]
- 8.Mulsant BH, Blumberger DM, Ismail Z, et al. : A systematic approach to pharmacotherapy for geriatric major depression. Clin Geriatr Med 2014; 30(3):517–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchalter ELF, Oughli HA, Lenze EJ, et al. : Predicting remission in late-life major depression: a clinical algorithm based upon past treatment history. J Clin Psychiatry 2019; 80(6):18m12483 [DOI] [PubMed] [Google Scholar]
- 10.Deng Y, McQuoid DR, Potter GG, et al. : Predictors of recurrence in remitted late-life depression. Depress Anxiety 2018; 35 (7):658–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenze EJ, Mulsant BH, Blumberger DM, et al. : Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet 2015; 386 (10011):2404–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kellner CH, Husain MM, Knapp RG, et al. : A novel strategy for continuation ECT in geriatric depression: phase 2 of the PRIDE Study. Am J Psychiatry 2016; 173(11):1110–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conelea CA, Philip NS, Yip AG, et al. : Transcranial magnetic stimulation for treatment-resistant depression: naturalistic treatment outcomes for younger versus older patients. J Affect Disord 2017; 217:42–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snyder HR: Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull 2013; 139(1):81–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexopoulos GS, Murphy CF, Gunning-Dixon FM, et al. : Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry 2008; 165(2):238–244 [DOI] [PubMed] [Google Scholar]
- 16.Cristancho P, Lenze EJ, Dixon D, et al. : Executive function predicts antidepressant treatment noncompletion in late-life depression. J Clin Psychiatry 2018; 79(3) [DOI] [PubMed] [Google Scholar]
- 17.Kaneriya SH, Robbins-Welty GA, Smagula SF, et al. : Predictors and moderators of remission with aripiprazole augmentation in treatment-resistant late-life depression: an analysis of the IRL-GRey randomized clinical trial. JAMA Psychiatry 2016; 73(4):329–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plante DT, Hagen EW, Ravelo LA, et al. : Impaired neurobehavioral alertness quantified by the psychomotor vigilance task is associated with depression in the Wisconsin Sleep Cohort study. Sleep Med 2020; 67:66–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yun CH, Kim H, Lee SK, et al. al: Daytime sleepiness associated with poor sustained attention in middle and late adulthood. Sleep Med 2015; 16(1):143–151 [DOI] [PubMed] [Google Scholar]
- 20.Murphy MJ, Peterson MJ: Sleep disturbances in depression. Sleep Med Clin 2015; 10(1):17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroder CM, O’Hara R: Depression and obstructive sleep apnea (OSA). Ann Gen Psychiatry 2005; 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holanda FWNJ, de Almondes KM: Sleep and executive functions in older adults: a systematic review. Dement Neuropsychol 2016; 10(3):185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry RBBR, Gamaldo CE, Harding SM, et al. : The AASM Manual for Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.3. Darien, Illinois: American Academy of Sleep Medicine, 2015 [Google Scholar]
- 24.Akerstedt T, Hume K, Minors D, et al. : Good sleep–its timing and physiological sleep characteristics. J Sleep Res 1997; 6(4):221–229 [DOI] [PubMed] [Google Scholar]
- 25.Tasali E, Leproult R, Ehrmann DA, et al. : Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A 2008; 105(3):1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molle M, Yeshenko O, Marshall L, et al. : Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. J Neurophysiol 2006; 96(1):62–70 [DOI] [PubMed] [Google Scholar]
- 27.Deuschle M, Schredl M, Wisch C, et al. : Serum brain-derived neurotrophic factor (BDNF) in sleep-disordered patients: relation to sleep stage N3 and rapid eye movement (REM) sleep across diagnostic entities. J Sleep Res 2018; 27(1):73–77 [DOI] [PubMed] [Google Scholar]
- 28.Riedner BA, Vyazovskiy VV, Huber R, et al. : Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep 2007; 30(12):1643–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasch B, Buchel C, Gais S, et al. : Odor cues during slow-wave sleep prompt declarative memory consolidation. Science 2007; 315(5817):1426–1429 [DOI] [PubMed] [Google Scholar]
- 30.Marshall L, Helgadottir H, Molle M, et al. : Boosting slow oscillations during sleep potentiates memory. Nature 2006; 444 (7119):610–613 [DOI] [PubMed] [Google Scholar]
- 31.Iliff JJ, Wang M, Liao Y, et al. : A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 2012; 4(147):147ra111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fultz NE, Bonmassar G, Setsompop K, et al. : Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 2019; 366(6465):628–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohayon MM, Carskadon MA, Guilleminault C, et al. : Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep 2004; 27(7):1255–1273 [DOI] [PubMed] [Google Scholar]
- 34.Iliff JJ, Chen MJ, Plog BA, et al. : Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 2014; 34(49):16180–16193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucey BP, McCullough A, Landsness EC, et al. : Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease. Sci Transl Med 2019; 11(474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Achermann P, Dijk DJ, Brunner DP, et al. : A model of human sleep homeostasis based on EEG slow-wave activity: quantitative comparison of data and simulations. Brain Res Bull 1993; 31(1–2):97–113 [DOI] [PubMed] [Google Scholar]
- 37.Pittenger C, Duman RS: Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 2008; 33(1):88–109 [DOI] [PubMed] [Google Scholar]
- 38.Kupfer DJ, Frank E, McEachran AB, et al. : Delta sleep ratio. A biological correlate of early recurrence in unipolar affective disorder. Arch Gen Psychiatry 1990; 47(12):1100–1105 [DOI] [PubMed] [Google Scholar]
- 39.Gillin JC, Duncan WC, Murphy DL, et al. : Age-related changes in sleep in depressed and normal subjects. Psychiatry Res 1981; 4(1):73–78 [DOI] [PubMed] [Google Scholar]
- 40.Duncan WC Jr, Selter J, Brutsche N, et al. : Baseline delta sleep ratio predicts acute ketamine mood response in major depressive disorder. J Affect Disord 2013; 145(1):115–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buysse DJ, Frank E, Lowe KK, et al. : Electroencephalographic sleep correlates of episode and vulnerability to recurrence in depression. Biol Psychiatry 1997; 41(4):406–418 [DOI] [PubMed] [Google Scholar]
- 42.Kupfer DJ, Reynolds CF 3rd, Weiss BL, et al. : Lithium carbonate and sleep in affective disorders. Further considerations. Arch Gen Psychiatry 1974; 30(1):79–84 [DOI] [PubMed] [Google Scholar]
- 43.Suzuki H, Yamadera H, Nakamura S, et al. : Effects of trazodone and imipramine on the biological rhythm: an analysis of sleep EEG and body core temperature. J Nippon Med Sch 2002; 69(4):333–341 [DOI] [PubMed] [Google Scholar]
- 44.Doghramji K, Jangro WC: Adverse effects of psychotropic medications on sleep. Psychiatr Clin North Am 2016; 39(3):487–502 [DOI] [PubMed] [Google Scholar]
- 45.Jindal RD, Friedman ES, Berman SR, et al. : Effects of sertraline on sleep architecture in patients with depression. J Clin Psychopharmacol 2003; 23(6):540–548 [DOI] [PubMed] [Google Scholar]
- 46.Ehlers CL, Havstad JW, Kupfer DJ: Estimation of the time course of slow-wave sleep over the night in depressed patients: effects of clomipramine and clinical response. Biol Psychiatry 1996; 39 (3):171–181 [DOI] [PubMed] [Google Scholar]
- 47.Duncan WC, Sarasso S, Ferrarelli F, et al. : Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol 2013; 16(2):301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quera Salva MA, Vanier B, Laredo J, et al. : Major depressive disorder, sleep EEG and agomelatine: an open-label study. Int J Neuropsychopharmacol 2007; 10(5):691–696 [DOI] [PubMed] [Google Scholar]
- 49.Rantamaki T, Kohtala S: Encoding, consolidation, and renormalization in depression: synaptic homeostasis, plasticity, and sleep integrate rapid antidepressant effects. Pharmacol Rev 2020; 72 (2):439–465 [DOI] [PubMed] [Google Scholar]
- 50.Murphy M, Bruno MA, Riedner BA, et al. : Propofol anesthesia and sleep: a high-density EEG study. Sleep 2011; 34(3):283–291A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rabelo FA, Braga A, Kupper DS, et al. : Propofol-induced sleep: polysomnographic evaluation of patients with obstructive sleep apnea and controls. Otolaryngol Head Neck Surg 2010; 142 (2):218–224 [DOI] [PubMed] [Google Scholar]
- 52.Tung A, Bergmann BM, Herrera S, et al. : Recovery from sleep deprivation occurs during propofol anesthesia. Anesthesiology 2004; 100(6):1419–1426 [DOI] [PubMed] [Google Scholar]
- 53.Xu Z, Jiang X, Li W, et al. : Propofol-induced sleep: efficacy and safety in patients with refractory chronic primary insomnia. Cell Biochem Biophys 2011; 60(3):161–166 [DOI] [PubMed] [Google Scholar]
- 54.Mickey BJ, White AT, Arp AM, et al. : Propofol for treatment-resistant depression: a pilot study. Int J Neuropsychopharmacol 2018; 21(12):1079–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fritz BA, Kalarickal PL, Maybrier HR, et al. : Intraoperative electroencephalogram suppression predicts postoperative delirium. Anesth Analg 2016; 122(1):234–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cristancho P, Lenard E, Lenze EJ, et al. : Optimizing Outcomes of Treatment-Resistant Depression in Older Adults (OPTIMUM): study design and treatment characteristics of the first 396 participants randomized. Am J Geriatr Psychiatry 2019; 27(10):1138–1152 [DOI] [PubMed] [Google Scholar]
- 57.Kafashan MM, Hyche O, Nguyen T, et al. : Perioperative sleep in geriatric cardiac surgical patients: a feasibility study using a wireless wearable device. Br J Anaesth 2021; 126(6):e205–e208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith SK, Nguyen T, Labonte AK, et al. : Protocol for the Prognosticating Delirium Recovery Outcomes Using Wakefulness and Sleep Electroencephalography (P-DROWS-E) study: a prospective observational study of delirium in elderly cardiac surgical patients. BMJ Open 2020; 10(12):e044295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delorme A, Makeig S: EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 2004; 134(1):9–21 [DOI] [PubMed] [Google Scholar]
- 60.Mitra PP, Bokil H: Observed brain dynamics. 1st ed. New York: Oxford University Press, 2008 [Google Scholar]
- 61.Kafashan M, Brian Hickman L, Labonte AK, et al. : Quiescence during burst suppression and postictal generalized EEG suppression are distinct patterns of activity. Clin Neurophysiol 2022; 142:125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luscher B, Mohler H: Brexanolone, a neurosteroid antidepressant, vindicates the GABAergic deficit hypothesis of depression and may foster resilience. F1000Res 2019; 8. F1000 Faculty Rev-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
These data were presented by poster at the Center on Biological Rhythms and Sleep Research Symposium in St. Louis, MO, May 2022 and during the Foundation for Anesthesia Education and Research poster session at the American Society of Anesthesiologists Annual Meeting in New Orleans, LA, October 2022.