Abstract
Congenital heart disease (CHD) is the leading organ-specific birth defect, as well as the leading cause of infant morbidity and mortality from congenital malformations. Therefore, a comprehensive screening examination of the fetal heart should be performed in all women to maximize the detection of CHD. Four-dimensional sonography with spatiotemporal image correlation (STIC) technology displays a cine loop of a complete single cardiac cycle in motion. A novel method known as Fetal Intelligent Navigation Echocardiography (or FINE) was previously developed to interrogate STIC volume datasets using “intelligent navigation” technology. Such method allows the automatic display of nine standard fetal echocardiography views required to diagnose most cardiac defects. FINE considerably simplifies fetal cardiac examinations and reduces operator dependency. It has both high sensitivity and specificity for the detection of CHD. Indeed, FINE has been integrated into several commercially available ultrasound platforms.
Recently, eight novel and advanced features have been developed for the FINE method and they will be described herein. Such features can be categorized based upon their broad goals. The first goal is to simplify FINE further, and consists of the following features: 1) Auto fetal positioning (or FINE align); 2) Skip points; 3) Predictive cursor; 4) Static mode volume; and 5) Breech sweep. The second goal is to allow quantitative measurements to be performed on the cardiac views generated by FINE: 6) Automatic cardiac axis; and 7) Cardiac biometry. Finally, the last goal is to improve the success of obtaining fetal echocardiography view(s) and consists of (8) Maestro planar navigation.
Keywords: congenital heart disease, fetal echocardiography, fetal heart, STIC, ultrasound
Introduction and Background
The most common birth defect is congenital heart disease (CHD) [1–4], which is also the leading cause of infant morbidity and mortality from congenital malformations [5]. All pregnancies should undergo prenatal sonographic screening for CHD, since up to 90% of cases occur in the absence of high-risk features [6–8]. Unfortunately, the prenatal detection of CHD via two-dimensional (2D) sonography has remained low (15-39%) [9,10]. This is due to various factors such as the complex anatomy and small size of the fetal heart, as well as the high-level of expertise and skill required to evaluate this organ [11]. Indeed, the fetal heart may be the most difficult organ to examine by ultrasound [12].
A growing body of scientific work has shown that four-dimensional (4D) sonography with spatiotemporal image correlation (STIC) not only facilitates examination of the fetal heart [13–16], but also has the potential to increase the detection rate of CHD [13,17,18]. Therefore, investigators have proposed the application of this modality in prenatal cardiac screening and diagnosis of CHD [19–21], since it can shorten examination time and improve the identification of complex intracardiac relationships [13,16]. STIC technology allows acquisition of a volume dataset from the fetal heart and displays a cine loop of a complete single cardiac cycle in motion [22]. Such dataset contains all the necessary information to adequately examine the fetal heart, since an unlimited number of cardiac images are available to review in any plane or orientation [23]. However, manual navigation [24] through STIC volume datasets using software requires a comprehensive understanding of fetal cardiac anatomy, and is highly operator dependent and difficult to perform, especially when the heart is abnormal [11,25–27]. Manual navigation allows retrieval and display of relevant cardiac diagnostic planes; however, this requires operator interaction with the STIC volume dataset (e.g. operating the x, y, z controls; parallel shifting; scaling) [24]. Indeed, operator dependency has been reported to be the main problem when STIC volumes are analyzed [17].
To address such issues, we recently developed intelligent navigation technology as a new method to interrogate sonographic volume datasets, whereby identification and selection of key anatomical landmarks allows the technology to: 1) generate a geometrical reconstruction of the organ of interest (e.g. fetal heart); and 2) automatically navigate, find, extract, and display specific diagnostic planes [24,28]. This is achievable using operator-independent algorithms that are both predictable and adaptive [24]. Intelligent navigation refers to the system’s ability to automatically analyze a volume dataset and efficiently extract diagnostic information.
We then applied intelligent navigation technology to STIC volume datasets, and developed a novel method known as Fetal Intelligent Navigation Echocardiography (FINE) that automatically generates and displays nine standard fetal echocardiography views required to diagnose most cardiac defects [11,24]. Manual manipulation or standardization of the STIC volume dataset and cardiac planes is not required [26]. As a result, the FINE method considerably simplifies the fetal cardiac examination and reduces operator dependency. Moreover, it has the potential to improve clinical efficiency and workflow. FINE is comprised of various developments and features that are innovative, and a detailed summary of the method and its applications are described in Table 1. Indeed, the FINE method has been successfully integrated into several commercially available ultrasound platforms (UGEO WS80A and HERA W10; Samsung Healthcare, Seoul, Korea), and is commercially known as 5D Heart technology.
Table 1.
Summary of Characteristic Features and Applications of Fetal Intelligent Navigation Echocardiography (FINE)
| Characteristic | FINE |
|---|---|
| General | • Gestational age: second to third trimesters (recommended) • Intelligent navigation technology applied to STIC volume datasets (vs. manual navigation): ○ Seven anatomical structures of fetal heart are marked (or clicked) in volume dataset using Anatomic Box® ○ Generates a geometrical model of fetal heart ○ Automatically and immediately rotates, aligns, dissects, and scales volume dataset ○ Automatically navigates, finds, extracts, and displays nine cardiac diagnostic planes simultaneously in the same template ○ VIS-Assistance® • Automatic realignment of STIC volume, and reorientation and standardization of the anatomical position: ○ Fetus and cardiac diagnostic planes are consistently displayed in the same manner each time, regardless of fetal position or initial orientation (e.g. converts a true breech to a “vertex” presentation, places the spine at 6 o’clock) ○ Allows structures, anatomy, and relationships to be more easily recognized as normal or abnormal • Predictable method (i.e. diagnostic planes are generated in a consistent manner) • Adaptive method (“fits” the anatomy of each particular fetus under examination) • Incorporates cardiac phase recognition ○ Facilitates marking of anatomical structures (e.g. closed atrioventricular valves) and is an important feature to increase the success of obtaining echocardiography views • No manual navigation required ○ Manual standardization or manipulation of the STIC volume dataset and reference planes is not required (e.g. manual alignment or rotation) ○ Leads to reduced operator dependency and examination of the fetal heart is standardized and simplified |
| STICLoop™ | • 2D cine loop tool that scrolls in a continuous fashion ○ STIC volume is automatically converted into a 2D cine loop and displayed as individual frames in a scrolling loop ○ Operator independent and runs automatically at a constant speed • Aid to determine the appropriateness of STIC volumes before applying the FINE method to such volumes; provides immediate feedback • STICLoop™ criteria: 1. Fetal spine located between the 5 and 7 o’clock positions (reducing the possibility of shadowing from the ribs or spine) 2. Minimal or absent shadowing (which could obscure visualization of cardiac anatomy) 3. Adequate image quality 4. Upper mediastinum and stomach included within the volume and clearly visible 5. Minimal or no motion artifacts observed in the STICLoop™ (i.e. smooth sweep without evidence of abrupt jumps or discontinuous movements) 6. Chest circumference contained with the ROI 7. Sequential axial planes parallel to each other, similar to a sliced loaf of bread (i.e. no drifting spine from the four-chamber view down to the stomach 8. No azimuthal issues observed (i.e. atria/ventricles do not appear foreshortened in the four-chamber view) 9. Minimal or no motion artifacts observed in the sagittal plane • Developed to facilitate detection of: ○ Discontinuity or undulating movements that could modify anatomic structure representation and are due to motion artifacts or errors in STIC assembly ○ Azimuthal issues (i.e. tilted acquisitions) ○ Drifting spine (fetal spine location “migrates” on the screen during the automatic STICLoop™ scroll) |
| Anatomic Box® | • Tool used to mark (or click) seven anatomical structures of fetal heart in sequential order within the STIC volume: 1. Cross-section of aorta at level of stomach 2. Cross-section of aorta at level of four-chamber view 3. Crux 4. Right atrial wall 5. Pulmonary valve 6. Cross-section of superior vena cava 7. Transverse aortic arch • System automatically scrolls through the STIC volume to level of most likely location of anatomical structure to be marked • Menu and reference image(s) are displayed for each anatomical structure to be marked, and the order of marking is also specified |
| Fetal echocardiography views | • Nine views 1. Four chamber 2. Five chamber 3. Left ventricular outflow tract 4. Short-axis view of great vessels/right ventricular outflow tract 5. Three vessels and trachea 6. Abdomen/stomach 7. Ductal arch 8. Aortic arch 9. Superior and inferior vena cava |
| Cardiac diagnostic planes | • All nine planes (including transverse and sagittal) are displayed simultaneously in a single template approximately 3 seconds after marking process is completed • Successful display occurs despite the gestational age and in the presence of anatomical variability (e.g. cardiac axis and geometry) • Cases of CHD: ○ Abnormal cardiac anatomy and anatomic relationships are successfully demonstrated ○ Various features of the CHD can be visualized and compared side-by-side (e.g. overriding aorta, ventricular septal defect, pulmonary stenosis in tetralogy of Fallot) ○ Same abnormal cardiac feature(s) can be confirmed in multiple cardiac views at the same time (e.g. hypoplasia of the pulmonary artery seen in the 3VT view, short-axis view of great vessels/right ventricular outflow tract, and ductal arch view) |
| VIS-Assistance® | • Video clip tool which can be activated for each cardiac diagnostic plane • Operator-independent (automatic) sonographic navigation and exploration of surrounding structures in a given cardiac diagnostic plane (“virtual” sonographer which “scans” the STIC volume in a purposeful and targeted manner) • Written callouts (e.g. “move cephalad”) appear at the top of each video clip to inform the operator of the purpose/action of the automatic navigational movements • Objective: ○ Improves success of obtaining fetal echocardiography view of interest when not initially obtained via the diagnostic plane ○ Provides more information about diagnostic plane and its surrounding structures (e.g. pulmonary veins) ○ Improves the quality of examination since the complexity of the fetal heart can be studied in greater detail and specific structures shown • Advantages (vs. 2D sonography or manual navigation of STIC volumes) ○ Automatic navigation through volume (decreases operator dependency) ○ Consistent navigational movements through volume each time VIS-Assistance® is activated ○ Types of navigational movements through volume are unique, fluid, and would be difficult or impossible to perform otherwise ○ Time duration is typically shorter • Informative and valuable in cases of CHD (e.g. delineates complex anatomical relationships) • Depiction of cardiac abnormalities when the diagnostic plane appears normal • Provides further diagnostic information even when the diagnostic plane is abnormal • Changes a suspected abnormal fetal cardiac view to one that is interpreted as normal (reduces false positive rate) • Can show the appropriate azimuth • Double VIS-Assistance technique: VIS-Assistance® is applied twice to a cardiac diagnostic plane, allowing expanded navigational movements and further exploration • Triple VIS-Assistance technique: VIS-Assistance® is applied three times to a cardiac diagnostic plane, allowing expanded navigational movements and further exploration • Any original diagnostic plane can be changed via VIS-Assistance® and replaced with the new plane |
| Automatic Labeling | • Fetal echocardiography views (i.e. diagnostic planes); left and right fetal sides; cranial and caudal ends; anatomical structures (atrial and ventricular chambers, great vessels, venae cavae, stomach) • Labeling stays with the corresponding anatomical structure(s), even as the image is increased or decreased in size • May be activated for grayscale, color Doppler, S-flow Doppler (bidirectional power Doppler) • Optional feature that can be activated or turned off • Assists sonologist to recognize anatomical structures and allow the images generated by FINE to be compared with what is considered normal • Not a standard template overlay applied to cardiac diagnostic planes, but rather is always unique to a particular fetus |
| Intelligent and Marking Alerts | • Intelligent Alerts: ○ Notifies user about potential issues with a STIC volume dataset ○ Captions and/or a movie which automatically appear: a) during marking of anatomical structures of fetal heart; or b) in specific situations ○ Three types: ▪ Breech Alert: notifies the user that the fetus appears to be in a breech presentation ▪ Possible Drifting Spine Alert: notifies the user that there may be a drifting fetal spine in the volume dataset (i.e. when the spine location migrates on the screen) ▪ Spine Location Alert: notifies the user that the fetal spine appears to be located at a position (e.g. 9 o’clock) that is different from what is recommended (i.e. between 5 and 7 o’clock) • Marking Alerts: ○ Notifies user that fetal anatomical structures for marking (e.g. pulmonary valve) may be in a different location than expected ○ Captions/movies which occur during the marking process and only appear after a spine location alert has been activated ▪ Depicts reference image example to guide/teach sonologist where to mark anatomic structure of interest ○ Three marking alerts appear in sequence: ▪ Pulmonary Valve Alert: notifies the user that the pulmonary valve may not be visualized well (thus, there may be difficulty in marking the pulmonary valve) ▪ Superior Vena Cava Alert: notifies the user about the possible location of superior vena cava ▪ Transverse Aortic Arch Alert: notifies the user about the possible location of transverse aortic arch |
| Color Doppler FINE | • Cardiac diagnostic planes (including transverse and sagittal) contain either color Doppler or S-flow information and are displayed simultaneously in a single template • Mimics real-time color Doppler examination of the fetal heart due to its motion characteristics • Same Doppler flow information can be viewed in multiple cardiac views at the same time • Option of turning off color and power Doppler information allows successful examination of the fetal heart through grayscale • Color VIS-Assistance: VIS-Assistance activated in color Doppler FINE for each of nine cardiac diagnostic planes ○ By improving success of generating a cardiac view, an appropriate Doppler signal becomes visible ○ Allows visualization of more anatomical structures (e.g. hepatic veins) ○ Reduces false-positive diagnoses (e.g. “pseudo” ventricular septal defect) • Clinical applications: ○ Informative method to examine normal and abnormal fetal hearts ○ Depicts cardiac structures successfully when grayscale does not (e.g. ductus arteriosus/ductal arch) ○ Provides information about cardiac structure and function in normal fetal hearts ○ In CHD cases, depicts abnormal fetal cardiac anatomy and/or hemodynamic flow characteristics • Known as 5D Heart Color on commercially available ultrasound systems |
| Technology | • Operates on conventional computers • Not dependent on the use of software to perform manual navigation of volume datasets • Diagnostic planes, and VIS-Assistance® video clips can be transmitted by telemedicine for expert consultation, thus extending the benefits of prenatal cardiac examination • Smartphones, tablets, and other devices can be used to receive transmitted information (diagnostic planes or VIS-Assistance® videoclips) • Prenatal cardiac screening and diagnostic tool in clinical setting • Educational and training tool • Known as 5D Heart on commercially available ultrasound systems |
2D, two-dimensional; 3VT, three vessels and trachea view; CHD, congenital heart disease; ROI, region of interest; STIC, spatiotemporal image correlation; VIS-Assistance®, Virtual Intelligent Sonographer Assistance
In the past several years, we have investigated the FINE method and reported the following: 1) FINE improves assessment of the normal and abnormal fetal heart [11,24,27,29]; 2) nine standard fetal echocardiography views can be automatically generated in 96-100% of normal fetal heart cases [11,25,26]; 3) color Doppler FINE provides clinically useful information about cardiac structure and function in both normal and abnormal fetal hearts [30,31]; and 4) FINE has high sensitivity (98%) and specificity (93%) for the detection of CHD, and is therefore considered a cardiac screening and diagnostic tool in the clinical setting [32].
Color Doppler FINE is a recent technological advance that allows STIC volume datasets to be acquired in combination with color or bidirectional power Doppler (known as S-flow) information, so that fetal echocardiography views generated by FINE can be displayed with either modality (Table 1) [30]. Color Doppler flow mapping allows identification of cardiac structures and vasculature, as well as the pattern and direction of blood flow throughout the heart [30,33]. Color Doppler FINE has also been successfully integrated into several ultrasound platforms (UGEO WS80A and HERA W10; Samsung Healthcare, Seoul, Korea), and is commercially known as 5D Heart Color.
Recently, other investigators have demonstrated that the FINE method: 1) has a high success rate in generating three specific abnormal cardiac views in cases of fetal D-transposition of the great arteries, and can therefore be used to screen for this cardiac defect [34]; 2) is a reliable and easily learned method [35]; and 3) is comparable in accuracy to conventional 2D fetal cardiac examination in normal second trimester fetuses, and is also characterized by a significant reduction in examination time [36].
With the overall aim of improving the FINE method further, eight new and advanced features have now been developed and the objective of this article is to describe them herein (Table 2). Such features can be categorized based upon their broad goals: 1) to simplify the method: a) Auto fetal positioning (or FINE align); b) Skip points; c) Predictive cursor; d) Static mode volume; e) Breech sweep; 2) to allow quantitative cardiac measurements: f) Automatic cardiac axis; g) Cardiac biometry; and 3) to improve the success of obtaining fetal echocardiography view(s): h) Maestro planar navigation. However, before reviewing such new technologies, it is necessary to briefly describe the FINE method.
Table 2.
New and Advanced Features of Fetal Intelligent Navigation Echocardiography (FINE) Method
| Category | Feature | Purpose |
|---|---|---|
| Simplify FINE method | 1. Auto Fetal Positioning (or FINE Align) | • Automatically reorients and standardizes the STIC volume dataset so that fetal spine is consistently placed at 6 o’clock position • Realigns STIC volume dataset to correct a fetal drifting spine |
| 2. Skip Points and Predictive Cursor | • Simplifies the marking process of anatomical structures via Anatomic Box® by automatically placing cursor near or at the location to be marked • Can choose to rely on predictive cursor placement and “skip points” (vs. intentionally marking anatomical structures as before) |
|
| 3. Static Mode Volume | • Three-dimensional static volume obtained via a very rapid acquisition time (i.e. 1 second) and is characterized by a high frame rate ○ Resulting nine echocardiography views are static and without motion |
|
| 4. Breech Sweep | • Activated for breech presentations only • Motor array within volumetric transducer switches directions and automatically sweeps over fetal ROI so that fetal upper mediastinum is seen at the start of sweep, and stomach at end of sweep |
|
| Quantitative cardiac measurements | 5. Automatic Cardiac Axis | • Fetal cardiac axis calculated and depicted automatically in the four-chamber view diagnostic plane • Measures the angle formed between ventricular septum and a line drawn between crux and cross-section of aorta in four-chamber view (i.e. Ao-crux-apex) |
| 6. Cardiac Biometry | • Measurements obtained via electronic calipers (i.e. distance, elliptical circumference) applied to cardiac diagnostic planes | |
| Improve success of obtaining fetal echocardiography views | 7. Maestro Planar Navigation | • Fine-tune navigation of a single cardiac diagnostic plane at a time (without affecting the other eight diagnostic planes) ○ Parallel shift; x, y, z rotations • No multiplanar display or reference dot tool depicted |
ROI, region of interest; STIC, spatiotemporal image correlation
For the images used in this report, patients were examined at the Detroit Medical Center/Wayne State University and the Perinatology Research Branch of NICHD, NIH, DHHS. All women had been enrolled in research protocols approved by the Institutional Review Board of NICHD, NIH, and by the Human Investigation Committee of Wayne State University. All participants provided written informed consent for the use of sonographic images for research purposes.
FINE Method and Characteristic Features
Using STIC technology, after a 4D volume dataset of the fetal heart has been acquired from an apical four-chamber view, FINE will automatically convert the volume into a 2D cine loop available for assessment (i.e. STICLoop™) [11]. Such cine loop scrolls in a continuous fashion, and was developed to aid the user in determining the appropriateness of STIC volumes before applying the FINE method to such volumes. Using STICLoop™, the operator observes whether or not certain criteria are met (e.g. minimal or absent shadowing) (Table 1). This step is beneficial, because FINE may not be successful in generating fetal echocardiography views if the STIC volume: 1) is characterized by inadequate quality; 2) does not contain information about the echocardiography view(s) (e.g. upper mediastinum is not contained within the volume); and 3) is not acquired from a true four-chamber view (e.g. proper alignment in the axial plane) [11]. High quality STIC volume datasets are necessary so that they are informative (i.e. able to display cardiac planes and structures) [25, 37]. Therefore, by examining the automatically scrolling STICLoop™, one can determine the presence or absence of these issues.
Once an appropriate STIC volume has been selected for analysis, the operator then marks (or clicks) seven anatomical structures of the fetal heart in sequential order using the Anatomic Box® tool (Figure 1, Supplementary Video S1). The marked structures or points within the volume dataset generate spatial coordinates that allow reliable and accurate reconstruction of the heart (i.e. geometric modeling) that “fits” the anatomy of a given fetus under examination. As a result, the successful display of cardiac planes occurs in the presence of anatomical variability (e.g. geometry and cardiac axis), as well as different gestational ages [24].
Figure 1: Seven anatomical structures within the heart are marked using the Anatomic Box® tool.
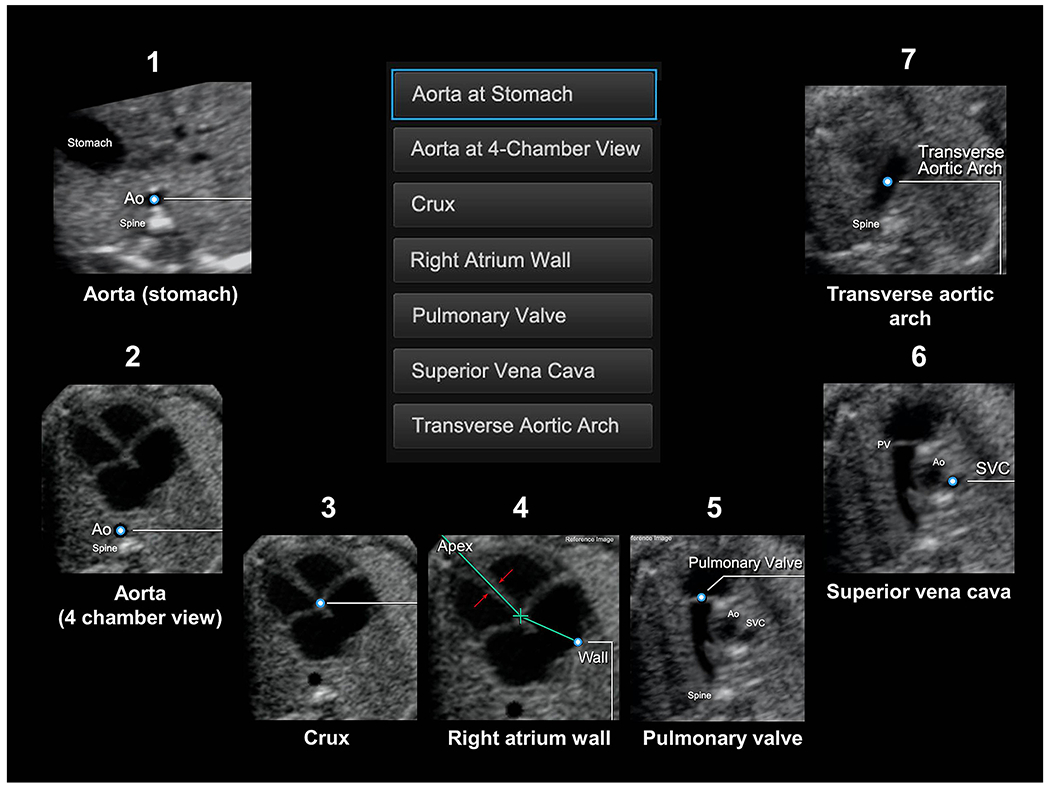
(also see Videoclip S1): 1) cross-section of the aorta at the level of the stomach; 2) cross-section of the aorta at the level of the four-chamber view; 3) crux; 4) right atrial wall; 5) pulmonary valve; 6) cross-section of the superior vena cava; and 7) transverse aortic arch. Ao, aorta; PV, pulmonary valve; SVC, superior vena cava.
After marking of structures is completed, FINE will automatically and immediately rotate, align, dissect, and scale the STIC volume dataset to display nine cardiac views (i.e. diagnostic planes) as a cine loop of a complete single cardiac cycle in motion (Figure 2, Supplementary Video S2). The diagnostic planes are displayed simultaneously in the same template approximately three seconds after marking is completed [11]. The nine planes are: 1) four chamber; 2) five chamber; 3) left ventricular outflow tract; 4) short-axis view of great vessels/right ventricular outflow tract; 5) three vessels and trachea; 6) abdomen/stomach; 7) ductal arch; 8) aortic arch; and 9) superior and inferior vena cava. An innovative feature of FINE is that automatic labeling occurs for the nine diagnostic planes, left and right fetal sides, cranial and caudal ends, as well as anatomical structures (Figure 2). Such labeling stays with the corresponding anatomical structure(s), even as the image is increased or decreased in size (Supplementary Video S2). Moreover, the labeling of anatomical structures is not a standard template overlay applied to the cardiac diagnostic planes, but rather is always unique to a given fetus.
Figure 2: Application of the FINE method to a fetus with a normal heart (spatiotemporal image correlation volume).
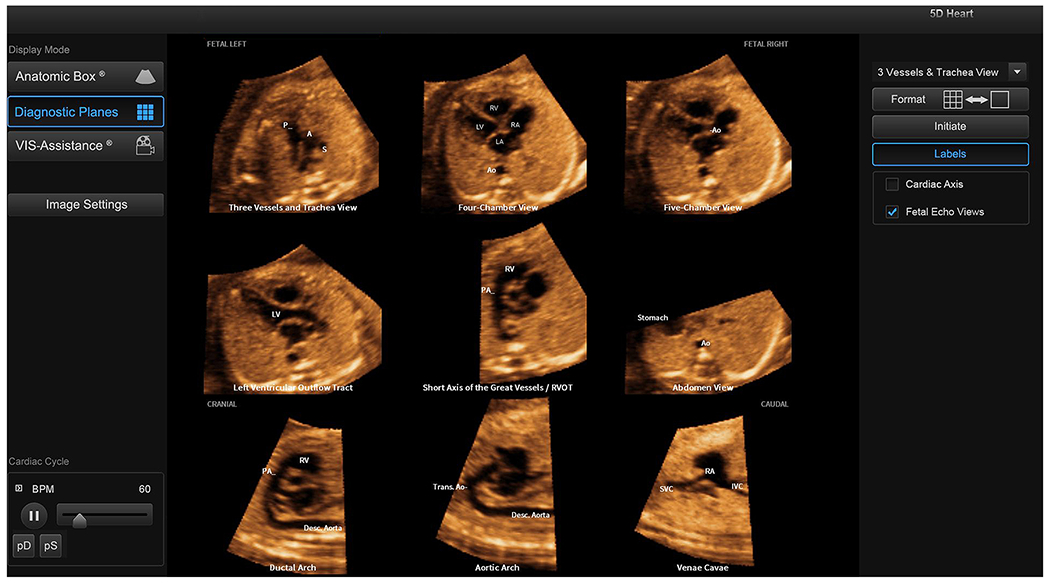
Nine normal cardiac diagnostic planes in a single template are shown with the unique feature of automatic labeling (through intelligent navigation) of each plane, anatomic structures, fetal left and right sides, and cranial and caudal ends (also see Video S2). The labeling is distinctive because it stays with the corresponding anatomical structure(s), even as the image is increased or decreased in size. A, transverse aortic arch; Ao, aorta; Desc., descending; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; P, pulmonary artery; PA, pulmonary artery; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract; S, superior vena cava; SVC, superior vena cava; Trans., transverse
Intelligent navigation technology automatically realigns the STIC volume dataset, and reorients and standardizes the anatomical position, so that the fetus, diagnostic planes, and anatomical structures are displayed consistently in the same manner each time, regardless of the fetal position or initial orientation [11,24], allowing their relationships to be more easily recognized as normal or abnormal.
Upon the development of FINE, we recognized that the complex anatomy of the fetal heart and its anatomical variations would require additional interrogation of cardiac views [11]. Therefore, we developed a novel video clip tool known as Virtual Intelligent Sonographer Assistance (VIS-Assistance®), which allows operator-independent (automatic) sonographic navigation and exploration of surrounding structures in a given cardiac diagnostic plane (Supplementary Video S3). Such “virtual” sonographer has several objectives (Table 1): 1) improve the success of obtaining the fetal echocardiography view of interest; 2) provide more information about the diagnostic plane and its surrounding structures (e.g. pulmonary veins); and 3) improve the quality of examination, since the complexity of the fetal heart can be studied in greater detail and specific structures shown. VIS-Assistance® has distinct advantages compared to 2D sonography or manual navigation of STIC volumes (Table 1) [24], and is especially informative in cases of CHD [27,32].
To assist sonologists in recognizing potential issues for a given STIC volume dataset, intelligent alerts were developed as part of the FINE method [26]. These alerts are composed of captions and/or a movie which automatically appear either during the marking of anatomical structures of the fetal heart, or in specific situations (Table 1). There are three types of intelligent alerts: 1) Breech alert: notifies the user that the fetus appears to be in a breech presentation. A question appears that will ask if the STIC volume can be reoriented as if the fetus is in a “vertex” presentation. If the user clicks “Yes”, then the system will automatically realign the volume dataset, reorient, and standardize the anatomical position so that the fetus is “converted” to a vertex presentation; 2) Possible drifting spine alert: notifies the user that there may be a “drifting” fetal spine in the STIC volume dataset (i.e. when the spine location migrates on the screen) (Supplementary Video S4). Further information about drifting spines is provided below. The caption states that marking of anatomical structures may be difficult, and successful visualization of echocardiography views may be affected; and 3) Spine location alert: notifies the user that the fetal spine appears to be located at a position (e.g. 9 o’clock) that is different from what is recommended (i.e. between 5 and 7 o’clock) (Supplementary Video S5) [26]. The caption states that this STIC volume is not recommended, and the spine location may lead to shadowing and suboptimal image quality.
Three marking alerts (i.e. pulmonary valve, superior vena cava, and transverse aortic arch) were also developed to notify the user that fetal anatomical structures for marking may be in a different location than expected, because the fetal spine is located outside the 5 to 7 o’clock range. Therefore, such alerts (i.e. captions/movies) occur during the marking process and only emerge after a spine location alert has been activated (Table 1). The goal is to depict a reference image example (in the movie) that will teach the sonologist where to mark the anatomic structure(s) of interest. In our experience, the appearance of intelligent and marking alerts can be unsettling for sonologists, and generally leads to self-improvement in the acquisition of STIC volumes to avoid the appearance of future alerts.
New and Advanced Features of the FINE Method
A. Simplify the FINE Method
1). Auto Fetal Positioning (or FINE Align)
Among the STICLoop™ criteria (Table 1), there are currently two situations that will cause intelligent alerts to appear: 1) fetal spine not located between the 5 and 7 o’clock positions; and 2) presence of a fetal drifting spine from the four-chamber view down to the stomach. Both scenarios will lead to difficulty in marking anatomical structures using Anatomic Box®. Therefore, to optimize the chances for FINE to be successful, it is recommended that these two criteria are met, and the reasons for this will now be discussed.
During STIC volume acquisition, if the fetal spine is located posteriorly (i.e. between 5 and 7 o’clock), this minimizes acoustic shadowing from the ribs or spine so that cardiac views may be visualized and assessed [23,25,38]. Investigators have reported how a fetus lying supine can easily be accomplished during routine sonographic examinations [12,15,25]. Moreover, techniques have been reported to proactively alter the true fetal spine position or “convert” the fetal spine to a posterior position on the ultrasound monitor screen [22,23].
Yet, in some circumstances, the fetal spine will remain outside the 5 to 7 o’clock region, thus triggering the spine location alert and three subsequent marking alerts. For every fetus whose spine is at a different clock time, the same cardiac anatomy will be in a different location(s), which may lead to difficulty in marking anatomic structures for FINE. This is because interpretation of sonographic images depends upon pattern recognition, and the more permutations of the same cardiac plane the sonologist needs to remember, the more challenging is the interpretation [24]. This concept is demonstrated in the superior vena cava marking alert (Figure 3), which shows the location and orientation of the superior vena cava when the fetal spine is located at 6 o’clock, and then at 3 o’clock. While the information contained in both images is identical (i.e. anatomical structures and their relationships with each other), the sonologist may experience difficulty in understanding the location of the superior vena cava in the latter situation.
Figure 3: Superior vena cava alert.
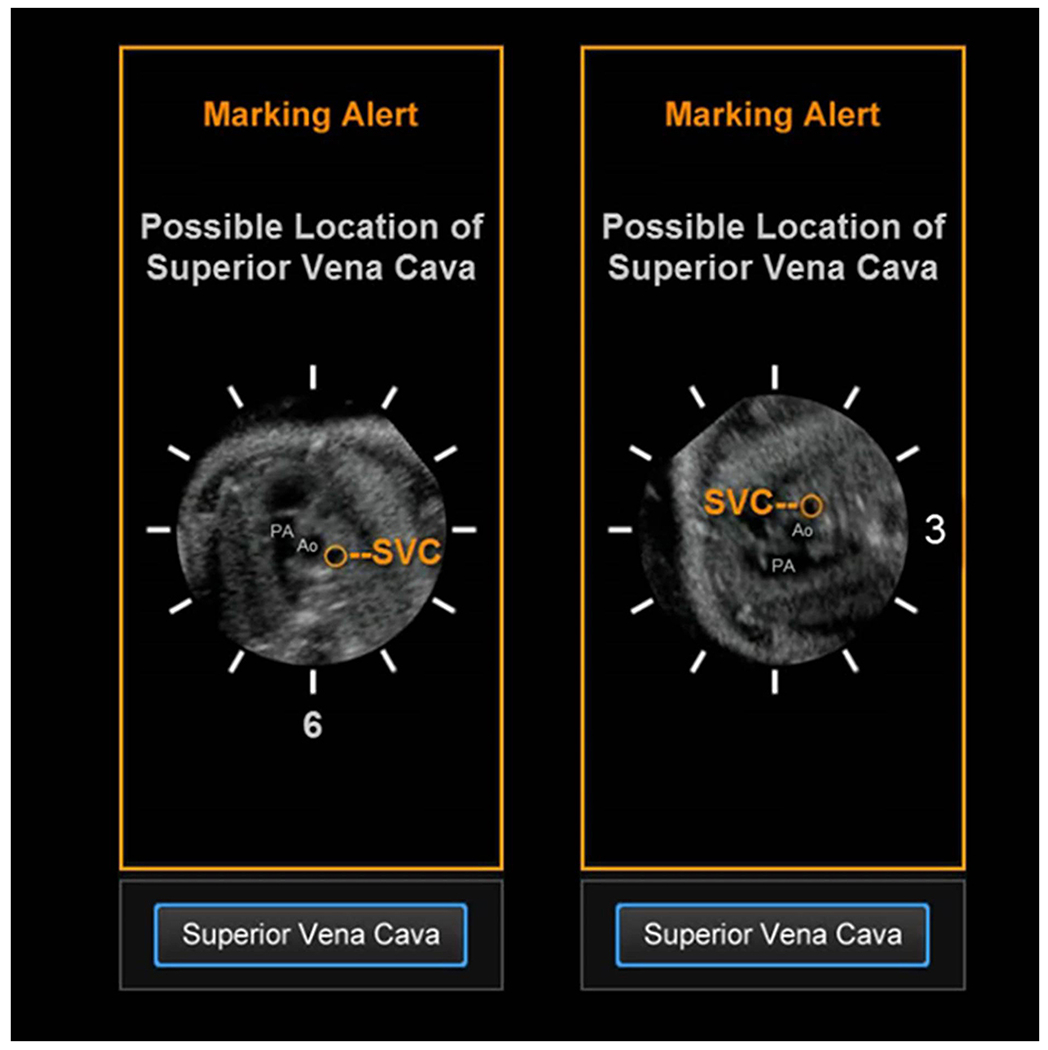
This type of marking alert notifies the user that the superior vena cava may be in a different location that what is expected. This is because the fetal spine in the STIC volume dataset being analyzed by FINE is at 3 o’clock. Thus, there may be difficulty in marking the cross-section of the superior vena cava using Anatomic Box®. This alert includes a reference movie that will automatically play and depicts the orientation/position of the pulmonary artery (PA), aorta (Ao), and superior vena cava (SVC) when the spine is at 6 o’clock (left image) and 3 o’clock (right image). The movie is intended to help the user recognize that for their STIC volume, the superior vena cava may be in a location similar to that depicted in the movie.
A drifting spine (from the four-chamber view down to the stomach) is defined when the fetal spine location “migrates” on the monitor screen during the STICLoop scroll [11,25,26]. There are two types of drifting spines: 1) horizontal (spinal ossification centers will appear to be moving in a lateral direction, either left or right) (Supplementary Video S4); and 2) vertical (spinal ossification centers will appear to be moving in a vertical direction, either up or down). The issue with drifting spines is that anatomical structures may be out of the plane of sight, leading to difficulty in marking them. In contrast, when sequential axial planes are parallel to each other (similar to a sliced loaf of bread) in a STIC volume dataset, there will be no drifting spine [23]. This situation occurs when during a STIC volume acquisition sweep, the transducer beam is exactly perpendicular (vs. oblique or tilted) to a fetus lying completely supine at zero degrees in a longitudinal lie. On the monitor screen, the three spinal ossification centers as a group will be visible in each serial transverse plane and located at the same area or point on the screen [23].
Auto fetal positioning (or FINE align) is a new feature of FINE that is activated in the two situations described above: 1) fetal spine located outside the 5 to 7 o’clock range, leading to the spine location alert; and/or 2) presence of a fetal drifting spine (either horizontal or vertical) from the four-chamber view down to the stomach, leading to the possible drifting spine alert. Auto fetal positioning affects the STIC volume dataset itself, occurs during the marking process, and will automatically: a) reorient and standardize the volume so that the fetal spine will consistently be placed at 6 o’clock; and/or b) realign the STIC volume dataset to correct a drifting spine (Table 2).
The FINE marking process will be simplified, since the fetal anatomical structures for marking will be: 1) consistently displayed in the same manner each time (i.e. spine moved to 6 o’clock); 2) thus, more easily recognized by the sonologist; and/or 3) brought into the plane of sight (i.e. drifting spine is corrected). Therefore, for any given fetus, each structure(s) should always be in the same location on the screen, and similar to what is depicted in the reference example images of Anatomic Box®. Another hidden benefit is that the accuracy of predictive cursor placement (see below) is improved, since anatomic structure locations are consistent among fetuses.
Auto fetal positioning will be activated after the third fetal structure (i.e. crux) is marked (clicked). A Volume Orientation message will appear (Figure 4, Supplementary Video S6) on the screen asking whether volume reorientation to a spine at 6 o’clock should occur (YES or NO). If the user clicks YES, reorientation will visibly occur, and the user will be asked to “Please mark crux again”, which should be performed (Supplementary Video S6). The consequence is that no marking alerts will appear. However, if the user clicks NO, the marking process will continue as usual (i.e. mark the right atrial wall), and the three marking alerts will now appear in sequence.
Figure 4: Auto fetal positioning in a fetus with the spine between 7 and 8 o’clock.
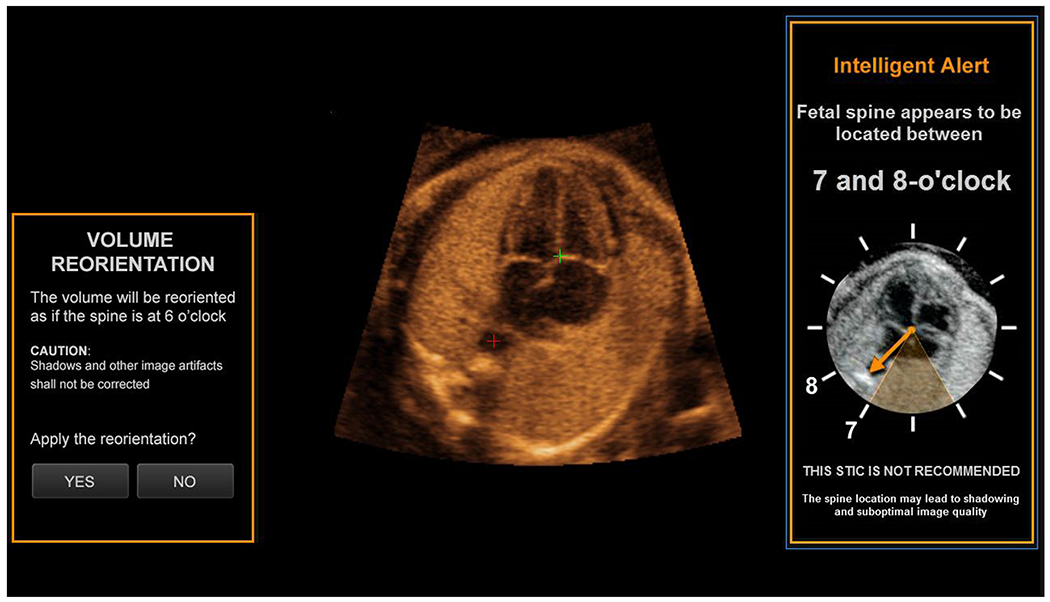
This new feature of FINE is activated automatically after the crux is marked, because the fetal spine is located outside the 5 to 7 o’clock range, leading to the spine location alert depicted (7 to 8 o’clock). A Volume Orientation message appears on the screen, asking whether volume orientation to a fetal spine at 6 o’clock should occur (YES or NO). If the user clicks YES, reorientation will visibly occur (not shown), and the user will be asked to “Please mark crux again”, which should be performed (also see Video S6).
It is noteworthy that Auto fetal positioning will artificially place the spine at 6 o’clock for marking; however, at the time of STIC volume acquisition, the fetus originally had a different spine location. Therefore, this feature will not “correct” inherent issues, such as acoustic shadowing or dropout from the ribs or spine. Such issues, along with any artifacts (e.g. motion), that were already captured during volume acquisition can never be “erased”, and may obscure visualization of fetal cardiac anatomy, structures, and views [23]. All cardiac views depicted via FINE are related to the original quality of the STIC volume dataset. Therefore, it is important that when performing the FINE method, the goal should still be to acquire high quality and appropriate volumes so that they are informative (i.e. able to display cardiac planes and structures) [25,37]. In other words, the availability of the Auto fetal positioning tool should not allow abandonment of such goal. Indeed, Veronese et al. reported the prevalence of spine location and possible drifting spine alerts in only 8.5% (n=21) and 0.8% (n=2) of their 246 cases, respectfully [26]. This indicates that the investigators acquired appropriate STIC volume datasets in the vast majority (>90%) of their patients, and that this performance can be achieved successfully.
The interested reader is referred to prior reports, which review in detail how to acquire STIC volumes for sonographic examination of the fetal heart [22,23].
2). Skip Points and Predictive Cursor
Currently, FINE helps sonologists to correctly identify and appropriately mark the seven anatomical structures of the fetal heart in the following manner: 1) a menu and reference image(s) are displayed for each anatomical structure to be marked, and the order of marking is specified (Figure 1, Supplementary Video S1); 2) the system automatically scrolls through the STIC volume to the level of the most likely location of the anatomical structure to be marked; and 3) FINE recognizes the cardiac phase, which facilitates marking of anatomical structures (e.g. automatically closes the atrioventricular valves) [24,26]. Users may need to perform minor scrolling adjustments to mark anatomical structures appropriately.
In some instances, there may be difficulty with the marking process (e.g. lack of familiarity with fetal anatomy or poor visualization). In such cases, FINE may not be successful in generating informative cardiac views (e.g. unobtainable or incorrect) [11,24]. One approach to address this is by activating VIS-Assistance®, which improves the success of obtaining the fetal cardiac view of interest when not initially obtained via the diagnostic plane (Table 1).
Yet, another alternative now available is to Skip points and instead, rely on Predictive cursor (or Intelligent cursor) placement by intelligent navigation technology, rather than relying on user judgement [29]. This technological advance simplifies the marking process by automatically placing the cursor near, or at the location to be marked [29]. Predictive cursor placement is available only for the last four anatomical structures to be marked: 1) right atrial wall; 2) pulmonary valve; 3) cross-section of superior vena cava; and 4) transverse aortic arch. Therefore, a user can now choose to rely on predictive cursor placement and “skip points” for one or more of these 4 structures, rather than intentionally marking them as before (Supplementary Video S7). The end result is an improvement in workflow speed and decreasing operator dependency.
When should a sonologist rely on Skip points and Predictive cursor placement? The following scenarios apply: 1) lack of familiarity with anatomy or uncertainty of where to mark structures; 2) suboptimal visualization or unclear structures; and 3) marking of the pulmonary valve when a static mode volume has been acquired (see below). Essentially, relying on predictive cursor placement through intelligent navigation technology may yield better results than using incorrect judgement or guessing a structure’s location, and subsequently marking the wrong area within the STIC volume.
Yet, implementing such technology may not be appropriate for some types of CHD, or if the overall image quality is poor. In the latter case, although predictive cursor placement may be accurate, the resulting cardiac views may still be uninterpretable and uninformative. For cases of CHD, predictive cursor placement may be incorrect due to the abnormal anatomy. Therefore, marking all seven anatomical structures as usual is recommended.
What if predictive cursor technology is implemented, and the resulting cardiac diagnostic plane(s) are not appropriate? There are three possible options to resolve this issue (besides choosing another STIC volume): 1) apply VIS-Assistance® to the diagnostic plane; 2) mark all seven anatomical structures based on operator judgment; or 3) maestro planar navigation (see later description).
3). Static Mode Volume
The FINE method was originally developed to interrogate STIC volume datasets using intelligent navigation [11,24]. The main advantage of 4D STIC technology is that it displays a cine loop of a complete single cardiac cycle in motion. Therefore, analysis of cardiac planes can occur at any specified time during the cardiac cycle (e.g. systole, diastole) by interrogating the volume dataset frame-by-frame [22]. Moreover, STIC volumes can be acquired in combination with color or bidirectional power Doppler information, so that fetal cardiac views generated by FINE can be displayed with either modality [30].
Yet, obtaining STIC volume datasets requires setting an acquisition time (e.g. 5 to 15 seconds). The more images that are stored per acquisition period, the greater the number which are available for volume reconstruction, and the better the image resolution [23]. Therefore, the longer the acquisition time (or duration of acquisition), the greater the number of frames obtained for the STIC volume dataset. However, disadvantages include: 1) a higher likelihood of introducing artifacts related to fetal breathing/motion into the volume dataset (which can compromise quality) [23,39]; and 2) some patients have difficulty remaining immobile and holding their breath during the acquisition timeframe. When fetuses are very active (i.e. breathing and/or motion), this can pose great challenges in acquiring volume datasets without motion artifacts and which are high quality.
Therefore, with the goal of establishing an acquisition time (or transducer sweep speed) that optimizes image resolution but minimizes the introduction of artifacts, a static mode volume was developed for the FINE method. This is a three-dimensional (3D) static volume obtained via a very rapid acquisition time (i.e. 1 second), and characterized by a high frame rate, which leads to a dataset with improved quality. The array in the volumetric transducer performs an automatic single sweep over the fetal heart, and then the FINE method is applied to this static mode volume. The resulting nine echocardiography views are static and without motion (Figure 5, Supplementary Video S8). One advantage is that the image resolution of the sagittal planes (e.g. aortic arch) generated by FINE may show an improvement in quality compared to that of STIC volume datasets.
Figure 5: Application of the FINE method to a fetus with a normal heart (static mode volume) and maestro navigation of cardiac diagnostic planes.
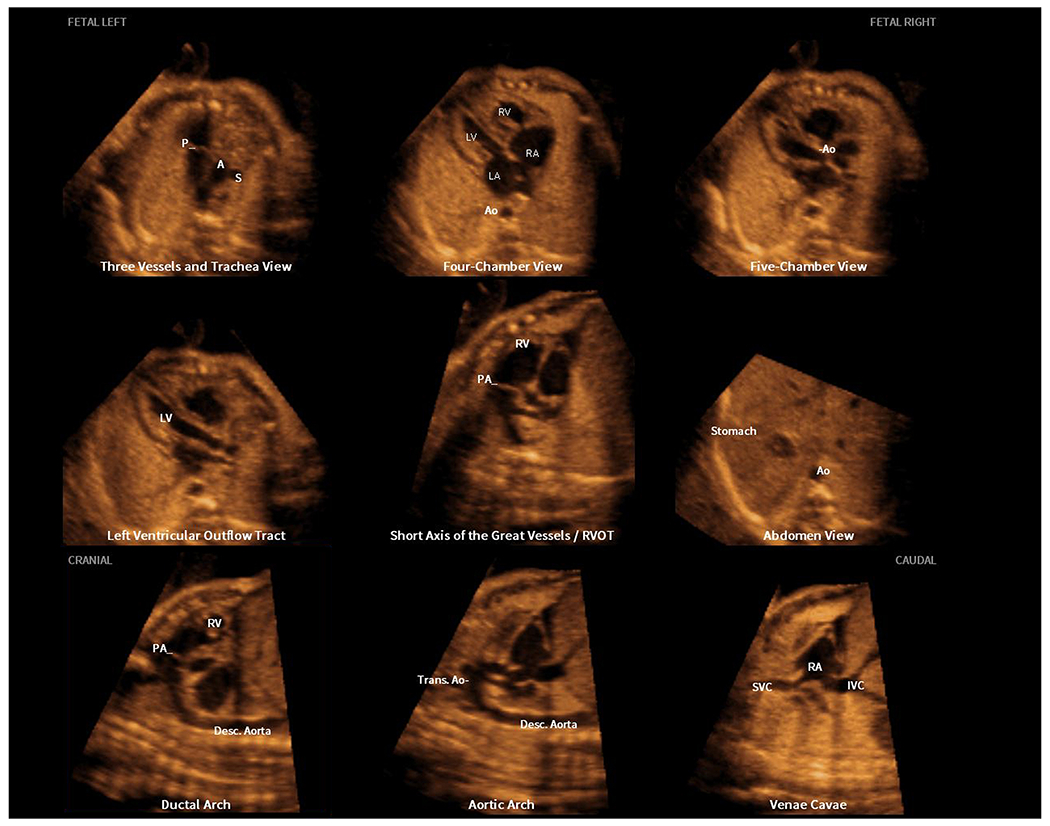
A static mode volume was acquired in which the fetal spine was originally located between 4 and 5 o’clock. Therefore, a spine location alert and Volume Orientation message appeared on the screen. Reorientation occurred so that the fetal spine was placed at 6 o’clock. The marking process continued, and nine normal cardiac diagnostic planes in a single template were depicted by FINE. Next, maestro navigation was used to fine-tune three planes (one at a time): 1) left ventricular outflow tract; 2) short axis of the great vessels / right ventricular outflow tract; and 3) ductal arch. The resulting nine cardiac diagnostic planes with the unique feature of automatic labeling (through intelligent navigation) of each plane, anatomic structures, fetal left and right sides, and cranial and caudal ends are depicted (also see Video S8). A, transverse aortic arch; Ao, aorta; Desc., descending; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; P, pulmonary artery; PA, pulmonary artery; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract; S, superior vena cava; SVC, superior vena cava; Trans., transverse
The main advantage of static mode volume acquisition is that even in the presence of fetal motion, hiccups, or maternal breath hold issues, it may still be possible to capture an informative volume. However, this action should be well timed by the sonologist. Since the acquisition speed is very rapid, motion as well as other artifacts should be reduced or avoided. The overall time required to perform the FINE method can also be decreased, since time spent on acquiring volume datasets is also shortened. Cardiac views may be reviewed while the patient is still present, and therefore, additional volumes may be acquired as necessary. Images may also be reviewed in the patient’s absence.
For static mode volumes, the region of interest (ROI) box is still set around the entire fetal chest circumference, so that it contains all the anatomic information of the fetal heart. The ROI box determines the height (y-plane) and width (x-plane) of the volume dataset. The fetal heart should occupy the maximum proportion of the image to be acquired, and the acquisition plane is the apical four-chamber view. Although there is no acquisition time to set, the acquisition angle should still be determined prior to obtaining the volume. Such angle determines the acquisition depth; therefore, a wide acquisition angle is equivalent to a longer distance covered during the transducer sweep. It is imperative that the angle be programmed to a minimum value, such that all (and only) the fetal anatomic structures of interest are encompassed in the sweep (i.e. upper mediastinum through the stomach). A good rule of thumb is to set the acquisition angle at least 5 degrees more than the gestational age; however, the angle should not be too wide, since this can actually decrease image resolution. Therefore, depending on whether the fetus is smaller or larger in size, the angle will need to be adjusted.
There are some limitations when applying the FINE method to static mode volume acquisitions. When fetal cardiac volumes are acquired using a static 3D approach, neither the heart rate nor motion is considered during acquisition [22]. Therefore, this cannot be used to assess events related to the cardiac cycle (e.g. diastole or systole), as well as valvular and myocardial wall motion. Indeed, the static mode volume is not synchronized with the fetal heart; therefore, it is unknown if the resultant cardiac images generated by FINE are related to diastole, systole, or something in between. Moreover, because the sweep captures cardiac structures randomly within the cardiac cycle, measurements of cardiac and outflow tract dimensions at a specific phase (e.g. end diastole) cannot be performed. A second limitation is that with static mode volumes, FINE cannot incorporate cardiac phase recognition to facilitate marking of anatomical structures. Specifically, the atrioventricular and pulmonary valves may be open, and therefore, it may be more difficult to mark the crux and/or pulmonary valve using Anatomic Box®. One way to address this is to rely on predictive cursor placement for marking these areas. Another alternative is to examine more than one static mode volume to determine which is most optimal for marking.
In general, we do not recommend obtaining static mode volumes in the presence of regular and frequent fetal breathing or very active fetuses, since this may still result in artifacts being introduced within the volume dataset. A suggested overall approach when performing FINE is that STIC volume datasets should initially be obtained, due to the advantages described above. However, if this is not possible, or fetal movements/breathing preclude obtaining appropriate STIC volume datasets, static mode volume acquisitions may be attempted, and FINE applied to such volumes. In our experience, at least several static mode volumes should be obtained, to increase the likelihood that it will be informative (i.e. able to display cardiac planes and structures). Similarly to 4D sonography with STIC and also conventional 2D sonography, the quality of cardiac images derived from static mode volumes will still be affected by the presence of shadowing, maternal habitus, and other factors. Therefore, STICLoop™ criteria (Table 1) are still relevant for these volumes. For example, the FINE method in combination with static mode volumes may not successfully generate fetal echocardiograpy views if the [11]: 1) quality of the original volume dataset is inadequate; 2) volume does not contain information about the echocardiography view(s) (e.g. a narrow angle of acquisition that does not include the upper mediastinum will not depict the three vessels and trachea view using FINE); and 3) volume has not been acquired from an appropriate four-chamber view [23] (true cross-section of the fetal thorax, proper alignment in the axial plane).
4). Breech Sweep
Once a STIC volume has been activated, the array within the volumetric transducer begins an automatic single sweep over the ROI [22]. During such sweep, the volume dataset comprises thousands of 2D images acquired through this area of interest. Setting higher acquisition times (e.g. 15 vs. 7 seconds) is optimal, since there will be a greater number of frames obtained for the STIC volume dataset. For the FINE method, the starting 2D plane for the STIC volume acquisition (i.e. acquisition plane) should be the four-chamber view [23], which is the sonographic plane most easily obtained in the fetal heart [40]. Once an acquisition plane has been obtained by the sonologist, and the acquisition angle set (e.g. 30 degrees), the STIC volume dataset is initially created by a mechanical sweep of the beam 15 degrees from this acquisition plane. The acquisition process then starts, coming back toward the acquisition plane and then continuing for another 15 degrees, to create a total volume of information of 30 degrees [23]. Thus, a total sweep of 30 degrees cranial and caudal to the apical four-chamber view is obtained, in which the acquisition plane is located in the middle of the sweep box.
As the actual STIC volume sweep is being obtained via the transducer, sequential images on the ultrasound monitor screen are displayed. For a four-chamber view acquisition plane and vertex fetal presentation, if the acquisition angle is wide enough, the fetal upper mediastinum (start of the sweep) and the stomach (end of the sweep) will be visible on the screen. However, for a four-chamber view acquisition plane but breech fetal presentation, the opposite is the case; instead, the stomach will be seen at the start of the sweep, and the upper mediastinum will be seen at the end of the sweep. For both scenarios, if all conditions are identical (except for the fetal presentation), equivalent anatomical information will be contained within the STIC volume dataset. However, because the transducer sweep starts from different anatomical locations of the fetus, there is a higher likelihood that fetal motion/breathing will affect cardiac images in breech presentations. Therefore, in reality, the quality of STIC volumes may differ depending upon the fetal presentation.
Why is this the case? In practice, at the exact moment a sonologist presses the machine button to activate the STIC volume sweep in a vertex fetus, the fetus will probably be quiescent (otherwise the volume acquisition would not have been performed). Therefore, cardiac views (e.g. three vessels and trachea, four-chamber view) are unlikely to be affected by breathing/motion artifacts. Even if they occur by the time the sweep reaches the stomach/abdominal area (e.g. 8 seconds later), this is unlikely to affect the cardiac views generated by FINE. Yet for the breech fetus, the opposite is the case. In the quiescent fetus, the transducer sweep will start at the stomach/abdomen. However, as the acquisition time passes, there is an increased likelihood that fetal breathing/motion artifacts will appear, and this could coincide with a sweep occurring of the cardiac structures. Therefore, this could affect the cardiac views generated by FINE.
To address this matter, a novel technology was introduced on ultrasound machines, and is known as breech sweep. If a fetus is breech, and the sonologist plans to acquire a STIC volume dataset for analysis by FINE, the breech sweep button can be pressed before activating the acquisition sweep. The motor array within the volumetric transducer will switch directions and automatically sweep over the fetal ROI so that the fetal upper mediastinum is seen at the start of the sweep, and the stomach at the end of the sweep. However, although the sweep itself has been changed, the fetus is still in a true breech presentation. Therefore, when marking anatomical structures using Anatomic Box®, the breech alert (see above) will still be activated by FINE. In summary, the goal of breech sweep is to minimize the probability that fetal breathing/motion artifacts will coincide with the transducer sweep of cardiac structures, which could adversely affect the cardiac images generated by FINE.
B. Quantitative Cardiac Measurements
1). Automatic Cardiac Axis
Fetal cardiac axis and position can be evaluated easily even if there is poor visualization of detailed cardiac structures [41]. Evaluation of the cardiac axis during the mid-second and third trimesters is important in both fetal cardiac screening and echocardiography exams [42,43]. Normally, the cardiac apex points to the left by 45 ± 20 (2 standard deviations) degrees in relation to an anteroposterior line drawn from the spine to the anterior chest wall, so that the chest is divided into right and left halves equally [44]. These values are derived from the angle the interventricular septum makes with such line. Comstock found that the fetal cardiac axis did not vary with gestational age (13-40 weeks).
An abnormal fetal cardiac axis increases the risk of CHD (especially involving the outflow tracts) [41,45,46], as well as chromosomal abnormalities [47,48]. Shipp et al. reported that of their 75 fetuses with CHD, the mean cardiac axis was 56 ± 13 degrees (vs. 43 ± 7 degrees in normal fetal hearts; P<0.001) [45]. The authors proposed that a fetal cardiac axis greater than 57 degrees may be an indirect clue to conotruncal or great vessel abnormalities. Indeed, Zhao et al. reported that 45% (27/60) of fetuses with tetralogy of Fallot had an abnormal cardiac axis (>65 degrees), and this is attributed to over-rotation of the bulboventricular loop, along with other mechanisms [49]. Taken together, including assessment of the fetal cardiac axis with the four-chamber view may improve the ability to screen for CHD in low-risk populations [41].
A new feature of the FINE method is calculation and depiction of the fetal cardiac axis automatically (known as automatic cardiac axis). However, it differs by intelligently measuring the angle formed between the ventricular septum and a line drawn between the crux and cross-section of the aorta in the four-chamber view (i.e. Ao-crux-apex) (Figure 6). If the ventricular septum is located to the left or right of such line, the fetal cardiac axis angle will be positive or negative, respectively (where zero degrees is a vertical line and 90 degrees is a horizontal line). After marking the seven anatomical structures using Anatomic Box®, the cardiac axis will be depicted automatically in the four-chamber view diagnostic plane.
Figure 6: Automatic cardiac axis.
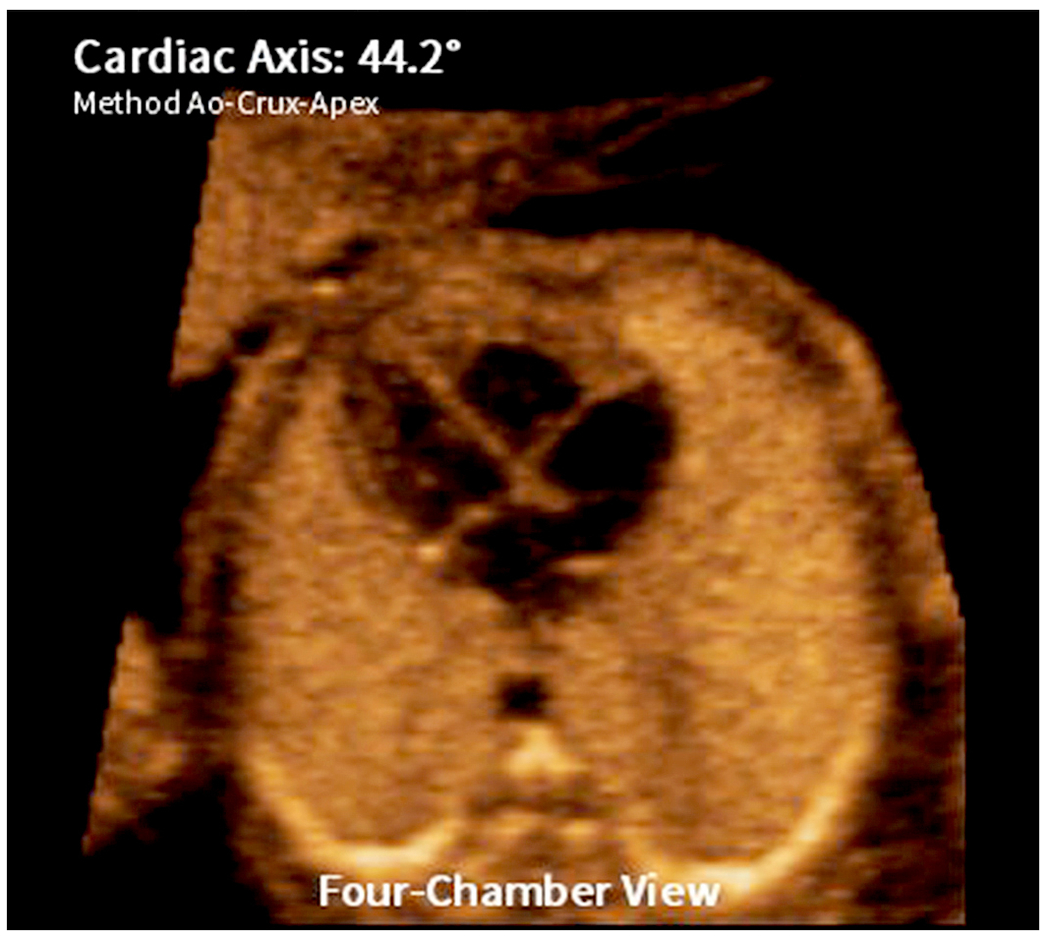
This new feature of FINE allows calculation and depiction of the fetal cardiac axis automatically in the four-chamber view diagnostic plane. Through intelligent navigation technology, the angle is automatically measured between the ventricular septum and a line drawn between the crux and cross-section of the aorta in the four-chamber view (depicted as “Method Ao-Crux-Apex” in the image). In this normal fetal heart, the cardiac axis is 44.2 degrees (normal).
Automatic cardiac axis has recently been evaluated in the clinical setting [50,51]. Weichert et al. conducted a retrospective cohort study in which more than 1500 second/third trimester STIC volumes were analyzed using FINE [50]. The cardiac axis in normal controls was 38.6 degrees (range 15.3 to 53.7 degrees). Significant differences from the normal cardiac axis were reported for conotruncal anomalies, as well as right and left heart defects [50]. In another study, investigators applied the FINE method to STIC volume datasets to calculate the cardiac axis in 160 fetuses with major CHD [51]. In approximately 80% of cases, an abnormal cardiac axis was detected (normal was defined as 40-45 degrees). Taken together, automatic cardiac axis derived from the FINE method may be a valuable tool in the identification of fetuses with CHD.
2). Cardiac Biometry
Measuring fetal cardiac biometry on ultrasound should be considered when there is a suspicion of structural or functional cardiac abnormalities, and some measurements are required when performing fetal echocardiography [43]. This is because fetal cardiac dimensions play an important role in the prenatal detection and evaluation of various cardiac defects [52,53], as well as adaptive changes in fetal cardiac shape and structure [54]. Examples of fetal cardiac parameters include: 1) diameters of the aortic and pulmonary valve annulus in systole; 2) diameters of the tricuspid and mitral valve annulus in diastole; 3) right and left ventricular length; 4) end-diastolic ventricular diameter just inferior to the atrioventricular valve leaflets; and 5) cardiac circumference [43]. Moreover, biometric measurements (e.g. valve diameter) in combination with Doppler echocardiography can be used to derive fetal cardiac function parameters (e.g. stroke volume, cardiac output) [55], which may provide useful information in cases of CHD.
Obtaining cardiac biometric measurements can be accomplished in several ways. However, when measurements are performed on a real-time (but frozen frame) image, it is difficult to define the pattern of valvular motion [56]. The fetal heart is rapidly contracting, and therefore, identifying the precise points of end-systolic and end-diastolic frames may be a challenge. Moreover, measurements of fetal cardiac dimensions by 2D sonography are not always precise, because the plane may not be the correct one [57]. Instead, fetal cardiac biometric measurements can be timed more precisely within the cardiac cycle when this can be viewed directly (e.g. M-mode echocardiogram) [56]. The same applies to cardiac measurements obtained from STIC volume datasets, since STIC technology allows acquisition of a volume dataset from the fetal heart, and displays a cine loop of a complete single cardiac cycle in motion [22]. From the cine loop, a specific cardiac phase can by identified and analyzed, by observing the opening and closing of the atrioventricular and semilunar valves throughout the cardiac cycle (frame-by-frame) [55].
Indeed, Wang et al. has shown that 4D sonography with STIC is a feasible and accurate method for obtaining fetal cardiac dimension measurements in the second and third trimesters [57]. STIC volumes were acquired from 150 low-risk pregnancies, and 11 cardiac dimensions were obtained using calipers and the ellipse function. The accuracy was assessed by comparing measurements obtained by STIC to that of conventional 2D echocardiography. The authors found that STIC-based measurements have good agreement with 2D echocardiography, and the former can be performed by different operators with good repeatability [57]. Other investigators have also evaluated fetal cardiac measurements derived from STIC technology [58]. When examining STIC volumes from 139 low-risk pregnancies, Bennasar et al. reported that right and left ventricular diameters, as well as aortic root and pulmonary artery diameters had intra- and interobserver ICC (intraclass correlation coefficient) values greater than 0.90 [58]. ICC was used to assess intra- and interobserver reliability, and was considered “almost perfect” for clinical application when values were 0.81-1.0.
FINE now allows the performance of quantitative cardiac biometry measurements (e.g. ventricles, atria, diameter of outflow tracts). Measurements are obtained via electronic calipers (i.e. distance, elliptical circumference) applied to the cardiac diagnostic planes (Figure 7). If any original diagnostic plane is changed through VIS-Assistance®, the updated diagnostic plane can also be measured. As emphasized by others, the ability to obtain biometric measurements depends upon STIC volume quality [57].
Figure 7: Cardiac biometry as a feature of FINE.
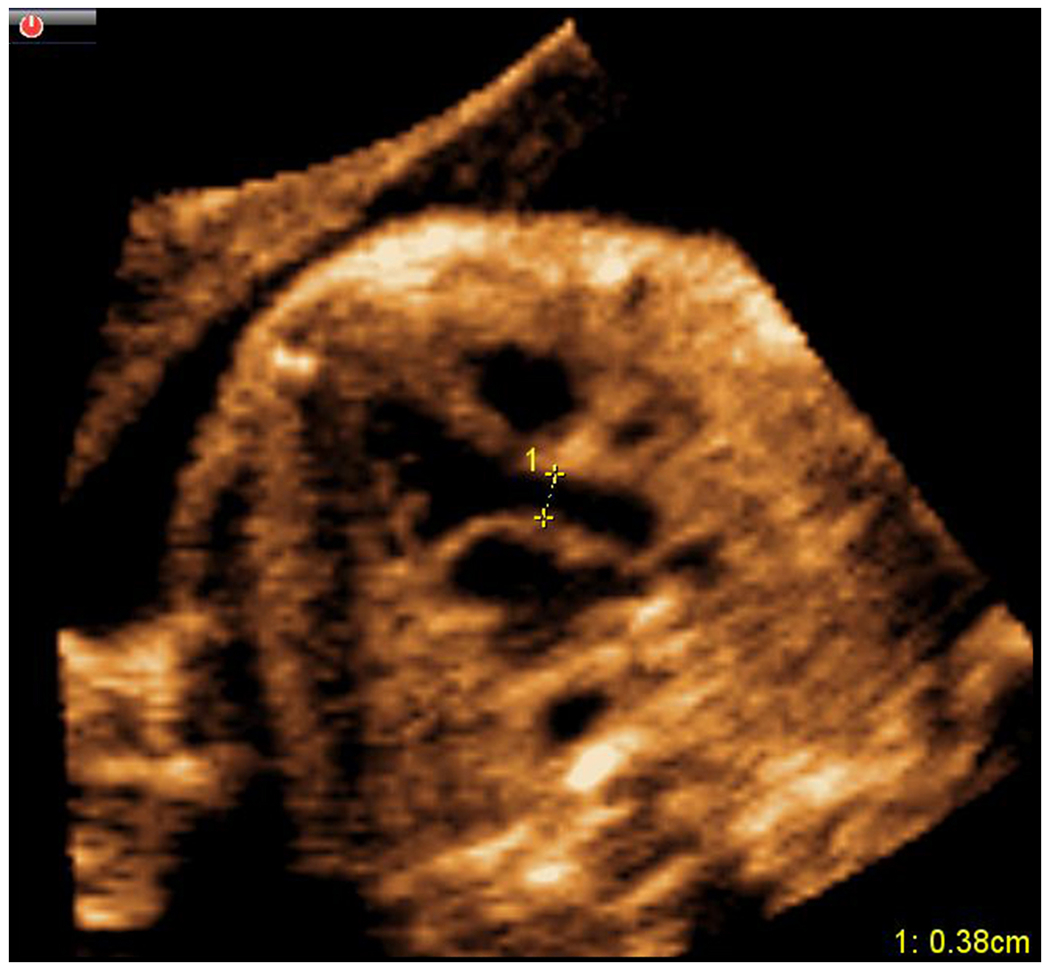
Quantitative fetal cardiac biometry measurements may now be performed on cardiac diagnostic planes generated by FINE. In this example, electronic caliper measurements were taken at end-systole from inner wall to inner wall (0.38 cm) at the level of the aortic valve in the left ventricular outflow tract view.
One distinct advantage of FINE is that the nine cardiac diagnostic planes are produced automatically, and then cardiac biometry can be measured. This is different than performing live 2D sonography, which requires the sonologist to obtain the cardiac view of interest first (i.e. operator dependent), before measurements are performed. Moreover, even if the diagnostic plane generated by FINE is not appropriate for cardiac biometry (e.g. improper alignment in the axial plane), it may be improved or corrected through VIS-Assistance® or maestro planar navigation (see below). This is in contrast to manual navigation through a STIC volume dataset to obtain an optimal (or correct) four-chamber view or left ventricular outflow tract [59]. Such navigation requires operator interaction with the dataset, such as moving the reference dot tool and then operating the x, y, and z controls. Therefore, this is highly operator dependent and can be cumbersome and challenging to perform [23,24].
C. Improve Success of Obtaining Fetal Echocardiography Views
1). Maestro Planar Navigation
This advanced technology allows an independent “fine-tune” navigation of each cardiac diagnostic plane generated by FINE, and is recommended for the more advanced sonologist without experience in manual navigation [24], or who has difficulty performing this in volume datasets. A distinct feature of FINE is that there is no multiplanar display or reference dot tool depicted, and this was done purposefully. The multiplanar display or view describes a volume (3D or 4D with STIC) that is displayed in three orthogonal planes simultaneously (representing the transverse, sagittal, and coronal planes of a reference 2D plane within this volume) [23]. The purpose of such a display is to allow correlation between image planes that are perpendicular to the main acquisition plane; the three planes intersect at a point (or reference dot), which is the tool used for manual navigation [22,23]. One must specifically decide where to move and place the reference dot on the image when performing x, y, or z rotations. Moreover, any given action (e.g. rotation around y-axis) will simultaneously affect all three orthogonal planes.
Maestro (or “master”) planar navigation applies to only a single cardiac diagnostic plane (e.g. four-chamber view) at a time, while the other eight diagnostic planes are unaffected. Therefore, maestro planar navigation differs from the multiplanar display mode. Moreover, it also contrasts with tomographic ultrasound imaging (TUI), a technology which slices a volume dataset (e.g. STIC) and displays planes that are parallel and equidistant to each other on the same screen [60]. With TUI, any manual navigation movements (e.g. parallel shift, x rotation) will also affect all planes on the screen simultaneously.
Maestro planar navigation allows the following movements (by 1 or 10 degrees) to fine-tune a given cardiac diagnostic plane (STIC or static mode volumes): 1) parallel shift; and 2) x, y, z rotations (Figure 5, Supplementary Video S8, Supplementary Video S9). We recommend that when performing such navigation, the sonologist should initially attempt parallel shift movements (known as Move View on ultrasound systems) before trying rotational movements, because the former is more likely to yield optimal information. It is the adjustment of depth (i.e. z-plane) that may improve the success of obtaining a cardiac view, and this is done via a parallel shift movement.
For a given cardiac diagnostic plane, maestro planar navigation may be implemented as a quick alternative to VIS-Assistance®. Therefore, it has similar objectives to that of VIS-Assistance®, which is to: 1) improve the success of obtaining the fetal echocardiography view of interest when not initially obtained via the diagnostic plane; 2) provide more information about the diagnostic plane and its surrounding structures; and 3) improve the quality of the examination. How then, is maestro planar navigation different from VIS-Assistance®? The latter (Table 1): 1) is a video clip tool with written callouts appearing at the top of each video clip; 2) allows automatic sonographic navigation and exploration of surrounding structures in the diagnostic plane (i.e. “virtual” sonographer); 3) is comprised of navigational movements through the STIC volume which are consistent each time VIS-Assistance® is activated; and 4) performs navigational movements through the STIC volume which are unique, fluid, and would be difficult or impossible to perform otherwise. For example, VIS-Assistance® consists of pivot points that automatically change, and around which sequential movements are centered [24]. Moreover, VIS-Assistance® allows a combination of rotations (e.g. x and y rotations simultaneously) around pivot points, and this is unique to the FINE method. In general, maestro planar navigation may be more appropriate to use (vs. VIS-Assistance®) when the sonologist wants to either quickly improve the success of obtaining a fetal echocardiography view or obtain further information about the view.
Comments
It is noteworthy that despite the development of new and advanced features for the FINE method, the following axiom about 4D sonography with STIC should still be followed. Specifically, this is the “garbage in, garbage out” principle used in the fields of computer science as well as information and communications technology [61]. A STIC volume dataset characterized by suboptimal or poor quality will produce undesired and uninformative “output” [22], since quality is essential for both post processing and assessment [57]. The new features of FINE described herein cannot solve this issue or may not be applicable to some volumes (e.g. cardiac biometry). On the flip side, when STIC volume datasets are of high quality, they are more likely to be informative (i.e. successful display of cardiac planes and anatomic structures).
Our group has previously described a practical and stepwise approach to performing 4D sonography with STIC, and also reviewed how to determine whether such volumes are appropriate before applying the FINE method [23]. Although sonologists do not have to be specifically experienced in 4D sonography to acquire high quality STIC volumes, they should be adequately trained.
Conclusions
The FINE method automatically generates and displays nine standard fetal echocardiography views required to diagnose most cardiac abnormalities. It considerably simplifies the fetal cardiac examination and reduces operator dependency. FINE improves assessment of both the normal and abnormal fetal heart, and can be considered a cardiac screening and diagnostic tool in the clinical setting. A summary of the method and its applications have been described herein. Recently, eight new and advanced features of FINE have been developed, and their goals broadly fall into three categories, which are to simplify the method, allow quantitative cardiac measurements, and improve the success of obtaining fetal echocardiography views. Our view is that such features will further contribute to the overall goal of FINE, which is to bring high quality prenatal screening and diagnosis of CHD to all pregnant women.
Supplementary Material
ACKNOWLEDGMENTS
This work was made possible by a partnership with two unique computer scientists, Mr. Gustavo Abella and Mr. Ricardo Gayoso. The work of Dr. Romero was supported by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. Dr. Romero has contributed to this work as part of his official duties as an employee of the United States Federal Government. Dr. Lami Yeo was funded by Wayne State University through a service contract in support of the Perinatology Research Branch. The contributions of Mr. Abella and Mr. Gayoso were funded by Medge Platforms, Inc., New York, NY, USA.
This article is a U.S. Government work and is in the public domain in the United States.
Funding
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NIH, DHHS; and in part with federal funds from NICHD, NIH, under Contract No. HHSN275201300006C.
Footnotes
DECLARATION OF INTEREST
The authors report no conflict of interest.
REFERENCES
- 1.Simeone RM, Feldkamp ML, Reefhuis J, et al. CDC Grand Rounds: understanding the causes of major birth defects: steps to prevention. MMWR MorbMortal Wkly Rep. 2015; 64(39):1104–1107. [DOI] [PubMed] [Google Scholar]
- 2.Słodki M, Soroka M, Rizzo G, et al. Prenatal atrioventricular septal defect (AVSD) as a planned congenital heart disease with different outcome depending on the presence of the coexisting extracardiac abnormalities (ECA) and / or malformations (ECM). J Matern Fetal Neonatal Med. 2018;1–191. [DOI] [PubMed] [Google Scholar]
- 3.Aydin E, Aypar E, Oktem A, et al. Congenital heart defects: the 10-year experience at a single center. J Matern Fetal Neonatal Med. 2020;33(3):368–372. [DOI] [PubMed] [Google Scholar]
- 4.Pavlicek J, Klaskova E, Prochazka M, et al. Congenital heart defects according to the types of the risk factors - a single center experience. J Matern Fetal Neonatal Med. 2019;32(21):3606–3611. [DOI] [PubMed] [Google Scholar]
- 5.Yang Q, Chen H, Correa A, et al. Racial differences in infant mortality attributable to birth defects in the United States, 1989–2002. Birth Defects Res A Clin Mol Teratol. 2006;76(10):706–713. [DOI] [PubMed] [Google Scholar]
- 6.Allan L. Antenatal diagnosis of heart disease. Heart. 2000;83(3):367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz-Lemini M, Nieto-Castro B, Luna-Garcia J, et al. Prenatal diagnosis of congenital heart defects: experience of the first Fetal Cardiology Unit in Mexico. J Matern Fetal Neonatal Med. 2021. May;34(10):1529–1534.. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Lodge J. Prenatal therapy for fetal cardiac disorders. J Matern Fetal Neonatal Med. 2019;32(22):3871–3881. [DOI] [PubMed] [Google Scholar]
- 9.Jaeggi ET, Sholler GF, Jones OD, et al. Comparative analysis of pattern, management and outcome of pre- versus postnatally diagnosed major congenital heart disease: a population-based study. Ultrasound Obstet Gynecol. 2001;17(5):380–385. [DOI] [PubMed] [Google Scholar]
- 10.Pinto NM, Keenan HT, Minich LL, Puchalski MD, Heywood M, Botto LD. Barriers to prenatal detection of congenital heart disease: a population-based study. Ultrasound Obstet Gynecol. 2012;40(4):418–425. [DOI] [PubMed] [Google Scholar]
- 11.Yeo L, Romero R. Fetal Intelligent Navigation Echocardiography (FINE): a novel method for rapid, simple, and automatic examination of the fetal heart. Ultrasound Obstet Gynecol. 2013;42(3):268–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizzo G, Capponi A, Muscatello A, Cavicchioni O, Vendola M, Arduini D. Examination of the fetal heart by four-dimensional ultrasound with spatiotemporal image correlation during routine second-trimester examination: the ‘three-steps technique’. Fetal Diagn Ther. 2008;24(2):126–131. [DOI] [PubMed] [Google Scholar]
- 13.DeVore GR, Falkensammer P, Sklansky MS, et al. Spatiotemporal image correlation (STIC): new technology for evaluation of the fetal heart. Ultrasound Obstet Gynecol. 2003;22(4):380–387. [DOI] [PubMed] [Google Scholar]
- 14.Goncalves LF, Lee W, Chaiworapongsa T, Espinoza J, Schoen ML, Falkensammer P, et al. Four-dimensional ultrasonography of the fetal heart with spatiotemporal image correlation. Am J Obstet Gynecol. 2003;189(6):1792–1802. [DOI] [PubMed] [Google Scholar]
- 15.Chaoui R, Hoffmann J, Heling KS. Three-dimensional (3D) and 4D color Doppler fetal echocardiography using spatio-temporal image correlation (STIC). Ultrasound Obstet Gynecol. 2004;23(6):535–545. [DOI] [PubMed] [Google Scholar]
- 16.Yagel S, Cohen SM, Shapiro I, et al. 3D and 4D ultrasound in fetal cardiac scanning: a new look at the fetal heart. Ultrasound Obstet Gynecol. 2007;29(1):81–95. [DOI] [PubMed] [Google Scholar]
- 17.Adriaanse BM, van Vugt JM, Haak MC. Three- and four-dimensional ultrasound in fetal echocardiography: an up-to-date overview. J Perinatol. 2016; 36(9):685–693. [DOI] [PubMed] [Google Scholar]
- 18.Espinoza J. Contemporary clinical applications of spatio-temporal image correlation in prenatal diagnosis. Curr Opin Obstet Gynecol. 2011; 23(2):94–102. [DOI] [PubMed] [Google Scholar]
- 19.Paladini D, Sglavo G, Greco E, et al. Cardiac screening by STIC: can sonologists performing the 20-week anomaly scan pick up outflow tract abnormalities by scrolling the A-plane of STIC volumes? Ultrasound Obstet Gynecol. 2008; 32(7):865–870. [DOI] [PubMed] [Google Scholar]
- 20.Gindes L, Hegesh J, Weisz B, et al. Three and four dimensional ultrasound: a novel method for evaluating fetal cardiac anomalies. Prenat Diagn. 2009; 29(7):645–653. [DOI] [PubMed] [Google Scholar]
- 21.Bennasar M, Martinez JM, Gomez O, et al. Accuracy of four-dimensional spatiotemporal image correlation echocardiography in the prenatal diagnosis of congenital heart defects. Ultrasound Obstet Gynecol. 2010; 36(4):458–464. [DOI] [PubMed] [Google Scholar]
- 22.Yeo L, Romero R. How to acquire cardiac volumes for sonographic examination of the fetal heart: Part 1. J Ultrasound Med. 2016;35(5):1021–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeo L, Romero R. How to acquire cardiac volumes for sonographic examination of the fetal heart: Part 2. J Ultrasound Med. 2016;35(5):1043–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeo L, Romero R. Intelligent navigation to improve obstetrical sonography. Ultrasound Obstet Gynecol. 2016; 47(4):403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia M, Yeo L, Romero R, et al. Prospective evaluation of the fetal heart using fetal intelligent navigation echocardiography (FINE). Ultrasound Obstet Gynecol. 2016; 47(4):450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veronese P, Bogana G, Cerutti A, et al. A prospective study of the use of fetal intelligent navigation echocardiography (FINE) to obtain standard fetal echocardiography views. Fetal Diagn Ther. 2017; 41(2):89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeo L, Luewan S, Markush D, et al. Prenatal diagnosis of dextrocardia with complex congenital heart disease using fetal intelligent navigation echocardiography (FINE) and a literature review. Fetal Diagn Ther. 2018; 43(4):304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kikuchi A. Medical ultrasound diagnosis in the near future as we move toward the era of the singularity. J Med Ultrason. 2016; 43(3):315–316. [DOI] [PubMed] [Google Scholar]
- 29.Yeo L, Markush D, Romero R. Prenatal diagnosis of tetralogy of Fallot with pulmonary atresia using: Fetal Intelligent Navigation Echocardiography (FINE). J Matern Fetal Neonatal Med. 2019;32(21):3699–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeo L, Romero R. Color and power Doppler combined with Fetal Intelligent Navigation Echocardiography (FINE) to evaluate the fetal heart. Ultrasound Obstet Gynecol. 2017;50(4):476–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeo L, Romero R. Prenatal diagnosis of hypoplastic left heart and coarctation of the aorta with color Doppler FINE. Ultrasound Obstet Gynecol. 2017;50(4):543–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeo L, Luewan S, Romero R. Fetal Intelligent Navigation Echocardiography (FINE) detects 98% of congenital heart disease. J Ultrasound Med. 2018;37(11):2577–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clerici G, Romanelli M, Tosto V, et al. Fetal transient tricuspid valve regurgitation: sonographic features and clinical evolution. J Matern Fetal Neonatal Med. 2021. Aug;34(15):2435–2439. [DOI] [PubMed] [Google Scholar]
- 34.Huang C, Zhao BW, Chen R, et al. Is fetal intelligent navigation echocardiography helpful in screening for d-transposition of the great arteries? J Ultrasound Med. 2020;39(4):775–784. [DOI] [PubMed] [Google Scholar]
- 35.Gembicki M, Hartge DR, Dracopoulos C, et al. Semiautomatic fetal intelligent navigation echocardiography has the potential to aid cardiac evaluations even in less experienced hands. J Ultrasound Med. 2020;39(2):301–309. [DOI] [PubMed] [Google Scholar]
- 36.Abdelrahman RM, Ramy ARM, Abdelhady AM. The diagnostic accuracy of automated fetal heart echocardiography by five dimensional compared to two-dimensional ultrasound in the second trimester of pregnancy. Open Journal of Obstetrics and Gynecology. 2018;8:513–520. [Google Scholar]
- 37.Uittenbogaard LB, Haak MC, Spreeuwenberg MD, et al. A systematic analysis of the feasibility of four-dimensional ultrasound imaging using spatiotemporal image correlation in routine fetal echocardiography. Ultrasound Obstet Gynecol. 2008;31(6):625–632. [DOI] [PubMed] [Google Scholar]
- 38.Gonçalves LF, Lee W, Espinoza J, et al. Examination of the fetal heart by four-dimensional (4D) ultrasound with spatio-temporal image correlation (STIC). Ultrasound Obstet Gynecol. 2006;27(3):336–348. [DOI] [PubMed] [Google Scholar]
- 39.Messing B, Cohen SM, Valsky DV, et al. Fetal cardiac ventricle volumetry in the second half of gestation assessed by 4D ultrasound using STIC combined with inversion mode. Ultrasound Obstet Gynecol. 2007;30(2):142–151. [DOI] [PubMed] [Google Scholar]
- 40.Allan LD, Tynan MJ, Campbell S, et al. Echocardiographic and anatomical correlates in the fetus. Br Heart J. 1980;44(4):444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith RS, Comstock CH, Kirk JS, et al. Ultrasonographic left cardiac axis deviation: a marker for fetal anomalies. Obstet Gynecol. 1995;85(2):187–191. [DOI] [PubMed] [Google Scholar]
- 42.International Society of Ultrasound in Obstetrics and Gynecology,Carvalho JS, Allan LD, Chaoui R, et al. ISUOG Practice Guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet Gynecol. 2013;41(3):348–359. [DOI] [PubMed] [Google Scholar]
- 43.American Institute of Ultrasound in Medicine. AIUM practice parameter for the performance of fetal echocardiography. J Ultrasound Med. 2020;39(1):E15–E16. [DOI] [PubMed] [Google Scholar]
- 44.Comstock CH. Normal fetal heart axis and position. Obstet Gynecol. 1987;70(2):255–259. [PubMed] [Google Scholar]
- 45.Shipp TD, Bromley B, Hornberger LK, et al. Levorotation of the fetal cardiac axis: a clue for the presence of congenital heart disease. Obstet Gynecol. 1995;85(1):97–102. [DOI] [PubMed] [Google Scholar]
- 46.Crane JM, Ash K, Fink N, et al. Abnormal fetal cardiac axis in the detection of intrathoracic anomalies and congenital heart disease. Ultrasound Obstet Gynecol. 1997;10(2):90–93. [DOI] [PubMed] [Google Scholar]
- 47.Vigneswaran TV, Kametas NA, Zinevich Y, et al. Assessment of cardiac angle in fetuses with congenital heart disease at risk of 22q11.2 deletion. Ultrasound Obstet Gynecol. 2015;46(6):695–699. [DOI] [PubMed] [Google Scholar]
- 48.International Society of Ultrasound in Obstetrics & Gynecology. Cardiac screening examination of the fetus: guidelines for performing the ‘basic’ and ‘extended basic’ cardiac scan. Ultrasound Obstet Gynecol. 2006;27(1):107–113. [DOI] [PubMed] [Google Scholar]
- 49.Zhao Y, Edington S, Fleenor J, et al. Fetal cardiac axis in tetralogy of Fallot: associations with prenatal findings, genetic anomalies and postnatal outcome. Ultrasound Obstet Gynecol. 2017;50(1):58–62. [DOI] [PubMed] [Google Scholar]
- 50.Weichert J, Hartge D, Gembicki M. Clinical validation of a semiautomatic volumetric approach for cardiac axis assessment in second and third trimester fetuses using FINE method. J Ultrasound Med. 2019;38(suppl):S49. [Google Scholar]
- 51.Weichert A, Gembicki MA, Hartge DR, et al. Semi-automatic assessment of the fetal cardiac axis in fetuses affected by congenital heart disease using fetal intelligent navigation echocardiography (FINE). Ultrasound Obstet Gynecol. 2019;54(Suppl 1):121. [Google Scholar]
- 52.Huang C, Zhao B, Lv S, et al. Smart-planes fetal heart (S-planes FH) software to quantitative evaluate the fetal great arteries. J Matern Fetal Neonatal Med. 2021. Jun;34(12):1932–1940. [DOI] [PubMed] [Google Scholar]
- 53.Schneider C, McCrindle BW, Carvalho JS, et al. Development of Z-scores for fetal cardiac dimensions from echocardiography. Ultrasound Obstet Gynecol. 2005;26(6):599–605. [DOI] [PubMed] [Google Scholar]
- 54.Sepúlveda-Martínez A, García-Otero L, Soveral I, et al. Comparison of 2D versus M-mode echocardiography for assessing fetal myocardial wall thickness. J Matern Fetal Neonatal Med. 2019;32(14):2319–2327. [DOI] [PubMed] [Google Scholar]
- 55.Yeo L, Romero R, Lee W. Assessment of cardiac geometry and stroke volumes by 4D fetal echocardiography. Bentham Science; 2010;160–177. [Google Scholar]
- 56.Allan LD, Joseph MC, Boyd EG, et al. M-mode echocardiography in the developing human fetus. Br Heart J. 1982;47(6):573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang N, Xie HN, Peng R, et al. Accuracy, agreement, and reliability of fetal cardiac measurements using 4-dimensional spatiotemporal image correlation. J Ultrasound Med. 2012;31(11):1719–1726. [DOI] [PubMed] [Google Scholar]
- 58.Bennasar M, Martínez JM, Gómez O, et al. Intra- and interobserver repeatability of fetal cardiac examination using four-dimensional spatiotemporal image correlation in each trimester of pregnancy. Ultrasound Obstet Gynecol. 2010;35(3):318–323. [DOI] [PubMed] [Google Scholar]
- 59.Tanis JC, Mohammed N, Bennasar M, et al. Online versus offline spatiotemporal image correlation (STIC) M-mode for the evaluation of cardiac longitudinal annular displacement in fetal growth restriction. J Matern Fetal Neonatal Med. 2018;31(14):1845–1850. [DOI] [PubMed] [Google Scholar]
- 60.Gonçalves LF, Espinoza J, Romero R, et al. Four-dimensional ultrasonography of the fetal heart using a novel tomographic ultrasound imaging display. J Perinat Med. 2006;34(1):39–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garbage in, garbage out [Internet]. Available from: https://en.wikipedia.org/wiki/Garbage_in,_garbage_out [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


