Abstract
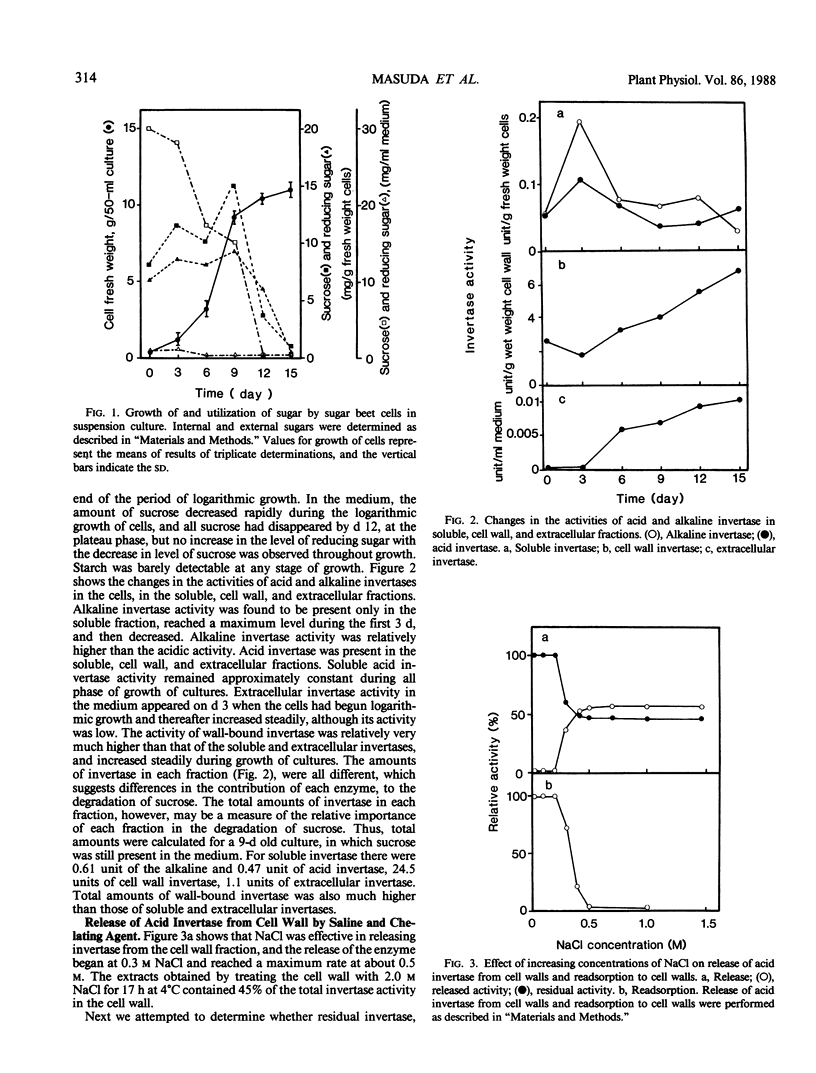
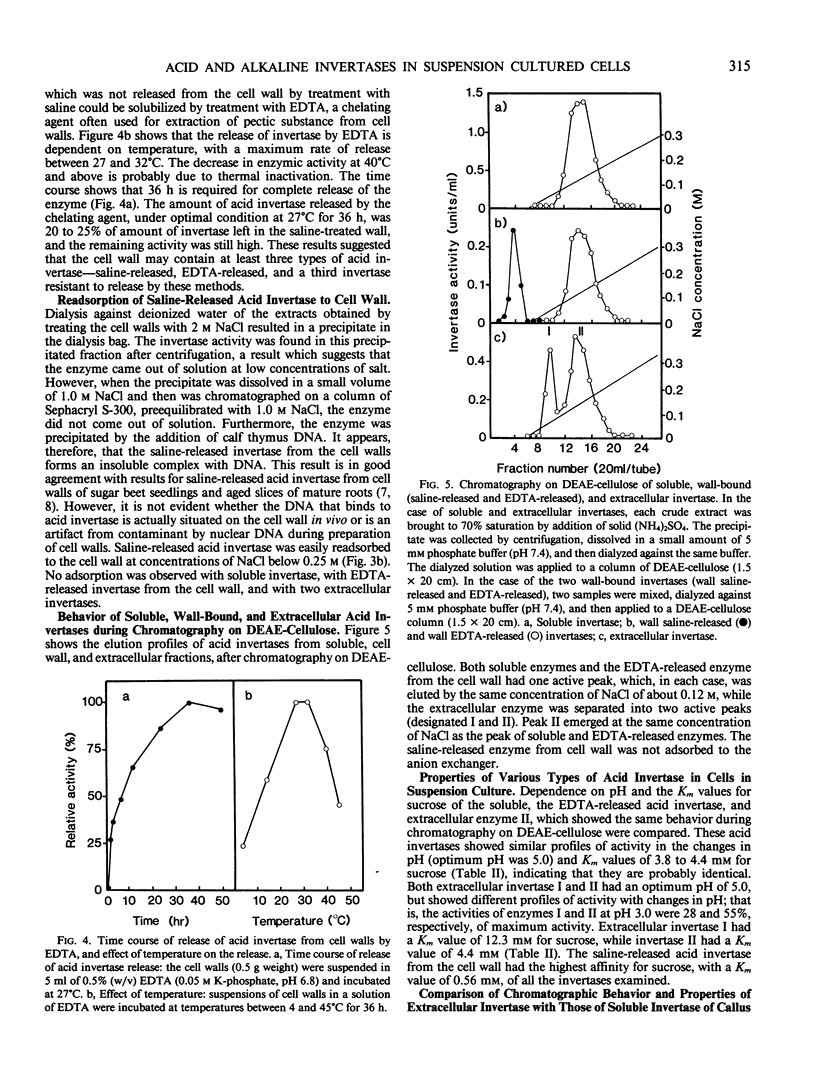
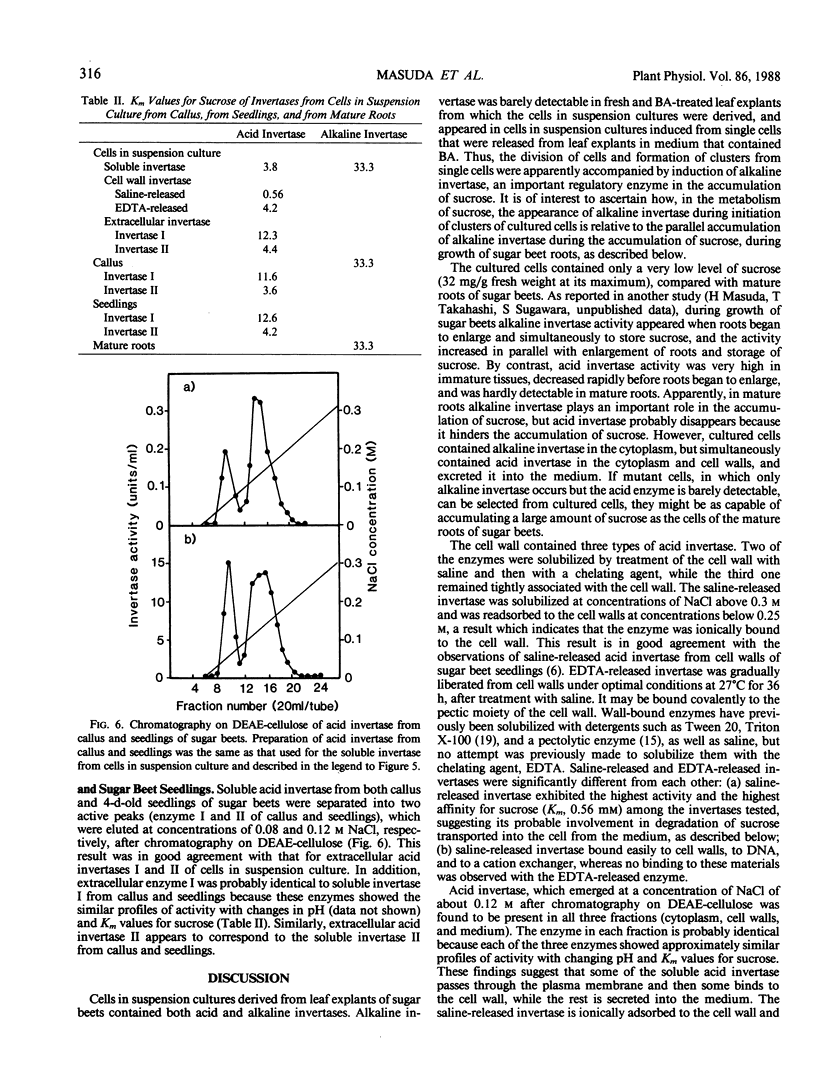
Alkaline invertase was induced during the initiation of suspension cultures of single cells from leaf explants of sugar beets in Murashige-Skoog liquid medium which contained benzyladenine. This activity was barely detectable in the leaves themselves. In suspension cultures, the presence of both acid and alkaline invertases was detected; alkaline invertase was only present in the cytoplasm of the cultured cells, whereas acid invertase was present in the cytoplasm and cell walls, and was also detected in the culture medium. The cell wall contained at least three types of acid invertase; two of these activities were solubilized by saline (saline-released) and EDTA (EDTA-released), respectively, and the third remained tightly associated with the cell wall. Saline-released and EDTA-released invertases from the cell wall showed the significant differences in their properties: the saline-released enzyme had the highest affinity for sucrose among the invertases tested, and was easily bound to cell walls, to DNA, and to a cation exchanger, unlike the EDTA-released enzyme. Sucrose is the source of carbon for plant cells in suspension culture and is probably degraded in the cell wall by the saline-released invertase, which had the highest activity and the highest affinity for sucrose. Hexose products of this degradation would be transported to cytoplasm. Soluble invertase, EDTA-released invertase from the cell wall, and one of two extracellular invertases behaved similarly upon chromatography on DEAE-cellulose. They had similar activity profiles with changing pH, and similar Km values for sucrose. Thus it appears that they are identical. Two extracellular invertases found in the growth medium of the suspension cultures were probably identical with those in the soluble fraction of callus and seedlings of sugar beets, because they showed similar behaviors during chromatography on DEAE-cellulose, and had similar activity profiles with changing pH and Km values for sucrose.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper R. A., Greenshields R. N. The partial purification and some properties of two sucrases of Phaseolus vulgaris. Biochem J. 1964 Aug;92(2):357–364. doi: 10.1042/bj0920357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanabus J., Bressan R. A., Carpita N. C. Carbon assimilation in carrot cells in liquid culture. Plant Physiol. 1986 Oct;82(2):363–368. doi: 10.1104/pp.82.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Sugawara S. Purification and Some Properties of Cell Wall-bound Invertases from Sugar Beet Seedlings and Aged Slices of Mature Roots. Plant Physiol. 1980 Jul;66(1):93–96. doi: 10.1104/pp.66.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K., Uritani I. Change in invertase activity of sweet potato in response to wounding and purification and properties of its invertases. Plant Physiol. 1974 Jul;54(1):60–66. doi: 10.1104/pp.54.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Stephens G. J., Wood R. K. Release of enzymes from cell walls by an endopectate-trans-eliminase. Nature. 1974 Sep 27;251(5473):358–358. doi: 10.1038/251358a0. [DOI] [PubMed] [Google Scholar]
- Zamski E., Wyse R. E. Stereospecificity of the glucose carrier in sugar beet suspension cells. Plant Physiol. 1985 Jun;78(2):291–295. doi: 10.1104/pp.78.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]


