Abstract
Executive function (EF) deficits are associated with depression. Given that few prospective studies have been conducted, it is unclear whether deficits contribute to depression or result from it. The present study examined whether self-reported EF prospectively predicted worsening of depression symptoms. Time 1 (T1) shifting, inhibition, and working memory (WM) were assessed in relation to T1 and time 2 (T2) depressive symptoms in participants pre-selected to range in risk for depression. Analyses indicated that poorer EF at T1 predicted increases in depressive symptoms and furthermore that this relationship was specific to WM. In contrast, a bidirectional relationship was not evident, as depressive symptoms did not prospectively predict changes in EF. Finally, T1 EF accounted for T2 depressive symptoms beyond two well established predictors of depression: depressive symptoms at T1 and rumination at T2. These findings suggest that EF deficits play an active role in depression onset, maintenance, and/or recurrence.
Keywords: Executive function deficits, Depressive symptoms, Depression
Introduction
Investigations of cognition-emotion interactions have revealed that cognitive deficits, such as problems with memory and attention, are common during depressive episodes (Gotlib and Joormann 2010; Levin et al. 2007). Furthermore, cognitive deficits have been found to persist beyond depressive episodes (Austin et al. 2001). Cognitive deficits associated with depression are typically evident during tasks that require effortful, rather than automatic, processing (Hartlage et al. 1993). Theorized to require effortful rather than automatic processing, top-down control of behavior is purported to help to guide behavior in line with one’s intentions and goals (Miller and Cohen 2001). Thus, basic cognitive processes (e.g., attention) may not be impaired in individuals with depression (Hertel and Rude 1991), but rather the deployment and control of these processes is impaired, which in turn may account for at least some of the cognitive impairments and biases associated with depression (Levin et al. 2007).
Broadly, the processes by which individuals effortfully guide behavior toward a goal have been referred to collectively as executive functions (EFs; Banich 2009). Deficits purported to fall within the domain of EF have commonly been found in individuals with depression using neuropsychological tests (Channon et al. 1993; Gohier, et al. 2009; Porter et al. 2003; Purcell et al. 1997; for a review see, Snyder 2013), other behavioral tasks (Joormann and Gotlib 2008; Rose and Ebmeier 2006), and self-report measures (Warren, Heller, and Miller, under review).
Psychophysiological data also suggest a relationship between depression and EF deficits. For example, individuals with depression commonly exhibit abnormal brain activity in brain regions purported to implement EF (Engels et al. 2010; Herrington et al. 2010; Warren et al. 2013). Hypoactivation in dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC), brain regions theorized to maintain task set and to select and evaluate responses respectively (Banich 2009), are common findings in the literature (for reviews, see Rogers et al. 2004; Murrough et al. 2011). Taken together, these findings suggest that depression is associated with a reduced and/or a less efficient ability to engage cognitive-control networks during tasks that involve EF.
Although EF is not a unitary phenomenon (Miyake et al. 2000; Banich 2009), this is not always considered in studies of depression. Many processes are thought to fall within the domain of EF, including maintaining a representation of task goals and prioritizing behaviors that facilitate achieving task goals (Banich 2009). However, not all purported EF components are easily distinguished from others (e.g., “planning”), which can make them difficult to study individually. Of the various processes that have been considered to fall under the umbrella of EF, one prominent EF framework suggests that there are at least three clearly definable EF sub-processes that are related but distinct; specifically, shifting, inhibition, and updating of working memory (WM) representations (Miyake et al. 2000). These components are defined as the ability to shift attention from one task or mental set to another (shifting), the ability to override or suppress dominant or automatic responses (inhibition, possibly not separate from a more general component accounting for shared variance across all types of EF tasks), and the ability to actively manipulate and/or maintain information during a brief time period (updating/WM: Miyake et al. 2000; Miyake and Friedman 2012).
Once considered an epiphenomenon of depression (Austin et al. 2001), it has been suggested that EF deficits play an active role in depressive episodes (Gotlib and Joormann 2010). For example, in older adults executive dysfunction has been associated with poorer outcomes, as well as depression recurrence and relapse (Alexopoulos 2003). One way EF deficits may contribute to depression over time is by interfering with the ability to complete tasks in daily life, which could be distressing and disruptive and hence foster depression.
EF deficits may also actively contribute to the course of a depressive episode by interfering with recovery from negative moods (Gotlib and Joormann 2010). For example, individuals with clinical depression have exhibited greater switch costs (shift costs) than controls during tasks involving affective words (Lo and Allen 2011). Difficulties switching or shifting attention away from affective information may lead to a prolonged focus on negative material, which could maintain or increase negative affect and in turn maintain or increase depressive symptoms. A prolonged focus on negative material could also be caused by inhibition deficits, as suggested by Joormann and Gotlib (2008) to explain findings that individuals with depression have difficulties preventing negative material from entering WM.
Finally, EF deficits may actively contribute to depression by playing a role in precipitating an initial depressive episode. In non-clinical samples, trait negative affect, a well-established risk factor for depression, has been associated with decreased activity in brain regions involved in top-down control of attention (an EF function) during emotional distraction (Crocker et al. 2012) and with disrupted top-down control of attention on a non-emotional, color-word Stroop in a negative affective context (Hur et al., under review).
Because the relationship between EF deficits and depressive symptoms has typically been investigated cross-sectionally, it is unclear whether EF deficits predict depressive symptoms over time. Therefore, the present study assessed the relationship between EF deficits and depressive symptoms prospectively. Furthermore, given that specific components of EF are not always distinguished in studies of depression, the present study examined the predictive power of shifting, inhibition, and WM. Additionally, because investigating EF deficits using clinical samples may not provide a sufficient sampling of such relationships in populations at risk, the present study used a non-clinical sample with a range of risk for depression. Finally, laboratory tasks of EF can be limited in their ecological validity by providing more structure and support for focus on a particular task than occurs in typical real-life settings and thus may not fully capture EF deficits experienced by individuals in daily life (Barkley and Murphy 2011). Therefore, EF deficits in the present study were assessed with a well-established self-report measure (the Behavior Rating of Executive Function; BRIEF; Roth et al. 2005).
The BRIEF provides information on a variety of functional impairments that individuals experience in their everyday lives. It includes items about behaviors that index commonly-recognized aspects of EF. Significant relationships between self-reported EF deficits and a variety of constructs that are associated with deficient implementation of EF in daily life have been reported, including academic procrastination (e.g., deficits in planning, organization, and initiation; Rabin et al. 2011), Attention Deficit Hyperactivity Disorder (ADHD; known to encompass multiple EF deficits, e.g., deficits in attentional control and inhibition, poor judgment, etc.; Yers et al. 2009), and traumatic brain injury (associated with documented deficits in EF tasks; see Toplak et al. 2013, for a review of studies that have used the BRIEF in conjunction with performance-based measures). Furthermore, in a sample of adults with mild cognitive impairment, BRIEF self-reports and informant reports revealed similar patterns of EF deficits, indicating that self-reports can capture perceived EF difficulties that are confirmed by others (Rabin et al. 2006). Thus, the BRIEF may be able to provide insight into EF deficits that individuals experience in daily life that may not be manifested on laboratory tasks.
Although EF deficits may predict depressive symptoms over time, a bidirectional relationship between EF and depressive symptoms may exist, whereby depressive symptoms contribute to EF impairment over time. Potential support for a bidirectional relationship between depressive symptoms and EF comes from a longitudinal study by Biringer et al. (2005), who found that EF was impaired in individuals with major depressive disorder compared to health controls at time 1 (T1), and that EF improved as individuals recovered. In addition, there was a direct association between decrease in depressive symptoms and EF improvement. Furthermore, for individuals who recovered from depression, neuropsychological task performance no longer significantly differed from that of healthy controls. An interpretation of these results is that depressive symptoms actively contribute to EF impairment. Therefore, the present study assessed whether depressive symptoms predicted change in EF over time.
The present study also assessed whether EF deficits at T1 prospectively predicted depressive symptoms distinct from variance associated with reported levels of rumination at time 2 (T2). Rumination has been defined as a perserverative focus on one’s symptoms of distress (Nolen-Hoeksema 1991) and has consistently been associated with higher levels of depressive symptoms (Michl et al. 2013; Nolan et al. 1998; Segerstrom et al. 2000). Given that a strong association between depressive symptoms and rumination has been established, it would be useful to determine whether prior EF deficits predict current depressive symptoms above and beyond rumination.
Finally, it has been suggested that some aspects of EF may mature during adolescence (e.g., inhibition of perseveration), while others may not mature until early adulthood (e.g., verbal fluency; Romine and Reynolds 2005). Given that EF deficits may play a role in depressive symptoms and that cortical immaturity associated with adolescence and early adulthood may contribute to less efficient EF, the present study examined age as a possible confound in the relationship between EF deficits and future depressive symptoms.
For the present study, depressive symptoms and perceived EF deficits were assessed at an initial screening session (T1) and approximately 3 months later (T2). Rumination was assessed at T2. It was hypothesized that worse EF in each component (shifting, inhibition, WM) at T1 would be associated with increases in depressive symptoms from T1 to T2. To investigate whether changes in depressive symptoms are independently associated with specific EF components, we examined the predictive power of each EF component adjusting for the others. Next, to investigate whether a bidirectional relationship between depressive symptoms and EF exists, T1 depressive symptoms were used to predict change in EF. Finally, EF deficits at T1 were used to predict T2 depressive symptoms controlling for T1 depressive symptoms and T2 rumination.
Method
Participants
Of 756 undergraduates screened for the study as a facet of enrollment in an introductory psychology course, participants were 52 (27 female) paid volunteers (mean age = 19.42 years, SD = 1.72) selected based on a range of levels of trait positive and negative affect using the Positive and Negative Affect Schedule (PANAS; Watson et al. 1988). Participants were contacted if they 1) scored at or above the 80th percentile on one of the dimensions and at or below the 50th percentile on the other dimension, or 2) scored at or below the 50th percentile on both dimensions. This recruitment strategy was employed to ensure the sample would include participants with a range of risk for depression, since with random sampling it would be less likely that high, low, and average levels of risk would be equally represented. One recruited participant was excluded from the present study due to missing data. Of the 51 participants included in this study, 20 had high positive affect, 16 had high negative affect, and 15 had positive and negative affect scores below the 50th percentile. Positive and negative affect scores on the PANAS were not targets of interest in present analyses, so PANAS grouping was not utilized. All participants provided written informed consent at the initial group screening (T1) and were compensated with course credit. At the individual follow-up session approximately 3 months later (T2; M = 96 days, SD = 60 days), participants provided written informed consent and were financially compensated.
Questionnaires
At T1 and T2, participants completed the 22-item Anhedonic Depression subscale of the Mood and Anxiety Symptom Questionnaire (MASQ-AD; Watson et al. 1995a, b). The MASQ-AD allows both categorical and dimensional analytic strategies. For the present study, a dimensional approach was used to assess changes in depressive symptoms. Change scores were calculated by subtracting T1 from T2 MASQ-AD scores. A positive change score represented an increase in depressive symptoms over time.
At T1 and T2 participants completed the 75-item Behavior Rating Inventory of Executive Function (BRIEF)—Adult Version (Roth et al. 2005). The BRIEF is a self-report measure intended to assess EF over the past 6 months in an ecologically sensitive manner. The measure provides scores for nine subscales measuring different EF components. For the present study, the Shift (6 items; e.g., “I have trouble changing from one activity to another”), Inhibit (8 items; e.g., “I have problems waiting my turn”), and WM (8 items; e.g., “I forget what I am doing in the middle of things”) subscales were used to assess the three EF components discussed above. Higher Shift, Inhibit, and WM scores represent worse EF. Change scores were calculated by subtracting T1 from T2 EF scores. A positive change score represented a worsening of EF over time.
Rumination was measured at T2 using the Rumination subscale of the Rumination–Reflection Questionnaire (RRQ; Trapnell and Campbell 1999). In the RRQ, rumination is characterized as chronic self-focus, often involving recurrent negative thinking about the past (e.g., “I spend a great deal of time thinking back over my embarrassing or disappointing moments”; Trapnell and Campbell 1999).
Participants also completed the 10-item Drug Abuse Screening Test (DAST-10) which asks about drug use within the past 12 months (Skinner 1982) and the 10-item alcohol use disorders identification test (AUDIT) which assesses average alcohol use (Saunders et al. 1993).
Results
Table 1 reports means and standard deviations for the EF components and depressive symptoms at T1 and T2.1 Depressive symptoms increased for 10 individuals, stayed the same for 1 individual, and decreased for 40 individuals. Individuals whose MASQ-AD score increased versus decreased did not differ in MASQ-AD scores at T1 [t (50) = −0.28].
Table 1.
Descriptive statistics
| Mean (SD) | Total (N = 51) | Decreased in depressiona (N = 40) | Increased in depressionb (N = 10) | t valuec |
|---|---|---|---|---|
| BRIEF Shift | 9.41 (2.68) | 8.9 (2.24) | 10.7 (2.95) | −1.81 |
| BRIEF Inhibit | 13.47 (3.03) | 13 (2.64) | 14.6 (3.47) | −1.36 |
| BRIEF Working Memory | 12.51 (3.72) | 11.88 (3.12) | 13.9 (3.99) | −1.50 |
| MASQ-AD (T1) | 59.84 (12.02) | 59.43 (11.92) | 60.7 (13.33) | −0.28 |
| MASQ-AD (T2) | 52.14 (15.07) | 47.95 (12.36) | 67.3 (15.44) | 3.68d |
| MASQ-AD change score | −7.71 (10.73) | −11.48 (8.56) | 6.6 (5.02) | −8.67e |
| MASQ-AD (T2 residuals) | 0.00 (10.64) | −3.82 (8.25) | 14.40 (5.60) | −8.28e |
| RRQ-Rumination | 38.85 (11.47) | 36.36 (10.78) | 48.24 (9.86) | −3.34d |
BRIEF Behavior Rating Inventory of Executive Function, MASQ-AD Mood and Anxiety Symptom Questionnaire, Anhedonic Depression Subscale, RRQ Rumination and Reflection Questionnaire
Participants that decreased in depression from time 1 (T1) to time (T2)
Participants that increased in depression from T1 to T2
t Tests compare the N = 40 and N = 10 samples; equal variances not assumed
p < 0.01
p < 0.001
Zero-order correlations revealed that the T1 and T2 BRIEF Shift, Inhibit, and WM scores were each positively correlated with MASQ-AD scores at T1 and T2 (see Table 2).2 Thus, as predicted, higher depression was associated with worse self-reported EF cross-sectionally. Correlations between the MASQ-AD change scores and the T1 BRIEF Shift, Inhibit, and WM scores revealed that WM and MASQ-AD change scores were positively correlated [r (49) = 0.35, p < 0.05], indicating that worse self-reported WM at T1 was associated with an increase in anhedonic depression over time (Fig. 1). The correlations between Shift and MASQ-AD change scores and between Inhibit and MASQ-AD change scores were also in the expected direction though not significant (for both, p = 0.09). Neither DAST-10 nor AUDIT scores were significantly correlated with depression change scores or T1 BRIEF Shift, Inhibit, or WM. Furthermore, BRIEF WM continued to account for a significant portion of variance in MASQ-AD change scores with DAST-10 and AUDIT scores included in the first step of a hierarchical regression and MASQ-AD change scores entered in the second step.
Table 2.
Correlation coefficients
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. BRIEF Shift | – | 0.54c | 0.58c | 0.52c | 0.59c | 0.24 | 0.31a | 0.49c |
| 2. BRIEF Inhibit | – | – | 0.68c | 0.41b | 0.50c | 0.24 | 0.29a | 0.45b |
| 3. BRIEF Working Memory | – | – | – | 0.40b | 0.57c | 0.35a | 0.41b | 0.52c |
| 4. MASQ-AD (T1) | – | – | – | – | 0.71c | – | ||
| 0.13 | 0.00 | 0.54c | ||||||
| 5. MASQ-AD (T2) | – | – | – | – | – | 0.61c | 0.71c | 0.72c |
| 6. MASQ-AD change score | – | – | – | – | – | – | 0.99c | 0.41b |
| 7. MASQ-AD (T2 residuals) | – | – | – | – | – | – | – | 0.49c |
| 8. RRQ-Rumination | – | – | – | – | – | – | – | – |
Spearkman rank correlation coefficients are reported across the rows of the BRIEF Shift, BRIEF Inhibit, and BRIEF Working Memory scales, whereas for all other rows Pearson product moment correlation coefficients are reported
N = 51. BRIEF Behavior Rating Inventory of Executive Function, MASQ-AD Mood and Anxiety Symptom Questionnaire, Anhedonic Depression Subscale, RRQ Rumination and Reflection Questionnaire, T1 time 1, T2 time 2
p < 0.05
p < 0.01
p < 0.001
Fig. 1.
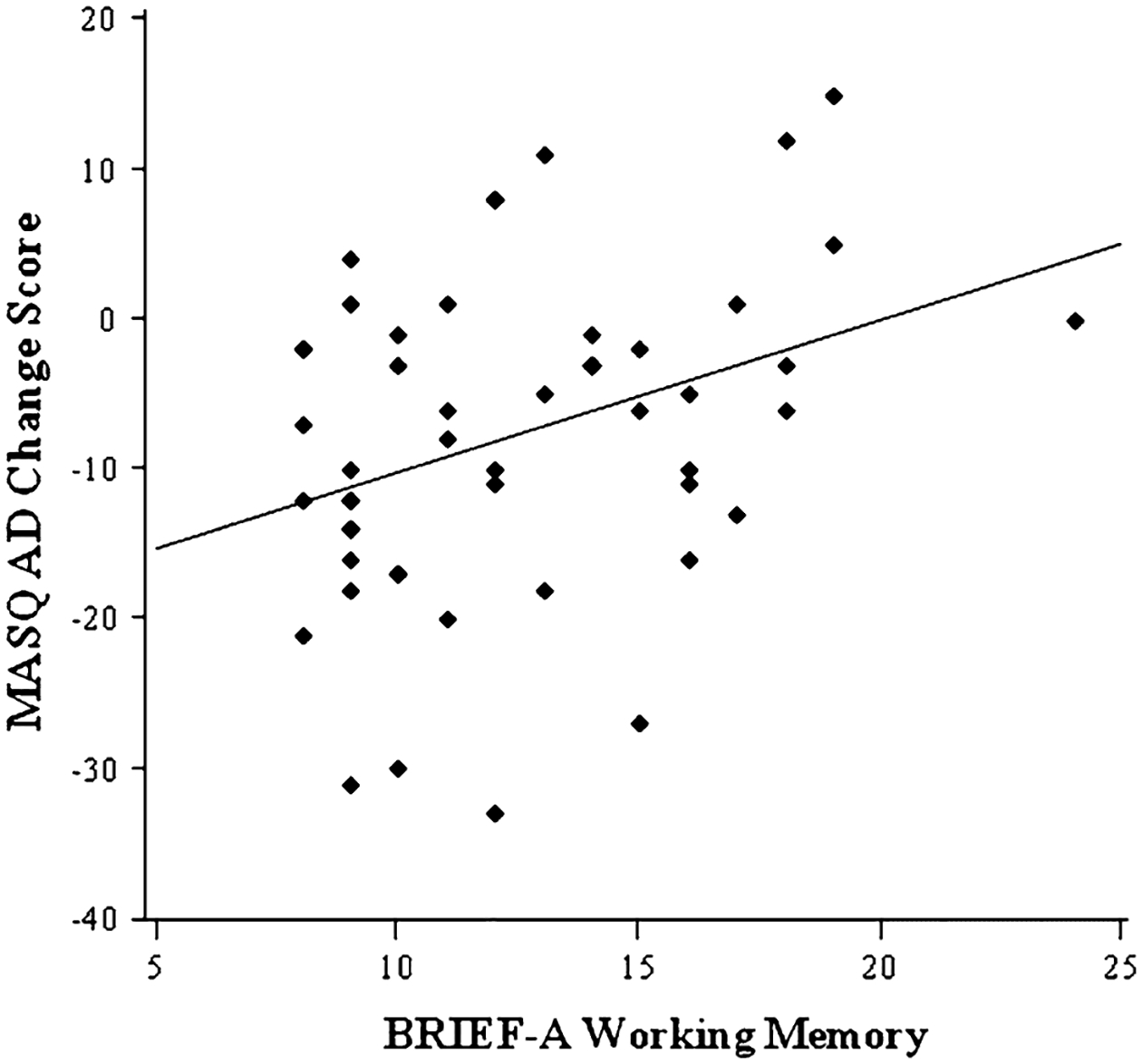
Scatterplot showing the association between working memory (WM) scores and anhedonic depression (AD) change scores. Higher WM scores represent worse self-reported WM, and positive Mood and Anxiety Symptom Questionnaire AD (MASQ-AD) change scores represent an increase in depressive symptoms from time 1 to time 2
The specificity of the zero-order relationship between T1 BRIEF WM scores and change in depression scores was evaluated in several ways. First, depression change was captured via a hierarchical regression with T1 MASQ-AD scores entered in the first step and WM scores entered in the second step, predicting T2 MASQ-AD scores. T1 BRIEF WM scores again accounted for significant variance [total R2 = 0.60, ΔR2 = 0.10, F-change (1, 48) = 11.67, p < 0.01].
Second, a hierarchical regression was conducted with all three T1 EF scores (BRIEF Shift, Inhibit, and WM) as predictors of depression change scores. When added last, BRIEF WM remained a marginally significant predictor of MASQ-AD change scores [total R2 = 0.13, ΔR2 = 0.06, t (50) = 1.68, p = 0.10], whereas the other two did not approach significance.
Third, T1 MASQ-AD scores were entered first, and all three T1 EF scores were entered second, predicting T2 MASQ-AD. BRIEF WM remained a marginally significant predictor of T2 MASQ-AD scores [total R2 = 0.61, ΔR2 = 0.11, t (50) = 1.89, p = 0.07]. In contrast, neither Shift nor Inhibit approached significance.
Next, whether depressive symptoms predict change in EF was assessed. Zero-order correlations revealed that T1 MASQ-AD was not significantly associated with any of the BRIEF change scores. Although zero-order correlations indicated that T1 MASQ-AD was a predictor of T2 BRIEF Shift, Inhibit, and WM [r (49) = 0.52, p < 0.001, r (49) = 0.28, p < 0.05, and r (49) = 0.28, p < 0.05, respectively], three separate hierarchical regressions with T1 BRIEF scores entered in the first step and T1 MASQ-AD scores entered in the second step predicting T2 BRIEF scores revealed that the latter did not add significant variance.
A hierarchical linear regression was employed to determine whether T1 BRIEF WM predicted T2 MASQ-AD scores above and beyond rumination reported at T2, with T1 MASQ-AD scores entered in the first step, T2 RRQ-Rumination in the second step, and T1 WM scores in the third step. Both RRQ-Rumination [total R2 = 0.67, ΔR2 = 0.17, F-change (1, 48) = 23.73, p < 0.001], and T1 BRIEF WM [total R2 = 0.69, ΔR2 = 0.03, F-change (1, 48) = 4.27, p = 0.04] added significant variance.
Finally, hierarchical regressions were utilized to evaluate age as a potential mediator of or confound in the relationship between EF and change in depressive symptoms. Age was not significantly related to depression change scores [R2 = 0.007, F (1,48) = 0.33, p = n.s.], and age did not predict WM [R2 = 0.001, F (1,48) = 0.05, p = n.s.], nor did it predict Inhibit or Shift. Furthermore, whether WM predicted depression change scores beyond age was examined, and it did [total R2 = 0.36, ΔR2 = 0.13, F-change (1, 47) = 6.78, p = 0.01].
Discussion
Prior studies have reported associations between EF deficits and depression cross-sectionally and this was confirmed in the present study. Using a prospective design, present results indicate that perceived EF deficits in daily life anticipate subsequent increased depression. In particular, present results provided support for the hypothesis that EF deficits, as measured by self-report, would predict subsequent increases in depressive symptoms. Furthermore, a specific component of EF, WM, was associated with an increase in depressive symptoms over time, above and beyond the effects of initial depression. Specifically, WM predicting depression was confirmed not only for concurrent (T1 WM and T1 depression, p < 0.01) and prospective (T1 WM and T2 depression, p < 0.001) measurement, but for change in depression over time (T1 WM and T2 depression minus T1 depression, p < 0.05). This relationship was not a result of mediation by or confound with age (T1 WM and T2 depression minus T1 depression, with age partialed out, p < 0.01), and it largely survived with the other two EF facets partialed out (p < 0.10). Thus, EF, and perhaps especially WM, anticipates changes in depression.
The predictive utility of self-reported T1 EF was specific (p = 0.10 and p = 0.07) to the WM facet of EF. This specificity is consistent with research by Harvey et al. (2004), who compared EF performance in controls to patients with clinical depression and investigated associations between shifting, inhibition, updating WM, and clinical measures (e.g., number of past hospitalizations) in the patient group. Shifting, inhibition, and WM were significantly impaired in patients. However, only the n-back task performance (purportedly an index of WM) was significantly negatively associated with patients’ number of previous hospitalizations. Although more hospitalizations may be an indicator of more depressive episodes, which may account for worse WM, it is also possible that WM deficits leave individuals at risk for subsequent depressive episodes. Although the present study and Harvey et al. (2004) support the possibility that deficits in certain EF components, such as WM, actively contribute to depressive symptoms, few prospective longitudinal studies have examined the relationship between EF and depressive symptoms and the predictive power of separate subcomponents of EF. More research is needed to determine whether EF deficits are a risk factor for the subsequent development of depression.
In contrast to perceived EF disruption predicting subsequent depressive symptoms, T1 depressive symptoms did not prospectively predict change in EF or EF at T2 beyond the effects of initial EF. Connolly et al. (2014) also found that depressive symptoms at baseline did not prospectively predict changes in EF approximately 15 months later. However, unlike the present study, Connolly et al. (2014) did not find that EF at T1 predicted depressive symptoms at T2. This discrepancy may reflect differences in procedures; Connolly et al. (2014) used conventional laboratory neuropsychological tasks to assess EF, whereas the present study used a self-report measure. Although both the present study and Connolly et al. (2014) found that depressive symptoms did not prospectively predict changes in EF, this could be attributable to the use of non-clinical samples. Higher levels of depressive symptoms may actively contribute to EF disruption. In the future, the value of depressive symptoms predicting EF should be assessed using samples with more representation of severe depressive symptoms.
That T1 predicted T2 depressive symptoms above and beyond initial depression and rumination reported at T2 suggests that prior WM deficits play an important role in current depressive symptoms. However, the mechanism by which impaired WM may contribute to future depressive symptoms is unclear. It is possible that impaired WM leads to an impaired ability to maintain and manipulate task-oriented material, which could lead to disruptions in task performance in daily life. Over time, recurrent task performance disruption could contribute to symptoms of distress, which may contribute to increases in depressive symptoms. It is also possible that WM impairments disrupt coping efforts, such as behavioral, cognitive, and/or emotional strategies (e.g., effective problem-solving; Nolen-Hoeksema et al. 2008) that serve to ameliorate negative thoughts and depressive symptoms (Gotlib and Joormann 2010). Finally, WM deficits may affect the facility with which negative material is removed from WM, which could foster a recurrent focus on negative material and in turn could contribute to an increase in depressive symptoms.
The present study does not provide information regarding the cause of EF deficits at T1. Past studies have found impairments in cognitive control in individuals with depression when confronted with emotional information (Beevers et al. 2010; Ditcher et al. 2009; Fales et al. 2008). Thus, cognitive deficits may be the result of negative cognition or sustained engagement with emotional information that may reduce the capacity to engage in or may divert attention away from tasks that require effortful processing (Ellis 1991; Hartlage et al. 1993; Hertel 1997; Hertel and Rude 1991). An example of engagement with emotional information that may be particularly disruptive to EF is rumination. EF deficits have been associated with higher levels of rumination and greater EF deficits in individuals with depression have been associated with higher levels of rumination (Davis and Nolen-Hoeksema 2000; Joormann and Gotlib 2008). Although it is possible that EF deficits contribute to rumination, it is also possible that a ruminative response style precedes EF deficits and, when activated by distress, contributes to EF impairment. Once impaired, EF deficits may play an active role contributing to depressive symptoms. However, even if rumination leads to EF deficits initially, EF deficits could also actively contribute to ruminative processes over time (e.g., by disrupting coping efforts). Hence it is possible that there is a bidirectional relationship between EF deficits and rumination. The relationship between EF deficits and rumination, as well as other variables that may potentially contribute to EF deficits, should be examined further.
A limitation of the present study was the reliance on self-report to assess EF. Although self-report can provide an indication of EF deficits in individuals’ daily lives, it is subject to biases including social desirability (Furnham 1986) and inaccurate recollection. For example, individuals (such as those with EF deficits) may not have insight into their functional impairments, or they may over-report impairment due to negative cognitive distortions and/or a pessimistic bias (Beck 1967; Strunk et al. 2006). Furthermore, they may be unable to infer cognitive processes such as EF (Nisbett and Wilson 1977). However, supporting the utility of the BRIEF, self-reported EF is associated with important functional outcomes as measured by both self-report and independent measures (e.g., driving records of DUI citations, informant reports), including criminal activity (Barkley and Murphy 2011), occupational impairment (Barkley and Murphy 2010), academic procrastination (Rabin et al. 2011), and psychopathology (Knouse et al. 2013). In the future, the impact that perceived EF deficits have on individuals’ daily function should be confirmed using other measures, such as informant reports, psychophysiological assessments, ecological momentary assessments, and/or activity/sleep trackers.
Another limitation of the present study was that although on average individuals completed T2 approximately 3 months following T1 (M = 96 days), there was variability in how long individuals completed T2 after T1 in the time between T1 and T2 (M = 96 days, SD = 60 days). The present study was part of a larger project, which involved recruiting individuals based on questionnaire scores from a group screening session (T1) to complete a multi-session laboratory study that included EEG/ERP and fMRI data collection (T2). Although all individuals were all recruited for T1 at approximately the same time, they were brought back for T2 at different intervals. In the future, time lapses should be held consistently across individuals to reduce the potential confounding effects of third variables. Exploratory analyses using time lapse as a predictor did not alter findings reported here.
The present study found that a perceived deficit in WM predicted increases in depressive symptoms. Recent work indicates that training can improve EF (for a review, see Crocker et al. 2013) and improve outcomes of psychopathology. Intervention strategies aimed at improving WM may prevent or reduce impairments in daily function, which may in turn lessen distress and/or rumination, and thus moderate the likelihood of subsequent depression. It is also possible that improvements in WM would enhance effective coping strategies which would reduce negative effects of stressors and contribute to the prevention of depression.
Acknowledgments
A portion of these results was reported at the annual meeting of the Society for Research in Psychopathology, 2013. Support for this work was provided by the National Institute of Mental Health (P50 MH07948, R01 MH61358, MH19554).
Footnotes
Conflict of Interest Allison M. Letkiewicz, Gregory A. Miller, Laura D. Crocker, Stacie L. Warren, Zachary P. Infantolino, Katherine J. Mimnaugh, Wendy Heller declare that they have no conflict of interest.
Informed Consent The present study was approved by the University of Illinois at Urbana-Champaign Institutional Review Board. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (University of Illinois at Urbana-Champaign) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all participants for being included in the study.
Animal Rights No animal studies were carried out by the authors for this article.
The sampling strategy of the present study included the vast majority of the range of depression symptoms and executive function scores and there was no evidence of bimodality. In a study published by Fisher et al. (2007) a similar recruitment strategy was used, and their sampling strategy also included the vast majority of the score distribution.
Because not all scores were normally distributed, Pearson and Spearman values were calculated when appropriate, as Spearman’s rho is a nonparametric correlation which deals with non-normal distributions.
Contributor Information
Allison M. Letkiewicz, Department of Psychology, University of Illinois at Urbana-Champaign, 603 E. Daniel St., Champaign, IL 61820, USA
Gregory A. Miller, Department of Psychology, University of Illinois at Urbana-Champaign, 603 E. Daniel St., Champaign, IL 61820, USA Department of Psychology and Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Los Angeles, CA, USA.
Laura D. Crocker, Department of Psychology, University of Illinois at Urbana-Champaign, 603 E. Daniel St., Champaign, IL 61820, USA
Stacie L. Warren, Department of Psychology, University of Illinois at Urbana-Champaign, 603 E. Daniel St., Champaign, IL 61820, USA Department of Psychology, Palo Alto University, Palo Alto, CA, USA.
Zachary P. Infantolino, Department of Psychology, University of Delaware, Newark, DE, USA
Katherine J. Mimnaugh, Department of Psychology, University of Illinois at Urbana-Champaign, 603 E. Daniel St., Champaign, IL 61820, USA
Wendy Heller, Department of Psychology, University of Illinois at Urbana-Champaign, 603 E. Daniel St., Champaign, IL 61820, USA.
References
- Alexopoulos GS (2003). The role of executive dysfunction in late-life depression. Journal of Clinical Psychiatry, 64, 18–23. [PubMed] [Google Scholar]
- Austin M-P, Mitchell P, & Goodwin GM (2001). Cognitive deficits in depression: Possible implications for functional neuropathology. The British Journal of Psychiatry, 178, 200–206. [DOI] [PubMed] [Google Scholar]
- Banich MT (2009). Executive function: The search for an integrated account. Current Directions in Psychological Science, 18, 89–94. [Google Scholar]
- Barkley RA, & Murphy KR (2010). Impairment in occupational functioning in adult ADHD: The predictive utility of executive function (EF) ratings versus EF tasks. Archives of Clinical Neuropsychology, 25, 157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, & Murphy KR (2011). The nature of executive function (EF) deficits in daily life activities in adults with ADHD and their relationship to performance on EF tests. Journal of Psychopathology and Behavioral Assessment, 33, 137–158. [Google Scholar]
- Beck AT (1967). Depression: Clinical, experimental, and theoretical aspects. New York: Harper and Row. [Google Scholar]
- Beevers CG, Clasen P, Stice E, & Schnyer D (2010). Depression symptoms and cognitive control of emotional cues: A functional magnetic resonance imaging study. Neuroscience, 167, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biringer E, Lundervold A, Stordal K, Mykletun A, Egeland J, Bottlender R, et al. (2005). Executive function improvement upon remission of recurrent unipolar depression. European Archives of Psychiatry and Clinical Neuroscience, 255, 373–380. [DOI] [PubMed] [Google Scholar]
- Channon S, Baker J, & Robertson M (1993). Working memory in clinical depression: An experimental study. Psychological Medicine, 23, 87–91. [DOI] [PubMed] [Google Scholar]
- Connolly SL, Wagner CA, Shapero BG, Pendergast LL, Abramson LY, & Alloy LB (2014). Rumination prospectively predicts executive functioning impairments in adolescence. Journal of Behavior Therapy and Experimental Psychiatry, 45, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker LD, Heller W, Spielberg JM, Warren SL, Bredemeier K, Sutton BP, et al. (2012). Neural mechanisms of attentional control differentiate trait and state negative affect. Frontiers in Psychology, 3, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker LD, Heller W, Warren SL, O’Hare AJ, Infantolino ZP, & Miller GA (2013). Relationships among cognition, emotion, and motivation: Implications for intervention and neuroplasticity in psychopathology. Frontiers in Human Neuroscience, 7, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RN, & Nolen-Hoeksema S (2000). Cognitive inflexibility among ruminators and nonruminators. Cognitive Therapy and Research, 24, 699–711. [Google Scholar]
- Ditcher GS, Felder JN, & Smoski MJ (2009). Affective context interferes with cognitive control in depression: An fMRI investigation. Journal of Affective Disorders, 114, 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis HC (1991). Focused attention and depressive deficits in memory. Journal of Experimental Psychology: General, 120, 310–312. [DOI] [PubMed] [Google Scholar]
- Engels AS, Heller W, Spielberg JM, Warren SL, Sutton BP, Banich MT, et al. (2010). Co-occurring anxiety influences patterns of brain activity in depression. Cognitive, Affective, and Behavioral Neuroscience, 10, 141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, et al. (2008). Altered emotional interference processing in affective cognitive-control brain circuitry in major depression. Biological Psychiatry, 63, 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JE, Heller W, & Miller GA (2007). Semantic associations, lateralized frontal function, and context maintenance in schizotypy. Neuropsychologia, 2007, 663–672. [DOI] [PubMed] [Google Scholar]
- Furnham A (1986). Response bias, social desirability and dissimulation. Personality and Individual Differences, 7, 385–400. [Google Scholar]
- Gohier B, Ferracci L, Surguladze SA, Lawrence E, Hage WE, Kefi MZ, et al. (2009). Cognitive inhibition and working memory in unipolar depression. Journal of Affective Disorders, 116, 100–105. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, & Joormann J (2010). Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology, 6, 285–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartlage S, Alloy LB, Vásquez C, & Dykman B (1993). Automatic and effortful processing in depression. Psychological Bulletin, 113, 247–278. [DOI] [PubMed] [Google Scholar]
- Harvey PO, Le Bastard G, Pochon JB, Levy R, Allilaire JF, Dubois B, et al. (2004). Executive functions and updating of the contents of working memory in unipolar depression. Journal of Psychiatric Research, 38, 567–576. [DOI] [PubMed] [Google Scholar]
- Herrington JD, Heller W, Mohanty A, Engels AS, Banich MT, Webb AG, et al. (2010). Localization of asymmetric brain function in emotion and depression. Psychophysiology, 47, 442–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel PT (1997). On the contributions of deficient cognitive control to memory impairments in depression. Cognition and Emotion, 11, 569–583. [Google Scholar]
- Hertel PT, & Rude SS (1991). Depressive deficits in memory: Focusing attention improves subsequent recall. Journal of Experimental Psychology: General, 120, 301–309. [DOI] [PubMed] [Google Scholar]
- Joormann J, & Gotlib IH (2008). Updating the contents of working memory in depression: Interference from irrelevant negative material. Journal of Abnormal Psychology, 115, 705–714. [DOI] [PubMed] [Google Scholar]
- Knouse LE, Barkley RA, & Murphy KR (2013). Does executive functioning (EF) predict depression in clinic-referred adults? EF tests vs. rating scales. Journal of Affective Disorders, 145, 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin RL, Heller W, Mohanty A, Herrington JD, & Miller GA (2007). Cognitive deficits in depression and functional specificity of regional brain activity. Cognitive Therapy and Research, 31, 211–233. [Google Scholar]
- Lo BCY, & Allen NB (2011). Affective bias in internal attentional shifting among depressed youth. Psychiatry Research, 187, 125–129. [DOI] [PubMed] [Google Scholar]
- Michl LC, McLaughlin KA, Shepherd K, & Nolen-Hoeksema S (2013). Rumination as a mechanism linking stressful life events to symptoms of depression and anxiety: Longitudinal evidence in early adolescents and adults. Journal of Abnormal Psychology, 122, 339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, & Cohen JD (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Miyake A, & Friedman NP (2012). The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science, 21, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iacoviello B, Neumeister A, Charney DS, & Iosifescu DV (2011). Cognitive dysfunction in depression: Neurocircuitry and new therapeutic strategies. Neurobiology of Learning and Memory, 96, 553–563. [DOI] [PubMed] [Google Scholar]
- Nisbett RE, & Wilson TD (1977). Telling more than we can know: Verbal reports on mental processes. Psychological Review, 84, 231–259. [Google Scholar]
- Nolan SA, Roberts JE, & Gotlib IH (1998). Neuroticism and ruminative response style as predictors of change in depressive symptomatology. Cognitive Therapy and Research, 22, 445–455. [Google Scholar]
- Nolen-Hoeksema S (1991). Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology, 100, 569–582. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, & Lyubomirsky S (2008). Rethinking rumination. Perspectives on Psychological Science, 3, 400–424. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Gallagher P, Thompson JM, & Young AH (2003). Neurocognitive impairment in drug-free patients with major depressive disorder. The British Journal of Psychiatry, 182, 214–220. [DOI] [PubMed] [Google Scholar]
- Purcell R, Maruff P, Kyrios M, & Pantelis C (1997). Neuropsychological function in young patients with unipolar major depression. Psychological Medicine, 27, 1277–1285. [DOI] [PubMed] [Google Scholar]
- Rabin LA, Fogel J, & Nutter-Upham KE (2011). Academic procrastination in college students: The role of self-reported executive function. Journal of Clinical and Experimental Neuropsychology, 33, 344–357. [DOI] [PubMed] [Google Scholar]
- Rabin LA, Roth RM, Isquith PK, Wishart HA, Pare N, Flashman LA, et al. (2006). Self- and informant reports of executive function on the BRIEF-A in MCI and older adults with cognitive complaints. Archives of Clinical Neuropsychology, 21, 721–732. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K, et al. (2004). Executive and prefrontal dysfunction in unipolar depression: A review of neuropsychological and imaging evidence. Neuroscience Research, 50, 1–11. [DOI] [PubMed] [Google Scholar]
- Romine CB, & Reynolds CR (2005). A model of the development of frontal lobe functioning: Findings from a meta-analysis. Applied Neuropsychology, 12, 190–201. [DOI] [PubMed] [Google Scholar]
- Rose EJ, & Ebmeier KP (2006). Pattern of impaired working memory during major depression. Journal of Affective Disorders, 90, 149–161. [DOI] [PubMed] [Google Scholar]
- Roth RM, Isquith PK, & Gioia GA (2005). BRIEF-A: Behavior rating inventory of executive function-adult version. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Saunders JB, Aasland OG, & Babor TF (1993). Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction, 88, 791–803. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Tsao JCI, Alden LE, & Craske MG (2000). Worry and rumination; repetitive thought as concomitant and predictor of negative mood. Cognitive Therapy and Research, 24, 671–688. [Google Scholar]
- Skinner HA (1982). The drug abuse screening test. Addictive Behaviors, 7, 363–371. [DOI] [PubMed] [Google Scholar]
- Snyder HR (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin, 139, 81–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunk DR, Lopez H, & DeRubeis RJ (2006). Depressive symptoms are associated with unrealistic negative predictions of future life events. Behavior Research and Therapy, 44, 861–882. [DOI] [PubMed] [Google Scholar]
- Toplak ME, West RF, & Stanovich KE (2013). Practitioner review: Do performance-based measures and ratings of executive function assess the same construct? Journal of Child Psychology and Psychiatry, 54, 131–143. [DOI] [PubMed] [Google Scholar]
- Trapnell PD, & Campbell JD (1999). Private self consciousness and the five-factor model of personality: Distinguishing rumination from reflection. Journal of Personality and Social Psychology, 76, 284–304. [DOI] [PubMed] [Google Scholar]
- Warren SL, Crocker LD, Spielberg JM, Engels AS, Banich MT, Sutton B, et al. (2013). Cortical organization of inhibition-related functions and modulation by psychopathology. Frontiers in Human Neuroscience, 7, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, & McCormick RA (1995a). Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology, 104, 15–25. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, & McCormick RA (1995b). Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology, 104, 3–14. [DOI] [PubMed] [Google Scholar]
- Yers BE, Wallace GL, Sokoloff JL, Shook DA, James JD, & Kenworthy L (2009). Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Research, 2, 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]


