Abstract
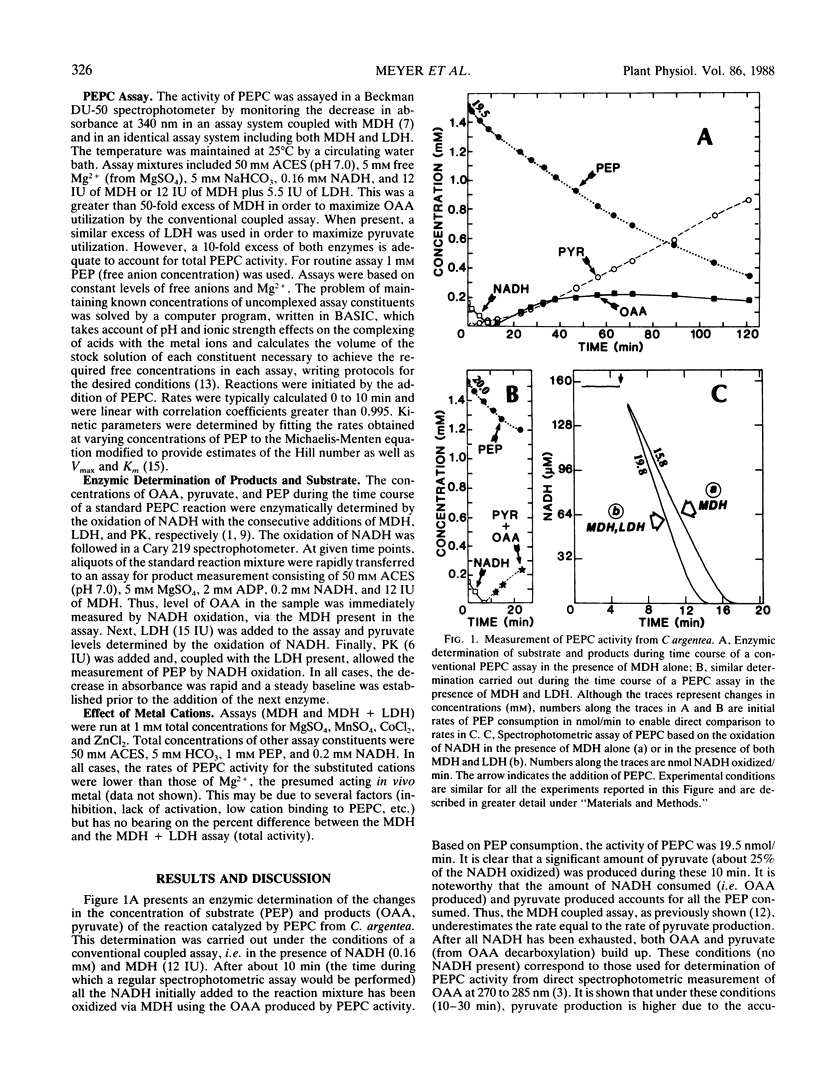
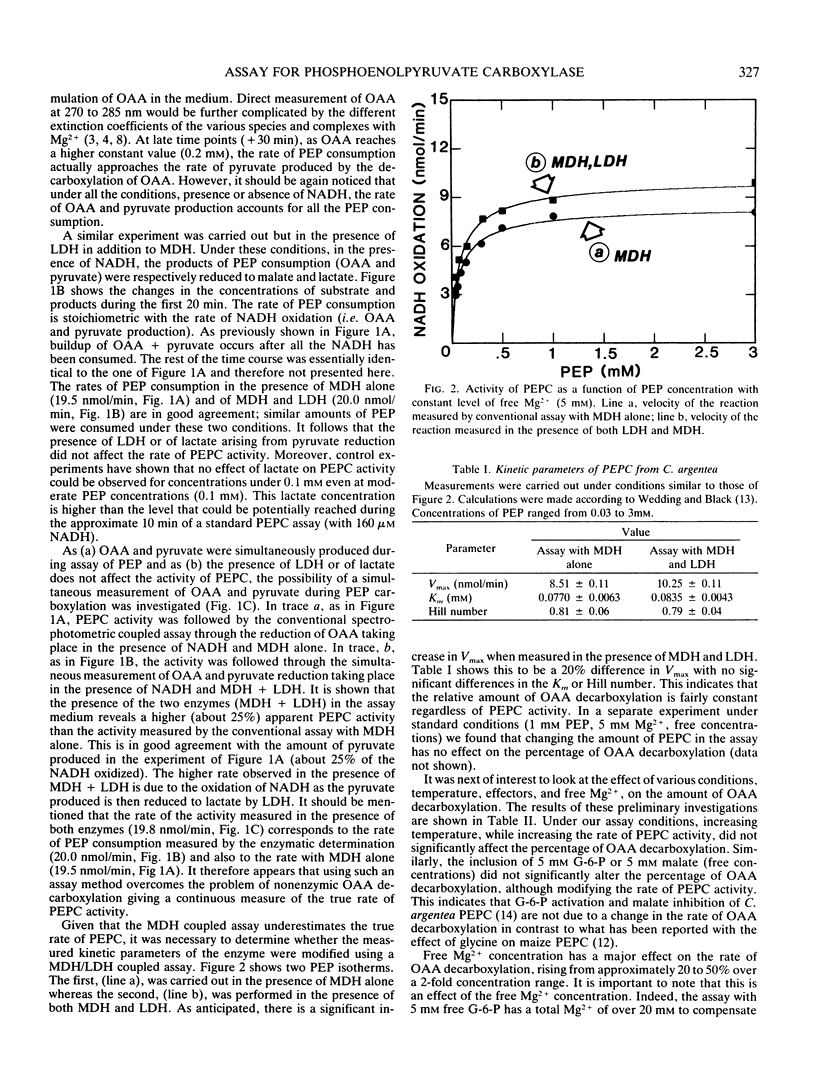
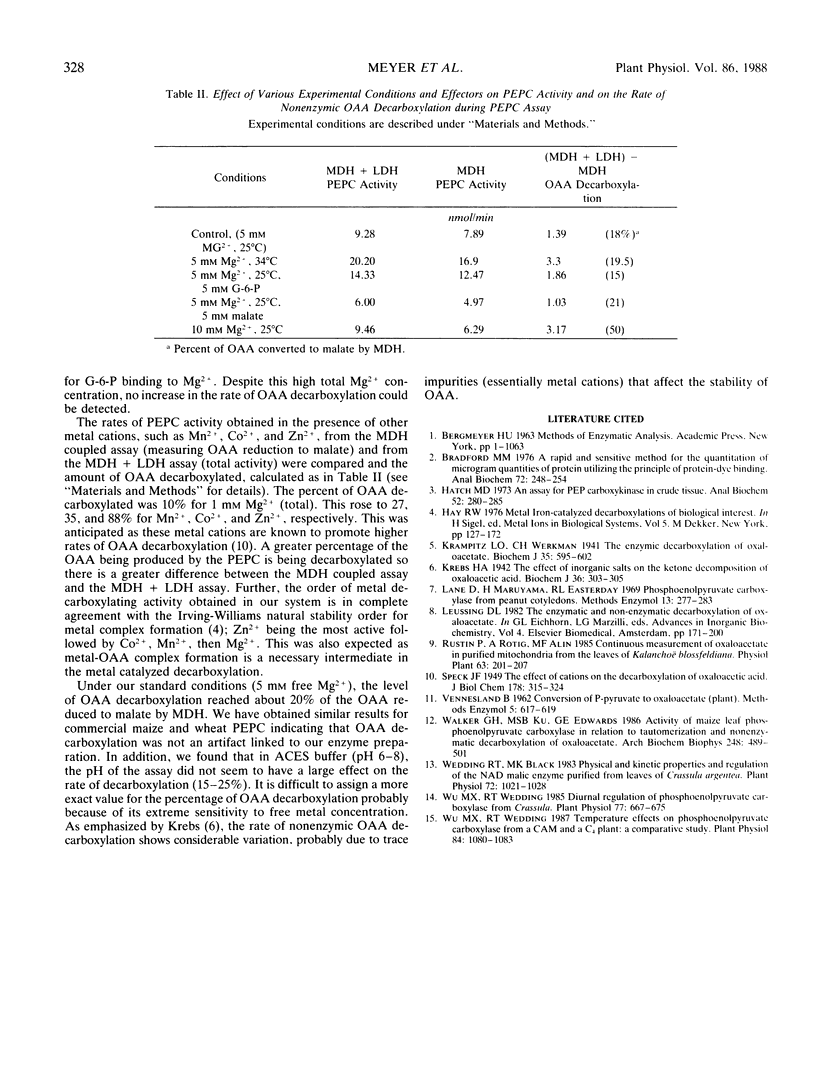
The rate of phosphoenolpyruvate carboxylase activity measured through the conventional coupled assay with malate dehydrogenase is underestimated due to the instability of oxaloacetate, which undergoes partial decarboxylation into pyruvate in the presence of metal ions. The addition of lactate dehydrogenase to the conventional assay allows the reduction of pyruvate formed from oxaloacetate to lactate with the simultaneous oxidation of NADH. Then, the enzymic determination of substrate and products shows that the combined activities of malate dehydrogenase and lactate dehydrogenase account for all the phosphoenolpyruvate consumed. The net result of the improved assay is a higher Vmax with no apparent effect on Km. The free divalent cation concentration appears to be the major factor in the control of the rate of oxaloacetate decarboxylation.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Hatch M. D. An assat for PEP carboxykinase in crude tissue extracts. Anal Biochem. 1973 Mar;52(1):280–285. doi: 10.1016/0003-2697(73)90350-3. [DOI] [PubMed] [Google Scholar]
- Krampitz L. O., Werkman C. H. The enzymic decarboxylation of oxaloacetate. Biochem J. 1941 Jun;35(5-6):595–602. doi: 10.1042/bj0350595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. The effect of inorganic salts on the ketone decomposition of oxaloacetic acid. Biochem J. 1942 Apr;36(3-4):303–305. doi: 10.1042/bj0360303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. H., Ku M. S., Edwards G. E. Activity of maize leaf phosphoenolpyruvate carboxylase in relation to tautomerization and nonenzymatic decarboxylation of oxaloacetate. Arch Biochem Biophys. 1986 Aug 1;248(2):489–501. doi: 10.1016/0003-9861(86)90502-3. [DOI] [PubMed] [Google Scholar]
- Wedding R. T., Black M. K. Physical and Kinetic Properties and Regulation of the NAD Malic Enzyme Purified from Leaves of Crassula argentea. Plant Physiol. 1983 Aug;72(4):1021–1028. doi: 10.1104/pp.72.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Diurnal regulation of phosphoenolpyruvate carboxylase from crassula. Plant Physiol. 1985 Mar;77(3):667–675. doi: 10.1104/pp.77.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Regulation of Phosphoenolpyruvate Carboxylase from Crassula argentea: Further Evidence on the Dimer-Tetramer Interconversion. Plant Physiol. 1987 Aug;84(4):1080–1083. doi: 10.1104/pp.84.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]


