Abstract
In multicellular systems, oriented cell divisions are essential for morphogenesis and homeostasis as they determine the position daughter cells within the tissue and also, in many cases, their fate. Early studies in invertebrates led to the identification of conserved core mechanisms of mitotic spindle positioning centred on the Gαi–LGN–NuMA–dynein complex. In recent years, much has been learnt on the way this complex functions in vertebrate cells. In particular, studies addressed how Gαi–LGN–NuMA– dynein complex dynamically crosstalks with astral microtubules and the actin cytoskeleton, and how it is regulated to orient the spindle according to cellular and tissue-wide cues. We have also begun to understand how dynein motors and actin regulators interact with mechanosensitive adhesion molecules sensing extracellular mechanical stimuli, such as cadherins and integrins, and with signalling pathways in order to respond to extracellular cues instructing the orientation of the division axis in vivo. In this Review, with the focus on epithelial tissues, we discuss the molecular mechanisms of mitotic spindle orientation in vertebrate cells, and how this machinery is regulated by epithelial cues and extracellular signals to maintain tissue cohesiveness during mitosis. We also outline recent knowledge gathered about how spindle orientation impacts tissue architecture in epithelia and its emerging links to the regulation of cell fate decisions. Finally, we describe how defective spindle orientation can be corrected or its effects eliminated in tissues under physiological conditions, and the pathological implications associated with spindle misorientation.
Introduction
Cell division is orchestrated by the mitotic spindle, a large intracellular structure organized in mitosis by polar tubulin filaments termed microtubules that emanate from the centrosomes 1. Bundles of microtubules connecting spindle poles to kinetochores [G] are essential for equal segregation of sister chromatids, whereas astral microtubules [G] protruding to the cell cortex dynamically position and stabilize the spindle within the cell, this way determining the division orientation.
The working principles and the biological relevance of spindle orientation for tissue morphogenesis and homeostasis have been intensively studied in the last two decades. Initial studies conducted in invertebrate systems and epithelial cells in culture led to the identification of molecular motors that power spindle positioning, and the conserved regulators that determine their position and activity2. More recently, genetic manipulation coupled to confocal and live imaging techniques have uncovered cell-autonomous and tissue-mediated regulatory mechanisms instructing division orientation in different organs in vertebrate systems. Parallel in silico modelling uncovered the key molecular players and functional principles underlying spindle positioning 3–6. These studies also revealed the identity of cell-type specific ancillary subunits and post-translational modifications modulating the activity of force generators, and demonstrated that these regulatory mechanisms are coupled to the execution of morphogenetic programmes and respond to cues from the environment, such as tissue injuries and challenges 7. Thus, we now appreciate that formation of the mitotic spindle must be coupled with its proper orientation for tissue development and function.
Early on in studies of spindle positioning it was appreciated that division orientation in a mother cell can impact fate choices of the progeny. Seminal studies in Drosophila melanogaster neuroblasts [G] and Caenorhabditis elegans zygotes provided crucial conceptual insights into the process of asymmetric stem cell division, defined as cell division leading to daughter cells with different identity 8–10. Notably, these initial studies were conducted in cells that always divide asymmetrically (with respect to fate), even in isolation, and set the stage to investigating the relevance of asymmetric oriented divisions in vertebrate stem cells. However, vertebrate stem cells are intrinsically more difficult to analyze in that they: do not obligatorily divide asymmetrically; they require specialized microenvironments known as niches to maintain stemness — and hence might be affected by isolation procedures from endogenous tissues; and they are endowed with high plasticity that allows cell identity changes during experimental manipulation.
In this Review, we describe mechanisms of oriented divisions in vertebrate cells, with emphasis on epithelial cells, where division orientation is tightly linked to tissue architecture. We also discuss the major research achievements that over the last few years contributed to the current understanding of how oriented divisions contribute to the morphogenesis and homeostasis of multicellular organisms.
Pulling machinery at the cortex
Oriented divisions are defined as mitoses occurring in a prototypic direction with respect to intrinsic cell polarity and overall tissue organization, and they ultimately determine positioning of daughter cells in the tissue. Division orientation is determined by the positioning of the mitotic spindle, which is governed by multiple distinct factors. In most vertebrate systems, the mitotic spindle first assembles and captures chromosomes, and then, at metaphase, it is centred in the cell and stabilized in an orientation that is maintained through anaphase and telophase, when the spindle elongates and sister chromatids are separated towards opposite spindle poles 1 (Figure 1). In this paragraph we outline the basic molecular machinery for spindle orientation, comprising the cortical Gαi–LGN–NuMA complex and cytoplasmic dynein-1 (hereon termed dynein), which together with astral microtubules couple the spindle poles to the cortex to orient the spindle and ultimately specify the division plane.
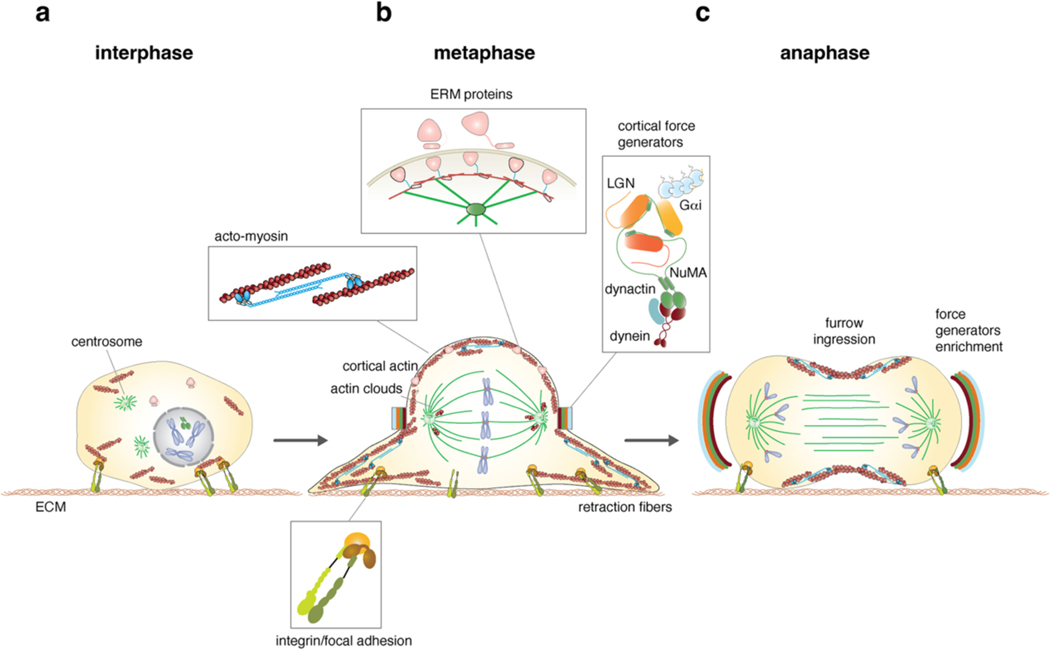
Figure 1. Morphological changes instructing spindle orientation during mitotic progression.
a) Distribution of integrin/focal adhesion adhesive cues, F-actin cytoskeleton and chromosomes in interphase and prometaphase. Centrosomes are positioned near the nucleus, NuMA is nuclear, and ERM (Ezrin-Radixin-Moesin) proteins are found in a closed inhibited conformation. b) Rounded-up morphology of cells in metaphase, with sister chromatids congressed on the metaphase plate and a bipolar spindle. The spherical shape is sustained by an actomyosin layer (1, which is connected to the plasma membrane near actin clouds by activated ERM proteins (2). Mitotic cells maintain contact to the substrate via mitotic β1-integrin containing focal adhesion complexes anchored to actin-based retraction fibres (3). The mitotic spindle axis is maintained aligned to the substratum by dynein–dynactin cortical force generators recruited to cortical crescent above the spindle poles by Gαi–LGN–NuMA complexes (4). Actin clouds formed by F-actin localizing near the spindle poles contribute to spindle orientation as well. c) At anaphase, force generators level increases symmetrically above the spindle poles. At this point, NuMA is anchored to the membrane independently of LGN, by binding to membrane phospholipids, after its phosphorylation by CDK1 is relieved (not shown). This allows generation of stronger dynein-based traction forces that are needed to separate sister chromatids in synergy with pushing and pulling forces acting at the kinetochore level. Anaphase is also associated with actomyosin cortex reorganization, whereby actomyosin enriches at the equatorial region of the cell to promote cytokinetic cleavage furrow ingression.
Centrosomes and microtubules.
In most cell types, centrosomes serve as spindle poles and the main microtubule organizing centres for spindle microtubules, including astral microtubules radiating to the cell cortex, that are fundamental players in spindle placement through their interactions with the cell cortex [G]. From prophase onwards, separation of duplicated centrosomes assisted by dynein and kinesin Eg5 enables the formation of an overlapping array of microtubules between the nascent spindle poles. Consistently, defects at the spindle poles resulting in abnormal centrosome separation in prophase11 and impaired astral microtubule nucleation from the centrosomes 12, often cause spindle assembly defects and misoriented divisions.
Astral microtubule dynamic instability [G] is a key factor in spindle orientation mechanisms 13,14. Consistently, treatment of mitotic cells with low doses of the depolymerizing drug nocodazole causes spindle misorientation defects 15,16, and oftentimes misorientation phenotypes can be rescued by stabilizing microtubules with taxol 16. Specific microtubule-associated proteins (MAPs) control microtubule plus-ends including plus-end tracking proteins (+TIPs), such as EB1, or polymerizing enzymes, including XMAP215/Stu2 family proteins [G] and CLIP170 [G] 17 (Figure 2a). They synergize to orchestrate spindle–cortex contacts.
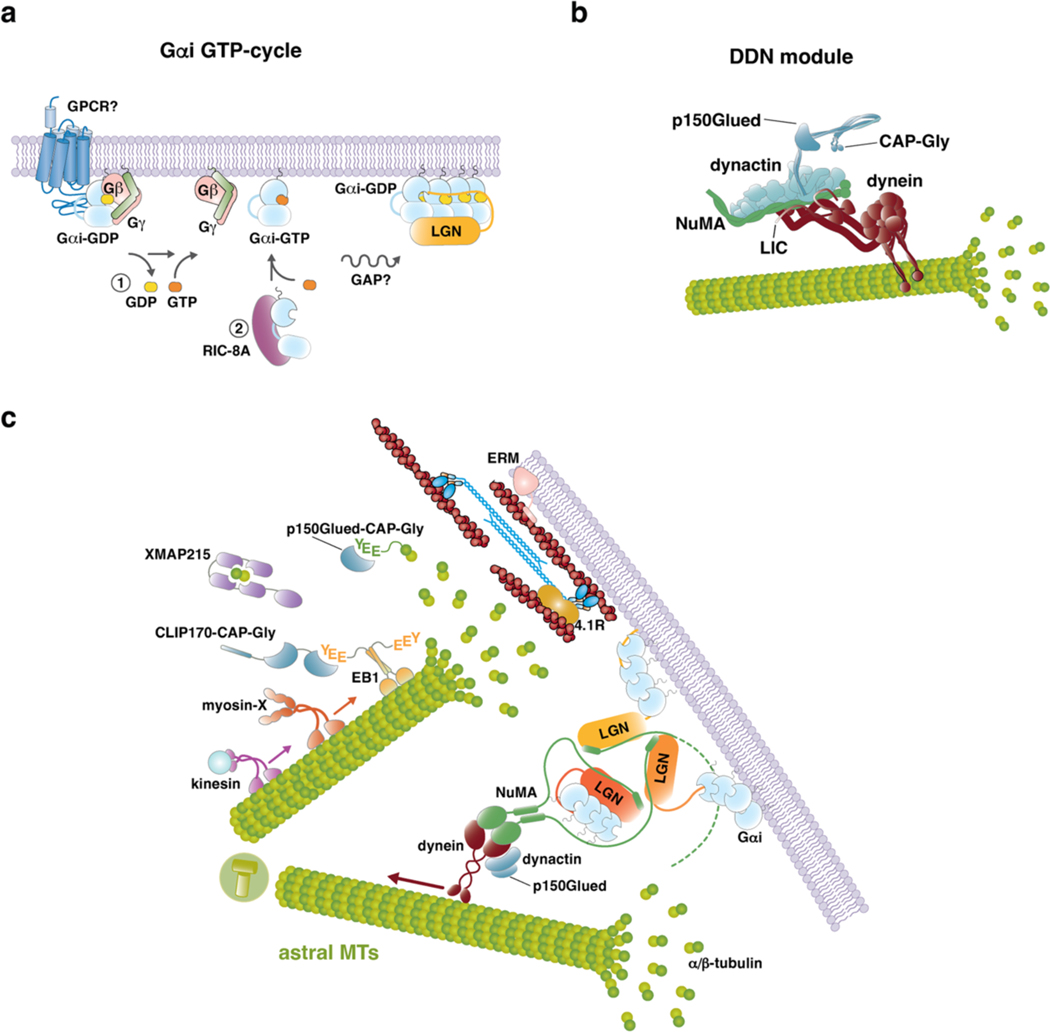
Figure 2. Working principles of cortical force generators.
a) Cartoon representation of the molecular players implicated in spindle positioning at the mitotic cortex. The force generating machines exerting traction forces on astral microtubules are organized on the dynein–dynactin–NuMA complexes, targeted to the membrane by Gαi–LGN via NuMA. Gαi-GDP accumulates at caveolin-rich membrane patches. The N-terminal portion of NuMA interacts with dynein, while the C-terminal region contacts LGN. A number of microtubule plus-TIPs binders modulate the dynamic instability of astral microtubules near the cortex, this way contributing to spindle positioning. These include: EB1, that binds with the C-terminal EEY motif to CAP-Gly containing proteins such as CLIP170 and p150Glued; XMAP215, that uses its four TOG domains [G] to assist tubulin incorporation into microtubules and stabilize the microtubule lattice at the plus ends; kinesins, such as kinesin-1. In addition, myosin X provides a physical link between astral microtubules and the cortical actin cytoskeleton, and generates pulling forces that cooperate with those produced by dynein–dynactin. Astral microtubule ends interact with the actin cortex also through mitotic interactor and substrate of PLK1 (MISP), which binds EB1 and p150Glued. The stiffness of the mitotic actomyosin cortex, needed to provide a rigid scaffold counterbalancing microtubule pulling forces exerted by dynein to position the spindle, is regulated by 4.1R proteins, likely associating to NuMA itself, and by ERM (Ezrin-Radixin-Moesin) proteins tethering F-actin to the plasma membrane b) Molecular events promoting cortical recruitment of dynein–dynactin force generators in metaphase. Top: In the unliganded form, LGN is kept inhibited by intra-molecular interactions between the N-terminal Tetratrico-Peptide-Repeat (TPR) domain and the C-terminal GoLoco region. Cooperative binding of the four GoLoco motifs to Gαi-GDP induces a conformational change allowing binding of LGN to NuMA C-terminus. Bottom: Structural evidence accumulated over the years documented the conformational rearrangement of LGN upon binding to Gαi and NuMA. In the closed conformation, the TPR domain of LGN folded as a helical cradle is occupied by the GoLoco helices, in purple (PDB-ID 4JHR). Binding of LGN to Gαi-GDP displaces the GoLoco motif from the TPR domain, that can form donut-shaped hetero-hexamers with the C-terminal portion of NuMA (PDB-ID 6HC2). Full-length NuMA dimerizes via its coiled-coil central region. The combination of the 3:3 stoichiometry observed in the LGN-TPR–NuMA-C-terminus donut-shaped structures with the dimeric nature of full-length NuMA implies that each donut connects at least two NuMA dimers — depicted by the dotted line of one of the NuMA chains departing from the hexamer — this way promoting the formation of LGN–NuMA–dynein cortical networks c) Gαi GTP-cycle associated with the assembly of Gαi–LGN–NuMA cortical complexes. In interphase, GDP-loaded Gαi forms hetero-trimeric G-protein complexes with Gβγ. To transfer Gαi from Gβγ to LGN, one possibility could be that, at mitotic entry, a localized pool of Gαi dissociates from Gβγ, possibly assisted by the GEF activity of a G-Protein-Coupled Receptor (GPCR), and generates a population of GTP-loaded Gαi molecules that, upon GTP hydrolysis, can bind and target LGN to the cortex by direct association with the four LGN-GoLoco motifs (path labelled 1 in the schematic). Alternatively (or in addition), recent evidence points at the chaperone-like activity of the scaffold RIC-8A to assist Gαi folding and GTP-loading, this way creating a GTP-bound pool of Gαi available for LGN after GTP hydrolysis (path labelled 2 in the schematic). c) Organizational principles of the dynein–dynactin–NuMA module responsible for the generation of microtubule-pulling forces orienting the spindle. Dynein is a multi-subunit complex comprising two-copies of six different chains, including a microtubule-binding stalk and an AAA+ ATPase domain. In mitosis, NuMA binds the light intermediate chain (LIC) subunit of dynein, likely sandwiching between dynein and the dynactin complex, which has been shown to confer processivity to the motor activity of dynein. Dynactin itself consists of several subunits, including a p150Glued subunit encompassing a CAP-Gly domain.
Considerable evidence demonstrated a role for microtubule dynamics in spindle positioning. For example, stabilization of microtubules with taxol inhibits spindle positioning in C. elegans embryos 18. Some aspects of the molecular control of microtubule dynamics that are important for spindle positioning have also been elucidated. In mitotic cells, phosphorylation of GTSE (G2 and S phase-expressed protein 1) by CDK1 [G] prevents its association with EB1, resulting in destabilization of astral microtubules, thereby preventing formation of long astral microtubules that prevent the preferential spindle orientation along the long cellular axis (phenomenon known as Hertwig’s rule) 19. Similarly, the depolymerizing kinesin, KIF18b, promotes catastrophe of astral microtubules and is required for robust spindle orientation in cultured cells, similar to the role of its yeast orthologue 20,21.
It recently became clear that the mitotic microtubules–MAPs interaction landscape is regulated by a specific tubulin post-translational modification (PTM), namely tyrosination of the α-tubulin tail 22. In mammalian cells, tyrosination is enriched on astral microtubules, whereas this modification is absent from inner spindle microtubules 23. Tyrosination enrichment at astral microtubule plus-ends, which is catalyzed by tubulin-tyrosine ligases (TTLs)24, is required for microtubule interaction with CAP-Gly containing proteins [G] recognize the EEY/F motif 25, which is present in key components of the force-generation machinery acting on the spindle, including the dynactin [G] subunit p150-Glued and CLIP-170 26 (Figure 2a) (see also next section). Mechanistically, these interactions are thought to stabilize the contacts between tyrosinated astral microtubule plus-ends and cortical dynein–dynactin complexes or the actomyosin cortex. Consistently, TLL-deficient mice, almost completely lacking tubulin tyrosination, display defective spindle orientation 27.
The dynein–NuMA–LGN–Gαi module.
The current view of spindle orientation has focused on pulling forces acting on astral microtubules, directed by restricted cortical localization of force generators and polarity proteins and modulated by astral microtubule dynamic instability. These traction forces act in concert with cell anisotropy factors and tissue-derived mechanical cues, such as compression and adhesion forces, to determine the final division orientation.
In most vertebrate cells, the activation of traction forces acting on the mitotic spindle starts in late prometaphase, when the microtubule cytoskeleton is reorganized in a bipolar spindle 28,29 (Figure 1a–b). Dynein is thought to be a major force generator on astral microtubules to position the spindle, and, once anchored at the mitotic cortex, it uses its movement towards microtubule minus ends (retrograde movement) to pull on the spindle poles 30,31. However, some kinesins and other MAPs play ancillary roles in spindle positioning by controlling microtubule dynamics and stability as discussed above. In addition, in mammary cells kinesin-1 has been reported to contribute to LGN, NuMA and dynein cortical targeting by direct transport on astral microtubules 32. To perform this role in force generation, rather than simply moving on microtubules, dynein needs to be stably connected to the cell cortex.
The first evidence in support of the existence of force generating macromolecular complexes acting on spindles came from studies in D. melanogaster and C. elegans 8–10, showing that in mitosis, GDP-loaded Gαi subunits of heterotrimeric G proteins [G] localize in cortical crescents above the spindle poles. In this location, they initiate an interaction cascade leading to the sequential recruitment of proteins named Pins in D. melanogaster, GPR1/2 in C. elegans and LGN in vertebrates (also known as GPSM2), and the dynein adaptor [G], termed Mud in D. melanogaster, LIN-5 in C. elegans and NuMA in vertebrates. NuMA then recruits and activates dynein, establishing a complete, cortical machinery capable of generating pulling forces on the spindle (see next section).
Metaphase crescents of Gαi–GDP target LGN and NuMA to the mitotic cortex33,31, supporting the notion of a conserved function of the Gαi–LGN–NuMA core module in spindle orientation 7,34 (Figure 1b). A number of observations led to the conclusion that the Gαi–LGN–NuMA module exerts this function by modulating the extent of microtubule pulling forces, which correlate with cortical LGN levels31. Accordingly, ectopic overexpression of LGN results in increased rotation of the spindle, termed rocking, caused by excessive cortical forces31, a phenomenon that is not observed upon NuMA overexpression 35.
Vertebrate LGN is a 77 kDa protein consisting of an N-terminal Tetratrico-Peptide-Repeat (TPR) domain [G] and a C-terminal region coding for four GoLoco motifs [G] interacting with Gαi–GDP. In the unliganded form, LGN is kept inhibited by intra-molecular interaction between the inner surface of the LGN-TPR domain and the GoLoco motifs 33,36 (Figure 2b). Cooperative binding of cortical Gαi–GDP to the LGN-GoLoco region induces a conformational opening of LGN that allows binding of the C-terminal region of NuMA in the same TPR cleft previously occupied by the LGN-GoLoco 37. Notably, in vertebrates, a homologue of LGN exists. This protein, termed AGS3 (Activator of G-protein-Signal 3), shares the same domain structure with LGN but is unable to localize cortically in dividing mouse and chick neuroepithelial progenitors. It is also unable to rescue misorientation phenotypes induced by LGN loss 38. Intriguingly, AGS3 has been implicated in oriented divisions39, perhaps by regulating cortical Gαi–GTP/GDP availability (see next section).
Control of oriented force generation
Once assembled, the mitotic spindle acts as a rigid structure, whose movement and rotation occurs without deformations, and that usually is localized at the centre of the cell. Importantly, the spindle adopts an orientation that responds to intracellular and extracellular cues. Unsurprisingly, this requires various levels of regulation of the cortical force generation complex, and we discuss below several mechanisms that have emerged to date including the Gαi GTP-cycle, the interaction of NuMA with dynein– dynactin, and NuMA post-translational modifications.
Role of nucleotide cycle in Gαi.
In D. melanogaster neuroblasts, the conformational rearrangement of Pins was shown to be triggered by association with the GTP-loaded pool of the G protein variant oα that is generated apically by the rhodopsin family G protein-coupled receptor [G] (GPCR) Tre1. This interaction primes Pins to bind Gαi-GDP 40. The evidence suggests that specific GPCRs might be implicated in generating a localized pool of Gαi molecules acting as initial positional cue that ultimately instructs cortical dynein recruitment. This provides a mechanism to coordinate the division orientation with the surrounding tissue that delivers the extrinsic signal for GPCR activation. There are several observations that suggest that GPCRs may play similar roles in some vertebrate cells, including in the kidney and neural progenitor cells 41,42, though this requires further examination. An additional unclear aspect of Gαi-dependent mechanism of spindle orientation relates to evidence that LGN associates only with GDP-loaded Gαi. This raises the possibility that the Gαi GTP/GDP cycle is important for spindle orientation, but the details of how the nucleotide state of Gαi contributes to spindle orientation require further investigation. In D. melanogaster neuroblasts and one-cell stage C. elegans embryos, the GTPase-activating protein (GAP) for Gαi, RGS-7, and the respective guanine nucleotide exchange factor (GEF), RIC-8, synergistically promote oriented cell divisions 7 43–45. The vertebrate RIC-8 homologue, RIC-8A (also known as synembryn-A), is required for LGN, NuMA and dynein cortical targeting in metaphase, and hence for oriented divisions of adherent HeLa cells and MDCK polarized cysts 46,47. Intriguingly, recent literature indicates that the working principles of RIC-8A differ profoundly from the canonical GEF factors, which induce a conformational rearrangement of the GEF nucleotide-binding pocket promoting GDP to GTP exchange. Structural studies revealed that RIC-8A rather acts as a chaperone cradle, that folds the nascent Gαi chain in a step-wise mechanism and assists GTP loading 48–52. However, how this chaperoning activity could favour LGN–NuMA recruitment at the cortex remains largely unclear (Figure 2c).
Function of NuMA as a dynein adaptor.
Functional studies on reconstituted dynein motors revealed that the processivity of mammalian dynein is enhanced by the presence of the macromolecular cofactor dynactin 53 and of different activating adaptors, which strengthen the dynein–dynactin binding affinity and physically link the motor to its cargoes conferring specificity (Figure 2d)54. HOOK2 was recently shown to be the dynein–dynactin activating adaptor promoting centrosome separation at mitotic entry, astral microtubule nucleation, and cytokinesis. Consistently, HOOK2 depletion results in spindle pole defects and therefore spindle misorientation 11.
Notably, elegant optogenetic studies conducted in HeLa and HCT116 cells showed that direct targeting of endogenous dynein to the cortex is insufficient to move the spindle 55, suggesting that dynein activators and/or other factors are required for its astral-microtubule pulling function. Several lines of evidence support the notion that NuMA is the dynein activating adaptor responsible for spindle orientation activities. Accordingly, binding of NuMA to dynein is required for dynein–dynactin cortical targeting, and hence for spindle positioning 31,55,56. NuMA shares domain structure with other dynein activating adaptors having an N-terminal hook domain followed by a dimerizing coiled-coil, and a C-terminal unstructured region. The NuMA C-terminus is responsible for the direct binding to plasma membrane 57,58, cortical proteins such as 4.1R [G] 56,59, LGN37,60,61 as well as microtubules and α/β-tubulin dimers 35,62–64 (Figure 2a), whereas, the hook domain and a recently identified CC1-like box motif [G] that resides within the N-terminus of NuMA have been found to the required for dynein–dynactin interaction 31 by directly contacting dynein65. The evidence that NuMA harbours two distinct dynein-interacting regions hints at the possibility of high-stoichiometry complexes with dynein, in which two dynein motors assemble with one NuMA dimer and one dynactin to ensure robust microtubule traction force, as already observed for other adaptors 66,67. Mutational analyses revealed that in HeLa cells the coiled-coil of NuMA is also necessary for dynein-dependent spindle positioning, and that shortening of its length results in NuMA molecules that are unable to promote spindle movement 55. An additional layer of supramolecular NuMA–dynein–dynactin ordering is promoted by the hetero-hexameric architecture of the NuMA–LGN complexes that was recently uncovered. In fact, the LGN–NuMA 3:3 stoichiometry (Figure 2b), combined with the dimeric nature of NuMA, might promote multivalent interactions leading to the assembly of cortical protein networks that are required for spindle orientation, as indicated in in HeLa cells and polarized Caco-2 cysts64. Interestingly, the NuMA C-terminal region has been shown to promote cortical dynein clustering that becomes visible as a punctate signal by confocal microscopy55 suggesting that a defined spatial organization of dynein motors concentrated in localized cortical areas is needed to effectively position the spindle. Whether the hexameric NuMA–LGN interactions are key in promoting the formation of cortical NuMA–dynein clusters observed by confocal microscopy in HeLa cells 55 remains an intriguing open question.
Of note, in spite of the number of evidences supporting the notion that NuMA can act as a dynein adaptor, a clear demonstration that NuMA can activate processive dynein–dynactin movement (for example, with reconstituted complexes) is still missing. Also, .in addition to its role as dynein adaptor, NuMA also binds microtubules and interactions of NuMA with astral microtubule plus-ends has been proposed to contribute to spindle orientation in keratinocytes 63.
Coordination with mitotic progression.
To coordinate spindle positioning with mitotic progression, the distribution of NuMA, and hence dynein–dynactin, between poles and the cortex is finely tuned by mitotic kinases. Phosphorylation by Aurora A [G] positively promotes the turnover of NuMA at the poles, preventing excessive accumulation at the poles in early mitosis and enabling cortical recruitment of NuMA specifically at metaphase 35,68. NuMA is also phosphorylated by CDK1, which is reversed by PP2A phosphatase activity, and these phosphorylation–dephosphorylation events regulate NuMA binding to the plasma membrane. At anaphase onset, when CDK1 activity decreases, NuMA is dephosphorylated, which promotes its LGN-independent binding to the plasma membrane phospholipids. Direct binding of NuMA to the plasma membrane above the spindle poles allows a much enhanced accumulation of cortical NuMA–dynein above the spindle poles in anaphase, and hence the generation of stronger dynein-based traction forces to assist spindle elongation and sister chromatids separation 56,69,70 71 (Figure 1b–c). Furthermore, the activity of PLK1 [G] disrupts the NuMA–dynein–dynactin interaction when the poles get too close to the cortex, this way sustaining spindle centring by oscillatory movements72,73. Furthermore, the activity of kinetochore-localized PLK1 was reported to prevent cortical LGN enrichment near chromosomes, hinting at the existence of an exclusion mechanism acting at the LGN level able to confine force generators in defined cortical crescents 74. In metaphase, the activity of PLK1 has also been shown to act as an additional layer negatively controlling cortical NuMA and dynein levels in an LGN-independent manner by direct phosphorylation of the C-terminal region of NuMA 73. Collectively, these evidences depict PLK1 as a master regulator of cortical pulling forces positioning the spindle.
In adherent cells, the chromosome derived Ran-GTP [G] gradient has also been shown to contribute to cortical exclusion of LGN–NuMA from the spindle middle zone; this might occur by local remodelling of the stiffness and contractility of the actomyosin cortex, but molecular details are still not clear 59. A similar mechanism of actomyosin contractility reshaping accompanied by relaxation of tension at the poles stabilizes the spindle position at anaphase onset to promote sister chromatid separation 75. Interestingly, NuMA harbours a nuclear localization sequence (NLS), which overlaps with its microtubule-binding domain. This initially suggested that Ran-GTP-dependent dissociation of NuMA from importin-b could initiate microtubule-dependent spindle assembly functions 76. However, recent studies revealed that the Ran-GTP gradient is not essential for spindle assembly activities that occur far from the chromosomes, including NuMA-dependent spindle pole focusing.
Role of WNT signalling in NuMA-dependent spindle orientation.
Increasing evidence points at the existence of NuMA-mediated LGN-independent mechanisms recruiting dynein–dynactin-based force generators at the cortex. So far, the best characterized pathway is linked to non-canonical WNT signalling [G] and converges on the putative direct interaction between NuMA and the WNT effector Dishevelled (DVL, Dsh in D. melanogaster). Initial hints of WNT-dependent spindle orientation came from studies in D. melanogaster sensory organ precursor cells in which the apico-basal vertical tilt of the spindle was shown to be regulated by Frizzled receptors acting upstream of Dsh and Mud 77,78. This link was later confirmed in zebrafish epiblast cells in which the NuMA–Dvl interaction promotes spindle orientation along the animal-vegetal axis during gastrulation 79,80. In HeLa cells, binding of NuMA to DVL depends on the deubiquitinase activity of CYLD, that also stabilizes astral microtubules81. The notion that WNT signals not only contribute but also suffice to drive oriented asymmetric mitoses was recently demonstrated by elegant single-cell analyses of cultured murine embryonic stem cells (mESCs) (see also Supplementary BOX-1), recapitulating the niche–ESC interaction in vitro 82,83. These experiments revealed that when exposed to a bead coated with a WNT ligand, WNT3, mESCs orient the mitotic spindle, and specifically the mother centrosome [G], towards the bead. They also polarize WNT components such as Lrp6, APC and β-catenin near the bead, this way preserving stemness of the daughter cell retaining contacts to the bead and promoting differentiation of the one positioned away from the bead. Interestingly, the concept of WNT3-mediated asymmetric oriented ESC divisions has recently found important application in bone regeneration strategies 84.
Implication of the actin cytoskeleton
The actin cytoskeleton is formed by double-helical actin filaments (F-actin), which are much thinner and less stiff than microtubules. The major cellular morphological changes observed in mitosis reflect the reorganization of the actin cytoskeleton in a contractile actomyosin cortex that first drive cell rounding by building a uniform high tension, and later, assist cytokinesis by forming a highly contractile ring at the midzone at the site of the cleavage furrow ingression 85 (Figure 1b–c). This reorganization of the actin cytoskeleton contributes to determine the spindle orientation in several ways.
Contribution of mitotic cell rounding.
The nearly spherical shape of mitotic cell is essential to provide 3D space for the assembly and the positioning of the mitotic spindle. Specifically, the rounded cell shape supports spindle movements: in metaphase and anaphase the actomyosin cortex of rounded cells provides a rigid scaffold counteracting the traction forces exerted by force generators on astral microtubules and preventing plasma membrane invaginations during spindle positioning and elongation 86. A critical factor for this scaffolding function is the stable anchoring of the actomyosin cortex to the plasma membrane, which is provided by the ERM (Ezrin-Radixin-Moesin) family proteins [G] 87 (Figure 1b and 2a). In cultured cells and murine neuronal progenitors, ERM proteins activated by the Ste20-like kinase [G] have been directly linked to spindle orientation by favouring enrichment of LGN and NuMA into cortical crescents88.
Recent evidence uncovered the existence of direct physical linkages between the actomyosin cortex and dynein motors orienting the spindle. Structural and functional studies showed that in cultured HeLa cells the actin-binding protein Afadin (Canoe in D. melanogaster) localizes at the mitotic cortex and promotes the recruitment of LGN by direct interaction with the TPR domain, this way favouring dynein–dynactin motor enrichment at the cortex 89 (Figure 3). A key role in directing NuMA to the cortex of metaphase cells has been also reported for the 4.1R protein family 56,59, although in this case it is not trivial to uncouple the structural role of 4.1R proteins at the cortex from their cortical NuMA-targeting function (Figure 2a).
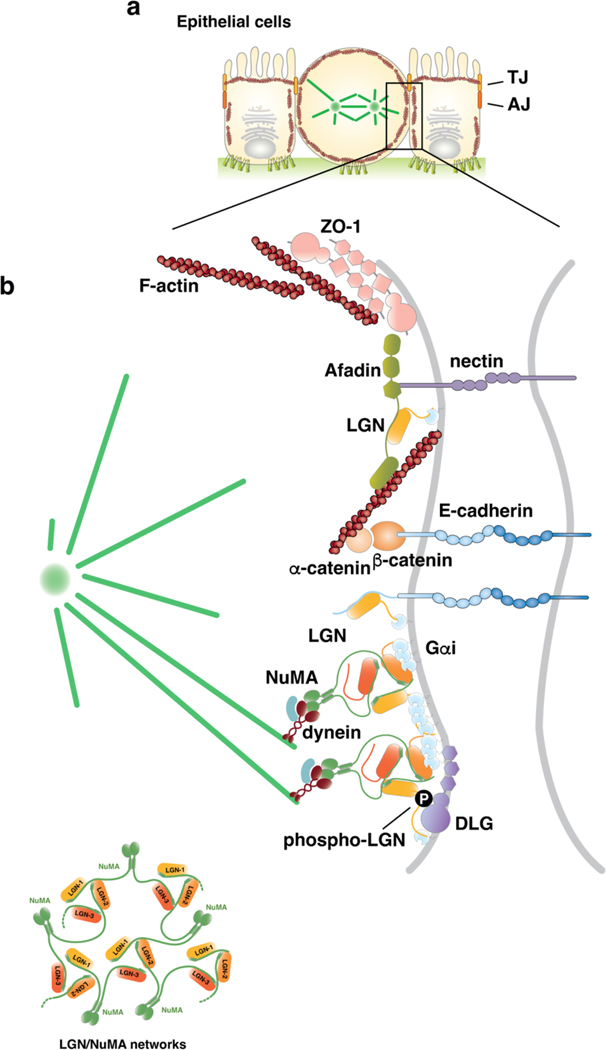
Figure 3. Molecular mechanisms of oriented planar cell divisions.
a) Schematic representation of epithelial mitosis in which the dividing cell rounds up maintaining adhesive junctions with neighbouring cells and with the extracellular matrix (ECM) at the basal site. Epithelial cell–cell contacts include adherens and tight junctions. b) Enlarged view of the molecular players accumulating at the lateral membrane during epithelial mitoses to promote planar cell divisions. Microtubule motors organized on dynein–dynactin–NuMA complexes are recruited laterally by Gαi–LGN–NuMA. Binding of NuMA to LGN and Gαi is compatible with the concomitant association of phospho (P)-LGN to the GK domain of the baso-lateral protein DLG because this LGN phosphorylation — mediated by the apical polarity kinase aPKC — occurs on the linker region between the Tetratrico-Peptide-Repeat (TPR) domain and the GoLoco motifs. In addition, the LGN-TPR interacts directly with the cytoplasmic tail of E-cadherin and with the actin-binding protein Afadin that localizes at the tight junctions with nectin [G] and ZO-1. Cell–cell junctions respond to mechanical forces by changing their organization and function (mechanosensing). Hence, forces acting tissue-wide, such as compression or stretching that can result from, for example, changes in cell number or morphogenetic movements, can be transmitted to regulate spindle orientation.
The profound cell shape change induced by the mitotic rounding-up has interesting implications for the cell division orientation with respect to extracellular cues. In epithelia, mitotic rounding counteracts the columnar shape that cells have in interphase, thereby preventing divisions occurring along the apico-basal axis — which may be unwanted, leading to, for example, cell delamination from the tissue — while allowing ‘memory’ of the cell shape via maintenance of cell–cell junctions. As we will describe better later, these events are key for oriented planar cell divisions, occurring with the spindle axis perpendicular to the apico-basal polarity axis and generating daughter cells positioned in the same epithelial layer as the mother cell. Interestingly, cultured cells that round-up for mitosis also retain memory of the shape they had in interphase, indicating the existence of adhesion cues granting memory thought the cell cycle. These cues have been associated with the assembly of actin-rich protrusions known as retraction fibres, that secure mitotic cells to their substrates (Figure 1b). Retraction fibres capture astral microtubule plus-ends in regions that are associated with subcortical actin clouds — mitosis-specific dynamic pools of subcortical filamentous actin that organize in response to the position of retraction fibers — (Figure 1b), by direct interaction of the protein MIPS (mitotic interactor and substrate of PLK1) with +TIPs such as EB1 and with the dynactin subunit p150-Glued 90,91, and by concomitant binding of myosin X [G] to F-actin and to astral plus-ends92 (Figure 2a). Establishment of retraction fibres also impinges on the membrane associated protein caveolin-1, which upon cell rounding enriches locally near retraction fibres and promotes formation of caveolae [G] -like structures. These membrane invagination are then able to recruit Gαi–LGN–NuMA complexes 93 (Figure 1a). Recent evidence pointed at the existence of mitotic actin clouds also near the spindle poles, that have also been proposed to regulate the growth of astral microtubules radiating from the poles and to transduce mechanical forces form the cortex to the spindle, possibly contributing in these ways to spindle positioning 94,95.
Cooperation between the microtubule and actin cytoskeleton.
Microtubule and actin interplay during many cellular processes, including in cell division, when these cytoskeletal systems work together in setting the division plane (reviewed in96). Molecularly, this interplay occurs in a number of different ways. On the one hand, actin and microtubule-interactors crosslink physically microtubule and actin filaments to guide microtubule growth and stabilize +TIPs at the cortex, as illustrated above. On the other hand, cortical actin acts as a barrier for astral microtubule to reach membrane-bound cortical anchors such as Gαi–LGN–NuMA complexes. Beside mechanical links, F-actin and astral microtubules share also common regulators of the RHO small GTPase family, controlling both F-actin and microtubules at the plus ends. In line with this, the RHO GTPase Cdc42 was among the first components of the spindle orientation pathway to be identified as an effector of planar divisions of Caco-2 cysts97.
Role of tissue architectural cues
Even though the machinery for spindle orientation that we have described so far functions intracellularly, it has the capacity to respond to extracellular cues guiding the recruitment of force generators to specific sites. This allows this machinery to regulate spindle orientation and in extension the cell division plane with respect to tissue architecture, thereby allowing an appropriate cell response to developmental and regeneration programmes. External cues impacting the spindle orientation pathway have been mostly studied in epithelial tissues and include cell adhesion to the extracellular matrix (ECM; or to the substrate in the case of cultured cells), and cell–cell contacts, including epithelial adherens junctions and tight junctions. In this paragraph, we will survey the current knowledge on the mechanisms transducing environmental cues to the spindle apparatus to orient cell divisions.
Cell–ECM adhesion.
As already mentioned in the previous paragraph, mitotic cells retain memory of interphase adhesion geometry via retraction fibres. Great insights into the molecular link between division orientation and substrate adhesion came from studies in cells cultured on adhesive fibronectin micropatterns of defined shapes 4,98 (see also Supplementary BOX-1). These elegant analyses not only led to the discovery that the mitotic distribution of retraction fibres is a key predictor of division orientation, but also provided knowledge of the molecular events underlying this phenotype. In interphase, canonical focal adhesion [G] complexes consisting of the focal adhesion kinase (FAK), talin and paxillin associate with the cytoplasmic tail of the β-integrin subunit of the integrin transmembrane receptor to form a layer connecting the cell to the substrate 99 (Figure 1b). Recent studies in HeLa cells suggested that focal adhesion complexes are not totally disassembled upon cell rounding, but that a thin signalling layer of paxillin, vinculin and FAK remains to maintain weaker adhesion 100. The relevance in vivo of adhesion to the ECM for oriented divisions is underscored by the evidence that β1-integrin ablation results in spindle misorientation in the murine developing skin and aberrations in keratinocyte stratification in the tissue (see also next section) 101.
Polarity and cell–cell adhesion.
Division orientation by definition implies an alignment of the cell division plane to a distinctive feature of the cell itself or its surrounding environment. As described above, for cultured cells in isolation the division orientation is oftentimes defined by mitotic spindle axis alignment with the plane of the substratum. However, in multicellular organisms, this is much more complex and division orientation is evaluated on many levels, for example with respect to tissue architecture or apico-basal polarity in the case of epithelial tissues.
Epithelial tissues are organized in monolayers of cells characterized by an asymmetrical distribution of cytoplasmic and membrane-associated cytoplasmic components, and a polarized actin and microtubule cytoskeleton. Intercellular adhesion occurs through junctional complexes positioned at the lateral sites of the cells, that also set the borders between apical and baso-lateral domains 102 (Figure 3a). As mentioned before, mitotic cells inherit polarity cues from interphase in the form of intercellular and ECM adhesive contacts; these ‘memory’ structures also contribute to determine the division orientation. Epithelial cells can orient their mitotic spindle parallel to the epithelial plane, this way promoting planar cell divisions in which daughter cells are positioned close to one-another and retain the same integrin-mediated contacts with the basement membrane [G]. Alternatively, they can undergo apico-basal divisions, which often induce delamination of one of the daughter cells and have been associated with the acquisition of asymmetric cell fates 29,101,103.
Polarity can be regarded as a prerequisite for regulated spindle orientation as defects in polarity invariably result in mislocalization of force generators and randomized orientation. A number of molecular mechanisms connects epithelial polarity to the spindle orientation pathway, mostly related to adhesive complexes. Astral microtubule plus-ends are captured by F-actin-rich adherens junctions. This occurs via E-cadherins, whose intracellular portion is also able to interact directly with the TPR domain of LGN 104. Specifically, in polarized monolayers of MDCK and enteroendocrine L-cells derived from the gut, the cytosolic tail of E-cadherins recruits LGN to adherens junction already in interphase, forming complexes with topology resembling the ones established for canonical interactions at adherens junctions between E-cadherin and catenins [G] (Figure 3b). Notably, in this experimental setting, planar spindle alignment along the underlying substrate not only requires binding of E-cadherin to LGN but also extracellular homotypic E-cadherin contacts, indicating a dual function of adherens junctions in sensing tissue organization — likely via transmission of tension, as adherens junctions are mechanosensitive 105— and concomitantly transducing this information to the spindle orientation machinery via LGN 104,106. Similarly, tight-junctions, which, in vertebrate cells are positioned apically to adherens junctions and are enriched in ZO-1 and Afadin, directs planar divisions by direct association of the Afadin C-terminal portion to LGN-TPR 89 (Figure 3b). Live cell imaging analyses of stretched Xenopus laevis embryonic tissues integrated with mathematical modelling revealed that tricellular junctions — where at least three cells meet — reorient in response to external tension, and instruct the orientation axis by accumulating LGN 107. This mirrors observations in the D. melanogaster notum [G] where NuMA was shown to instruct spindle orientation by localizing at tricellular junctions 108.
Consistent with the crosstalk between lateral cell–cell adhesion and spindle motors, planar divisions of chick neuroepithelial cells have been shown to rely on the Gαi-dependent distribution of LGN and NuMA in an equatorial belt at the lateral cortex, in accordance with the positioning of cellular junctions in these cells109. Of note, in addition to junctional proteins, also other lateral cues might contribute to secure this lateral NuMA–LGN localization, including the direct interaction of LGN with polarity protein DLG1 (a component of the Scribble polarity complex [G] ), promoted by mitotic phosphorylation of LGN by apical polarity-associated kinase aPKC 110 (Figure 3b).
An intriguing aspect of the junction-to-spindle connection relates to the evidence that E-cadherin and Afadin bind to LGN competitively with NuMA, as they all share a common negatively-charged motif that binds to the TPR domain of LGN37,89,104. A model of sequential binding of LGN first to the adhesive positional cues provided by cell–cell junctions and later, at mitotic entry, to NuMA in order to transfer the epithelial positional information to dynein–dynactin motors has been envisioned 89,104, although it is not straightforward to provide experimental validation for it.
Connections with tissue mechanics.
As mentioned above, division orientation is contributed not only by directional active forces pulling on astral microtubules towards specific cortical sites but also by tissue architecture. Another tissue-wide cue that impacts spindle positioning is tissue mechanics, which, in epithelia, is tightly related to tissue architecture via parameters such as cell crowdedness and cell shape.
Initial observations of the importance of cell shape on spindle orientation were made already in the late 19th century in artificially flattened amphibian eggs by Oscar Hertwig, who showed that the mitotic spindle could align passively to the longest cell axis111. However, the events instructing this kind of spindle movement were poorly understood for many years to come. Division orientation can also be influenced by tissue stretching that, in the case of uniaxial stretch, results in division along the deformation axis and serves to release mechanical tension at the lateral junctions, as elegantly documented in D. melanogaster pupal notum epithelium 108, in zebrafish epiboly [G] 112 as well as in vertebrate cells plated on deformable patterns 56,106,113. Mechanistically, it is plausible to assume that changes in cell shape generated by compression and tissue stretching instruct spindle axis alignment along the longest cell dimension. In parallel, during stretching, cell–cell contacts undergo remodelling, which includes actomyosin reorganization to favour accumulation of force generators at the contact under tension 98. Thus, one might say that cell shape and active spindle orientation mechanisms are coordinated in response to external mechanical stimuli, such as stretch and compression, which in turn are coupled to tissue dynamics, including proliferation, morphogenetic movements and mechanical stress/trauma.
We speculate that adhesion-dependent planar divisions might rely on molecular mechanosensitive platforms forming at adhesive sites, where multivalent dynamic interactions between adhesive molecules (such as ZO-1, Afadin and possibly also E-cadherin) and LGN determine spindle position in response to mechanical cues received. In such a model, LGN would cycle among different partners at the lateral site of epithelial cells alternating between junction-organized, positional complexes and NuMA–dynein–dynactin pulling ones (Figure 3b), this way recruiting dynein motors at adhesive sites instructed by the tissue organization and local tension. We conclude this paragraph by mentioning that throughout mitosis, cell shape, epithelial membrane tension and junctional mechanotransduction are affected by membrane trafficking and endocytosis (reviewed in 114). However, whether and how direct molecular links exist between membrane trafficking and division orientations remains to be explored.
Implications for tissue organization
Spindle orientation has well documented roles in regulating tissue organization and differentiation, although most of what we know about these mechanisms comes from invertebrate systems 115. Nevertheless, studies in recent years have also provided insights into the importance of spindle orientation in vertebrate tissues (see Table 1, which presents a list of vertebrate tissues and cell types in which regulated spindle orientation has been reported, with key examples from literature of the roles of oriented cell divisions in these systems). The two major functions of regulated spindle orientation are the control of tissue organization with the generation of distinct tissue architectures and cell fate specification via asymmetric divisions. These processes are often linked, but we discuss them in separate sections. Here, we focus on four, best described roles of spindle orientation in epithelial organization — stratification; branching/sprouting; elongation and turnover; and maintenance of a simple epithelium (Figure 4). In the following section, we will discuss in more detail connections between spindle orientation and establishment of tissue architecture.
Table 1.
Roles of oriented cell divisions in vertebrate tissues
| Tissue | Proposed role of spindle oreintation | References |
|---|---|---|
| Epidermis (Interfollicular) | Promotes stratification and differentiation | 101,103,116 |
| Epidermis (Hair placode) | Proposed to generate SOX9-positive stem cell precursors (division orientations have been observed but not disrupted) | 118 |
| Hair follicle matrix | Promotes the formation of differentiated hair cells | 63 |
| Oral epithelium | Stratification and differentiation | 119 |
| Oesophagus | Stratification and differentiation | 165 |
| Lung | Maintains epithelial architecture necessary for branching, planar divisions prevent stratification | 120,121 |
| Epicardium | Asymmetric division with apical cell remaining in epicardium and basal cell delaminating and entering the underlying myocardium | 166 |
| Endothelium | Occurs during sprouting, daughters have asymmetric fates and sizes, drives angiogenesis | 122 |
| T cells | Promotes asymmetric division, whereby Tbet transcription factor is asymmetrically inherited by effector T cells. | 131 |
| B cells | Promotes asymmetric division, BCL6 is asymmetrically inherited during division of germinal centre B cells. | 133 |
| Haematopoietic stem cells | Notch inhibitor Numb and lysosomes are inherited asymmetrically and may influence activation and differentiation decisions | 150,167 |
| Neural progenitors | Regulated spindle orientation during development, functional role remains controversial | 127,168,169 |
| Intestinal epithelium | Proposed to maintain simple epithelium and to promote linear arrays of cells moving up into the villus | 123 |
| Kidney epithelium | Disruption of spindle orientation is associated with cyst formation | 154,170–172 |
| Muscle (Satellite (stem) cells) | Asymmetric divisions of stem cells regulate production of progenitors | 173 |
| Oocytes | F-actin-dependent spindle positioning generates the large oocyte and small polar bodies | 61,143 |
| Mammary gland epithelium | Promotes branching | 174 |
| Prostate epithelium | Thought to promote luminal cell fates, branching and tissue polarity | 121,175,176 |
| Testes | Regulates germ cell fate, disruption may promote seminomas | 177,178 |
| Retina | Proposed to control cell fate and differentiation | 179,180 |
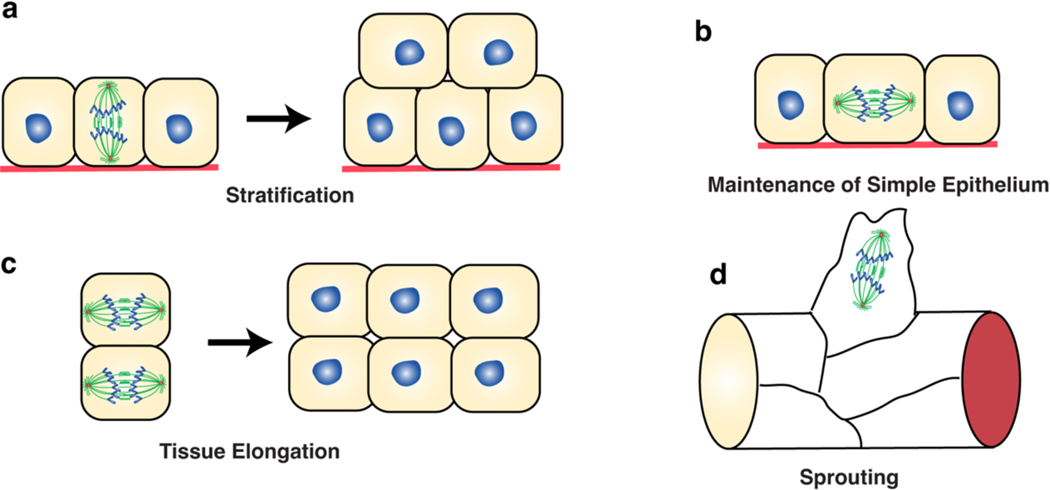
Figure 4. Roles of regulated spindle orientation in controlling tissue architecture.
a) Development of stratified epithelia is driven by spindle reorientation with respect to the basement membrane, from parallel to perpendicular. This directly generates an additional layer(s) of cell(s). These divisions are typically coupled to cell fate determination, whereby the basal daughter remains a progenitor and the apical daughter is fated for differentiation. b) Simple epithelia maintain their architecture by orienting their mitotic spindle parallel to the basement membrane. This prevents abnormal positioning of cells within the tissue, which would disturb tissue architecture and/or could lead to abnormal proliferation. c) Planar organization of spindles in one direction across the tissue can lead to elongation of a tissue (for example during morphogenetic growth or during tissue regeneration/homeostatic turnover). d) Spindle orientation can promote tissue branching in various ways. In the example shown, during angiogenesis, endothelial cells branch off of the main vessels, and orient their spindles along the branching axis. These divisions are also coupled to the acquisition of asymmetric cell fates, whereby the spindle is displaced proximal to the vessel, generating a smaller proximal and a larger distal cell, which become stalk cell and tip cell respectively. Tip cells are migratory and drive angiogenic branching of the vessel.
Oriented cell divisions in tissue stratification.
Stratified epithelia, including the epidermis of the skin, oral cavity, cornea and oesophagus, are characterized by multiple layers of epithelial cells in physical contact with one another. They are distinct from simple epithelia as the basal cells that sit on the underlying basement membrane do not have a free apical surface that characterizes simple epithelia. Despite this, these cells are polarized and use that polarity to orient their spindles. We highlight here three distinct roles for oriented cell divisions with a focus on mammalian epidermis, which has been extensively used to study the mechanism and functions of spindle orientation in vertebrates.
First, in the mouse embryonic epidermis, oriented divisions regulate tissue architecture and differentiation. In this case, aligning the spindle perpendicular to the basement membrane results in asymmetric division (see next section), resulting in one basal cell, which remains a progenitor and one apical daughter cell, which undergoes differentiation. In this way, these divisions promote both tissue stratification and connect that with cell fate decisions. By contrast, divisions with spindle aligned parallel to the basement membrane generate two basal daughters, which facilitates tissue growth and expansion of the basal progenitor pool, while preventing cell differentiation 101,116. The canonical spindle orientation machinery, described above, is required for these oriented divisions, as are adherens junctions and integrin-based adhesions 63,101,103. Promoting parallel spindle orientation results in a failure to stratify the tissue and perturbs tissue patterning, with randomization of division orientation leading to less severe defects. Accordingly, live imaging revealed that spindle orientation in the mouse embryonic epidermis is indeed a strong predictor of cell position, although oblique divisions can be resolved in distinct ways that can’t be predicted at late metaphase 117. Oriented cell divisions are also found during early stages of hair follicle morphogenesis118. In this case, cells divide with spindles oriented exclusively perpendicular to the basement membrane, which has been proposed to generate the bilayered placode with distinct cell types. Finally, during postnatal hair follicle morphogenesis, progenitors divide with spindles perpendicular to the underlying basement membrane to give rise to inner layers of the hair follicle 63.
Notably, the molecular requirements for the oriented divisions can differ depending on the epithelial tissue, suggesting molecular flexibility and context-specificity of spindle orientation machinery. For example, LGN is required in interfollicular epidermis, but not in hair placodes; similarly, different regions of the oral epithelium show differences in their reliance on LGN for tissue stratification119. Also, the perpendicular divisions are generally asymmetric in fate and coupled to the generation of distinct cell types, depending on the context. However, our understanding of molecular determinants of cell fate acquisition by vertebrate cells and how they are coupled to spindle orientation are only beginning to emerge (see next section).
Oriented cell divisions in tissue branching.
In addition to forming simple sheets, epithelia can also form highly branched networks that are required for their function. As notable examples, the developing mammalian lung and the gestational mammary gland undergo extensive branching that is required for their final form and function. In the developing lung epithelium, mitotic spindles fall into two categories: those that quickly align with the long axis of the cell and the airway axis, and those that continue to rotate throughout metaphase and contribute to branching 120,121. Cell shape, particularly length, appears to have an important role in regulating spindle position in these cells, as changing cell length disrupts the proportion of cells dividing in each mode and ultimately, disrupts the morphology of the branched epithelium 121. However, the molecular machinery that controls the differential spindle dynamics has not yet been described.
Of note, this function for spindle orientation is not limited to epithelia, but has also been reported to drive endothelial sprouting required for the elaboration of the vascular network during angiogenesis122. In this case, cells in angiogenic sprouts divide to generate a small tip cell enriched in VEGF [G] receptor, which is able to respond to angiogenic cues and initiate directed migration, and a larger stalk cell, which follow the path of tip cells while retaining high proliferative capacity. Thus, during angiogenic sprouting, oriented cell division is tightly connected to functional roles of the progeny, similar to scenario of epithelial stratification described above.
Oriented cell divisions in tissue elongation and turnover.
Alignment of spindle orientation along a defined axis (anterior–posterior or proximal–distal for example) in epithelia results in directional elongation of this tissue, by adding more cells consistently in a given plane 80. Similar mechanisms are responsible for homeostatic epithelial turnover in the small intestine. Here, tissue turnover relies on sustained cell divisions in the crypts, which serve as niches that house the stem cells and transit amplifying cells. Cell divisions in these cells align along the crypt axis, perhaps to maintain crypt diameter and to drive movement of these cells in a conveyor-belt fashion into the differentiated villus to replace cells that shed off from the tissue 123.
Oriented cell divisions in the maintenance of simple epithelia.
Simple epithelia, like those lining the intestines, kidney and lungs, orient their spindles parallel to the underlying ECM to maintain their simple architecture and prevent stratification. In epithelial cells cultured in vitro as 3D cysts, loss of planar spindle orientation leads to either multi-layering or multi-lumen phenotypes 89,97. In vivo, in the mouse lung epithelium, disruption of β1-integrin results in spindle misorientation and a multilayering phenotype, but whether spindle misorientation is sufficient to generate this phenotype is not known 124. More recently, in vivo studies in zebrafish embryos revealed the importance of planar cell divisions for the maintenance of integrity in the retinal neuroepithelium — a precursor tissue for the retina 125. The cells in the neuroepithelium are elongated and distribute their nuclei along the apico-basal axis in the interphase. However, for mitosis they redistribute their nuclei to the apical surface, where also cell–cell adhesions reside. During tissue proliferation, most of the divisions occur with spindles oriented parallel to the apical surface, resulting in two daughters that retain their apical contact and contact with each other. Spindle misalignment or induction of basal mitoses — whereby cells divide with random orientations, likely owing to the lack of positional cues delivered by cell–cell junctions — has been associated with the loss of apical contact by one of the daughters and cell delamination. This, in turn, led to the formation of aberrant, basal clusters of delaminated and perturbed tissue architecture126–128. Similar observations linking simple epithelium organization to spindle positioning have been made in intestinal organoids (see Supplementary BOX-1). Here, symmetric divisions guided by a parallel orientation of the spindle with respect to the apical surface were shown to result in both daughter cells maintaining their apical and basal attachments and staying close together after division. By contrast, asymmetric divisions, whereby the spindle is not aligned with the apical surface, were associated with the loss of basal attachment by one of the daughters and spatial separation of daughter cells in the tissue. This separation was then linked to the distribution of cells between tissue compartments (stem cell compartment versus transit amplifying cell compartment). In extension, if this distribution of cells is hampered, cells could gain the ability to colonize the stem cell niche more efficiently, potentially driving malignant growth127. In fact, mutations that result in misoriented divisions in the intestinal crypt were linked to oncogenesis129,130.
Remarkably, despite this evidence for the association between spindle orientation and simple epithelia organization and function, there is still no conclusive evidence for the roles of core spindle orientation machinery in most simple epithelia in vivo.
Connections to cell fate control
Spindle orientation can be used to generate cellular diversity when coupled to fate choices through asymmetric divisions 2,10. In this context, asymmetric division refers to the distinct fates of daughter cells, rather than size asymmetry. Importantly, this asymmetry can result from either intrinsic differences (that is, differential inheritance of cell fate determinants or from extrinsic differences (that is, differences in the microenvironment the daughter cells experience) that may result from the different positions of daughter cells within the tissue (Figure 5). Although very clear and elegant examples for intrinsic and extrinsic cues have been found in D. melanogaster and C. elegans (reviewed in 115), the mechanistic understanding of how spindle and division plane position translated to cell fate acquisition in vertebrate models, and in particular mammalian tissues, remains fragmentary, necessitating further studies in this area. This paucity of clear evidence may be due, in part, to robust and redundant mechanisms of spindle orientation in some cell types as well as potential rescue mechanisms (see next section). We describe the emerging themes below.
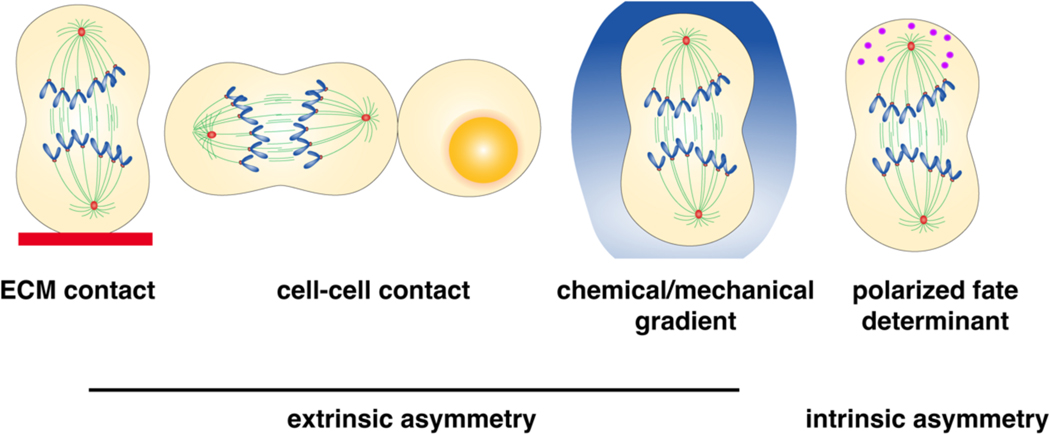
Figure 5. Cues implicated in asymmetric cell fate acquisition.
Cell fate asymmetry can be conferred by the daughters being exposed to distinct microenvironments (extrinsic cues) and/or via asymmetric segregation of cell fate determinants (intrinsic cues). Shown are three examples of extrinsic cues — the basement membrane, cell–cell contact and a gradient of cues, which can include both chemical and physical/mechanical signals. Intrinsic cell fate determinants may be proteins, RNA or organelles, which become asymmetrically positioned in the mitotic cell, accumulating towards one of the spindle poles or the cell cortex.
Asymmetric distribution of cell fate determinants in non-planar divisions.
Asymmetric inheritance of diverse cellular factors — these include proteins (such as transcription factors), RNAs, organelles, etc. — has been proposed to mediate cell fate decisions in invertebrate model systems (reviewed in115). The defining characteristic of such asymmetric divisions is that these fate-associated factors become enriched at one half or side of the cell defined by the spindle orientation (for example, cortical, cytoplasmic or enriched around one of the spindle poles), or that the spindle orientation is driven by the pre-existing polarity and/or the intracellular distribution of the cell fate determinant.
In vertebrates, these mechanisms have been described in T cells during their activation. In this process, a T-cell interacts with an antigen-presenting cell with which it establishes an immune synapse [G]. The immune synapse defines a polarity axis for the cell and the spindle aligns along this axis. The T-cell-specific T-box transcription factor T-bet, polarizes to the immune synapse, thus being preferentially inherited by the daughter cell proximal to the synapse. This then is linked to the acquisition of differential cell fates, specifying effector versus memory functions 131. This initial fate asymmetry in the naïve T cell is thought to be mediated by selective destruction of T-bet on the other end of the cell by the enrichment of the proteasome at this location 132. Similarly, during B cell activation, the BCL6 transcription factor is asymmetrically inherited 133, suggesting that these mechanisms are prevalent in the regulation of immune responses.
Another example of asymmetric distribution of intrinsic determinants is found in the early mouse embryo, where inheritance of the apical domain and/or apically associated intermediate filament cytoskeleton [G] component keratin drives cells toward the outer cell fate and extraembryonic tissue lineage, whereas cells that do not inherit the apical domain are more likely to internalize, becoming inner cell mass that generates the embryo proper 134.
Multiple studies across the years have also investigated asymmetric divisions in progenitors of the vertebrate central nervous system. These revealed that the inheritance of various components — including apical polarity proteins, primary cilium [G], the mother centrosome and the basal process that connects the cell to the ECM via integrin adhesions — is associated with retention of the proliferative state versus neurogenic differentiation (reviewed in 135). Having said so, the importance of spindle orientation in regulating cell fate decisions in neural progenitors is debated.
Extrinsic cues in the regulation of asymmetric fate acquisition.
Extrinsic factors can include contact-dependent cell–cell and cell–ECM interactions or gradients of signalling molecules. As epithelial cells sit on top of a basement membrane, this serves as an ideal extrinsic cue in many contexts. As mentioned above, the retention of the basal attachment confers proliferative potential on neural progenitor cells. Consistent with this idea, signals through integrins are essential for maintenance of proliferation in many tissues 136. Integrins are also important for proper spindle orientation in a number of cellular contexts 101, which complicates the genetic analysis of their roles in specific cell types. In stratified epithelia of the skin, expressing β1 integrin in the differentiated cells, from which it is normally absent, is sufficient to perturb the balance between proliferation and differentiation, consistent with the idea that integrins contribute to cell fate determination 137. That said, the pivotal experiment demonstrating the effects of disrupting integrin asymmetry upon cell division has not yet been accomplished. Further ahead, it will be required to decipher the signalling instigated by integrins to control cell fate.
Asymmetric distribution of signalling accompanying asymmetric divisions can also drive asymmetric cell signalling. A notable example here are the neural progenitors. In this case, Notch signalling components have been shown to be distributed unequally between the daughters, with cells inheriting high amounts of Notch receptor remaining progenitors and cells inheriting high amounts of Delta ligand being fated for differentiation. These experiments also revealed that through these mechanisms cells in the developing central nervous system generated their own micro-niche, in which neighbouring cells influence each other’s fate via regulation of Notch signalling 126,138–140
More recently, connections between asymmetric division and asymmetric cell signalling have been documented in the earliest steps of hair follicle formation141. As discussed above, during hair placode formation cells divide perpendicular to the basement membrane, thereby generating apical and basal progeny. In this case, the basal progenitor cells show polarized secretion of WNT signalling ligands and inhibitors, with the former accumulating basally and the latter apically. In consequence, when the early basal cell divides perpendicular to the tissue axis, its daughters experience a very different WNT-signalling environment, which favours maintenance of proliferative potential in the basal cell and differentiation of the supra-basal daughter. However, the respective contributions of intrinsic and extrinsic signals in cell fate specification in this cell type remain to be uncovered.
Cell size asymmetry.
An interesting, atypical example of spindle positioning/orientation in driving distinct cell fates is provided by the mammalian meiotic spindle in the oocyte. To generate the large oocyte, the spindle, rather than being positioned in the middle of the cell, is displaced to the cell cortex. There, the spindle rotates, with one spindle pole contacting the cortex, so that division results in the generation of one large cell, fated toward the oocyte and a small polar body [G]. Remarkably, this spindle displacement does not use the core spindle positioning molecular machinery and is dependent on F-actin but not microtubules 26,61,142,143. Myosin-mediated pulling forces, Arp2/3-mediated branched actin polymerization and resulting forces and cytoplasmic flows have all been implicated in this spindle movement. The subsequent anchoring and rotation of the spindle are also F-actin-dependent events. This example highlights the diversity in mechanisms for moving and placing spindles within the cells and suggests that other mechanisms may be elucidated by the further study of spindle orientation in diverse cell types. Asymmetry in cell size has also been linked to sprouting angiogenesis. Here, the asymmetric division that generates tip and stalk cells (see above) also produces a difference in size — with tip cell being considerably larger — which has been connected to the differences in migratory properties between these cells.
Dysregulation of spindle orientation
Spindle orientation is used in diverse organs to establish tissue architecture and cell fates. This leads to an expectation that spindle misorientation may contribute to human disease, including both developmental disorders, as well as diseases of stem cell dysregulation, like cancer. Here we discuss the reported roles of spindle orientation defects in human disease and in cancer. An important take away is that tissues have robust mechanisms to restore division orientation and/or tissue architecture after misoriented division, thereby limiting the negative consequences of cell division with aberrantly positioned spindles.
Spindle orientation and cancer.
Asymmetric cell divisions in stem and/or progenitor cells can give rise to one stem and one differentiated cell, maintaining the pool of progenitor cells. Disruption of asymmetric division would yield an imbalance between the pools of progenitors and differentiating cells, resulting in excessive differentiation and the exhaustion of progenitors or favouring the production of additional stem cells and potentially causing hyperproliferation and tumour formation. Work in D. melanogaster demonstrated that transplantation of neuroblasts that could not divide asymmetrically resulted in tumour formation and that loss of spindle orientation in a tumour-susceptible (p53 mutant) background also caused tumours 144,145
To date, there is little evidence that disruption of spindle orientation is sufficient to drive cancers in vertebrates. However, there is accumulating evidence that aberrant divisions associated with misaligned spindles may act in concert with the activation of oncogenes or inactivation of tumour suppressors (Figure 6a). For example, in skin tumours driven by increased VEGF signalling, there was a decrease in asymmetric divisions, consistent with more self-renewing and amplifying divisions 146–148. Other work suggests that asymmetric divisions are increased in epidermis in response to hyperproliferation149. Consistent with this, when cell division was randomized in a KRAS oncogene mutant background, tumours rapidly formed in the skin 149. One interpretation of these results is that perpendicular spindles, which generally generate one differentiated progeny are tumour suppressive in function, limiting the ability of oncogenes to expand progenitor cell numbers (Figure 6b).
Figure 6. Tumorigenic consequences of spindle misalignment and tissue responses to ameliorate spindle misorientation.
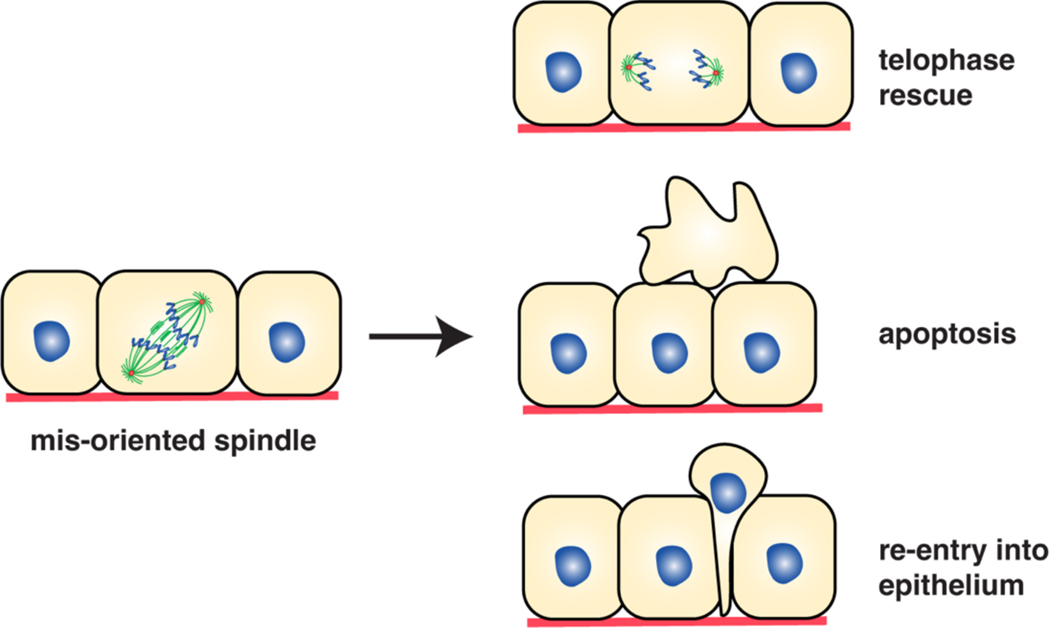
a) Loss of spindle orientation in a tissue is generally not sufficient to confer tumorigenic potential, likely via the existence of various rescue mechanisms operating in the tissue (see part c). However, as shown in this example, loss of spindle orientation (for example, owing to mutations associated with spindle positioning machinery or changes in intracellular or extracellular cues (see Figure 5)), and specifically, asymmetric cell division of a progenitor cell in a simple epithelium, might collaborate with oncogenes to promote tumour formation. Note that the order of eventscould be reversed, with spindle positioning defects preceding oncogenic insults. b) In a stratified epithelium, the tissue can respond to some oncogenic mutations by increasing divisions perpendicular to the basement membrane. This generates differentiated daughters, limiting the potential effect of hyperproliferation, resulting from the loss of asymmetric divisions and increased generation of stem/progenitor cells. However, when oncogenic mutations co-exist with the loss of spindle orientation, this may lead to tumour growth. c) Tissues have multiple ways to deal with spindles that are misoriented in anaphase. They may correct these errors later in telophase, undergo apoptosis to eliminate the misplaced cell, or re-integrate the cell back into the epithelium.
A positive role for asymmetric cell division in leukaemia has also been proposed. The dynein-regulator LIS1 was required for spindle positioning and the localization of putative cell fate determinants in adult haematopoietic stem cells and its loss blocked their progression in to leukaemia 150. However, as LIS1 has pleiotropic roles in cells, including in mitotic spindle assembly and various aspects of intracellular membrane trafficking, it remains unclear whether these effects are due to differences in asymmetric cell division or, mitotic and/or signalling defects downstream of LIS 1 loss.
Spindle orientation and morphogenetic defects.
Although spindle orientation has many roles during development, prominently including tissue patterning and morphogenesis, clear evidence for human diseases linked strictly to spindle orientation is still lacking. One developmental disorder with tight links to spindle orientation machinery is Chudley-McCollough syndrome [G], which results from mutations in the LGN-encoding GPSM2 gene151,152. The disease is characterized by deafness and architectural changes in the brain. The relatively mild disease suggests that these mutations are not associated with a complete loss of function of LGN. Indeed, testing of one of the most common mutant forms associated with the disease, which lacks the C-terminal GoLoco repeats, did not result in a dramatic defect in spindle orientation when expressed in the mouse developing epidermis103. Therefore, it is unclear whether the disease phenotypes result from a hypomorphic function of mutant LGN in spindle orientation or from possible other functions of this protein. In support of this last idea, GPSM2 expression has been reported to be important for stereocilia [G] function in the inner ear153, a spindle orientation-independent function.
It is also worth mentioning that defects in the planar cell polarity [G] (PCP) pathway result in multiple developmental alterations. As these also cause defects in spindle orientation, it is tempting to speculate that spindle orientation defects underlie some of these. For example PCP mutations which result in polycystic kidney disease have been shown to alter spindle orientation 154. Direct cause/consequence relationships are hard to detangle, however.
A final class of developmental disorders that has been proposed to be associated with disrupted spindle orientation is microcephaly [G]. The small brain size found in individuals with microcephaly has been ascribed to defects in generating correct numbers of neurons. Genes that are disrupted in microcephaly include centrosomal genes and early work suggested that spindle misorientation may underlie some of these defects (reviewed 155). However, contradictory evidence demonstrates that spindle misorientation does not necessarily result in microcephaly. For example, a number of microcephaly-causing mutations cause cell fate defects that have been reported to be caused by prolonged mitosis in the presence of aberrant spindles, rather than spindle misorientation 156
The reasons for the paucity of reported diseases that clearly result from spindle orientation defects may be several-fold. First, the core machinery is likely used in many developmental stages, starting from the early embryo as discussed above, likely resulting in embryonic lethality upon severe disruption. Second, as described below, the process of spindle orientation is quite robust, and mechanisms exist to ensure its fidelity.
Mechanisms to limit consequences of spindle misorientation.
Likely because of the great importance of spindle orientation in both tissue architecture and cell fate determination, there are safeguards to ensure this process occurs robustly. There have now been several mechanisms described that can, at least partially, rescue the effects of spindle misorientation (Figure 6c). First, aberrantly positioned spindle in the metaphase can still be re-positioned, thereby rescuing the proper plane of cell division. This process is known as telophase rescue and was first described in D. melanogaster neuroblasts 157. Subsequent work has hinted at a distinct form of this phenomenon occurring in vertebrates. Rather than repositioning the cell fate determinants with the spindle as occurs in flies, vertebrate cells appear to rely on re-positioning the spindle using tissue landmarks. In the mouse epidermis, misoriented metaphase/anaphase spindles can be re-aligned in telophase, in a tissue and adherens junction dependent manner 158. Oblique spindles were observed to be corrected into more clearly aligned perpendicular or parallel to the basement membrane during telophase. Second, cells that are mispositioned in the tissue after division, especially those displaced from the basement membrane, may undergo apoptosis due to loss of contact (anoikis) or loss of other micro-environmental survival signals. Further, work in D. melanogaster has demonstrated that in simple epithelia, divisions with improper orientations result in cells that are not fully integrated into the epithelium. These cells can use surrounding cells to ‘zipper back up’ and reform attachment with the basement membrane 159,160. Similarly, during embryonic gut development in the mouse, daughter cells born without the attachment to the basement membrane can reintegrate into the epithelium, for which they use filopodial pathfinding mechanisms161,162 In uteric buds, luminal mitoses are common, and the daughter cell that lacks a basal connection, is also able to reintegrate into the epithelium 163. Finally, progenitor cells in the vertebrate central nervous system can mitigate the loss of cell attachments resulting from oblique divisions, and were shown to be able to regrow both their apical connections and the basal attachment. This has important implications for tissue organization and integrity as well as tissue patterning and maturation. In the former case, uncontrolled cell delamination can displace progenitors, leading to proliferation in abnormal locations125. In the latter case, retention/regrowth of the basal cell attachment has been shown to be important for regulating cell fate: remaining a progenitor versus being directed towards neuronal differentiation (reviewed in 164). Together, these correction mechanisms safeguard proper tissue organization and development, and may also limit malignant growth by regulating the proliferative potential of the tissue.
Conclusions and perspective
As we look back on the last 20 years of studies on spindle orientation in mammalian systems, there are some clear lessons learned. We have made strides in understanding the function and regulation of the core LGN–NuMA–dynein–dynactin machinery. We are also beginning to appreciate that there is a diversity of mechanisms that can orient the spindle — even this core machinery can be used in distinct ways in different tissues, with new regulators awaiting discovery. We also now know that the very simple and elegant cell fate connections to spindle orientation that were elucidated in C. elegans and D. melanogaster are more complicated in mammals. Although it can be expected that new studies will unveil some contexts of cell fate allocation that are regulated in this way, the data on the role of spindle orientation in cell fate decisions and/or tissue architecture available to date is mostly correlative. Redundancy between extrinsic and intrinsic cues impacting spindle orientation may make it difficult to determine the precise connections. Also, owing to correction mechanisms, spindle misorientation appears to be fairly well-tolerated in some tissues. To progress, we need to identify the machinery specific for spindle orientation in distinct cell types, and determine the effects of specifically disrupting spindle orientation, without the impact on other cellular processes in the tissue of interest, at the same time closely examining the consequences of divisions with misoriented spindles on tissue architecture and cell fate decisions. Optogenetic methods for controlling spindle orientation combined with CRISPR-technologies to introduce specific point mutations into spindle orientation proteins will provide excellent ways of gaining more precise control over spindle orientation mechanisms and regulatory pathways.
Supplementary Material
Acknowledgments
This work was supported by a grant to MM from the Italian Association for Cancer Research (AIRC) (IG-25098). This was partially supported by the Italian Ministry of Health with Ricerca Corrente and 5×1000 funds. This work was supported by grants R01-AR067203, R01-DK117981, and R01-AR055926 from the National Institutes of Health to TL.
Glossary
- Kinetochores
Protein complexes that assemble on the centromeres of chromosomes that capture microtubules of the mitotic spindle, allowing for chromosome capture and segregation
- Astral microtubules
Populations of microtubules in the mitotic spindle that do not attach to kinetochores. A subset of these interacts with the cell cortex and are essential for spindle positioning
- Neuroblasts
Neural stem cells in Drosophila melanogaster development
- Cell cortex
Layer of actomyosin, F-actin and other cytoplasmic proteins forming on the inner side of the cell membrane and responsible for the mitotic round-up
- Dynamic instability
Dynamical switch between growing and shrinking phases occurring at the microtubule plus and minus ends
- XMAP215/Stu2 family proteins
Frog XMAP215 (Stu2 in budding yeast, TOG in human cells) are proteins containing TOG domains that binds tubulin and regulate microtubule dynamics, primarily by promoting microtubule growth
- CLIP-170
Cytoplasmic linker protein 170 contains two CAP-Gly domains binding to tubulin
- CDK1
Cyclin-dependent-kinase 1, activated by cyclin-B to promote G2/M transition. It is then inactivated upon bipolar kinetochore attachment by APC-dependent degradation of cyclin-B, which promotes metaphase to anaphase transition
- CAP-Gly containing proteins
Proteins containing a Cytoskeletal Associated Protein-Glycine rich domain associating to EEY motifs
- Dynactin
Macromolecular complex of 23 subunits acting as a cofactor for the microtubule motor cytoplasmic dynein 1
- Hetero-trimeric G proteins
Membrane-associated G-proteins consisting of trimers of Gα–Gβ–Gγ subunits, with a GTPase Gα subunit. Upon extracellular stimuli, transmembrane G-Protein-Coupled-Receptors (GPCRs) acts as GEF for the Gα subunits resulting in G-protein signaling activation
- Dynein adaptor
Coiled-coil protein promoting the formation of dynein–dynactin complexes and the processive movements of the dynein complex on the microtubule tracks
- Tetratrico-Peptide-Repeat (TPR) domain
34-residue long repeats consisting of two antiparallel helices connected by a short loop, generally organized in super-helical arrays
- GoLoco motifs
19-residue long motifs found in G-protein regulators acting as guanine-dissociation inhibitors of Gαi
- G protein-coupled receptor
Cell surface seven-pass-transmembrane receptor activating cellular response upon binding to extracellular signalling molecules
- 4.1 R
Member of the F-actin–binding 4.1 family that in mitosis binds to NuMA
- CC1-like box motif
Motif conforming to the CC1-box present in several dynein-activating adaptors but carrying a frameshift in the position of the Ala-Ala doublet and lacking the Gly at the central A-A-x-x-G sequence
- Aurora A
Mitotic kinase activated by microtubule-associated protein TPX2 that assists mitotic progression by phosphorylating key substrates at the spindle poles
- PLK1
Polo-like kinase 1, centrosomal kinase promoting spindle pole maturation and spindle assembly
- RAN-GTP
A small G-protein that plays roles in nuclear import–export as well as in regulating chromatin-dependent microtubule nucleation in mitosis
- Non-canonical WNT signalling
Signalling through secreted WNT proteins that activate pathways other than stabilization of β-catenin
- Mother centrosome
Centrosomes consists of a pair of centrioles and the pericentriolar material. The mother centrosome contains one centriole that is more than one cell cycle old, and another that was assembled during the current cell cycle, while the daughter centrosomes contain one centriole that was assembled in the previous cell cycle and one that was assembled in the current cell cycle
- ERM (Ezrin-Radixin-Moesin) family proteins
Conserved proteins consisting of a FERM domain interacting with membrane associated proteins and an F-actin-binding domain cross-liking actin filaments at the mitotic cortex. Moesin codes for a microtubule-binding domain stabilizing astral microtubules at the cortex
- Ste20-like kinase
Ser/Thr kinase that in mitosis activates ERM proteins to promote spindle orientation via LGN–NuMA cortical enrichment
- Myosin X
Microtubule-binding myosin with an unconventional dimeric motor domain
- Caveolae
Invaginations of the plasma membrane of about 50–100 nm diameter, that have been implicated in clathrin-independent endocytosis. In addition subcortical cavelin-rich patches generates proteinaceous platform for astral microtubule anchoring and mechano-transduction
- Focal adhesion
Integrin-containing macromolecular complexes mediating mechanical contacts between intracellular F-actin and the extracellular matrix
- Basement membrane
Thin flexible sheet-like extracellular-matrix found between the basal site of epithelial layers and connective tissues, providing support and signalling
- Catenins
Family of proteins binding to the intracellular portion of caderins at adherens junctions and to the actin cytoskeleton
- Notum
Dorsal portion of the thoracic segment of insects
- Scribble polarity complex
Macromolecular complex consisting of the evolutionarily conserved proteins Scribble, DLG and LGL, localising at the cytoplasmic site of the lateral membrane in polarized epithelial cells
- Epiboly
One of the types of cell movement occurring during zebrafish gastrulation
- VEGF
Vascular endothelial growth factor. A growth factor that promotes angiogenesis
- Immune synapse
The site of connection between an antigen presenting cell and a T or B lymphocyte
- Intermediate filament cytoskeleton
A cytoskeleton element, comprising filaments (8–10 nm in thickness) made up of diverse and cell-type specific proteins, such as keratins and vimentin
- Primary cilium
Microtubule-based structure that extends from the cell and that can act as a sensory antenna and signalling centre
- Polar body
A small haploid cell that result from meiotic divisions of the oocyte
- Chudley-McCollough syndrome
An autosomal recessive condition characterized by hearing loss and brain malformations
- Stereocilia
F-actin-rich protrusions of hair cells of the inner ear that sense fluid flow and are essential for hearing and balance
- Planar cell polarity
Polarization of a field of cells within the plane of the cell sheet
- TOG domains
Conserved tubulin-binding domains consisting of six HEAT repeats, i.e. helix-turn-helix repeats present in Huntingtin, Elongation factor 3, protein phosphatase PP2A and the kinase Tor1
- Nectin
Cell–cell adhesion molecule organizing epithelial junctions and interacting intracellularly with the F-actin-binding protein Afadin
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Prosser SL & Pelletier L. Mitotic spindle assembly in animal cells: a fine balancing act. Nat Rev Mol Cell Biol 18, 187–201, doi: 10.1038/nrm.2016.162 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Knoblich JA Asymmetric cell division during animal development. Nat Rev Mol Cell Biol 2, 11–20 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Kozlowski C, Srayko M. & Nedelec F. Cortical microtubule contacts position the spindle in C. elegans embryos. Cell 129, 499–510, doi: 10.1016/j.cell.2007.03.027 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Thery M, Jimenez-Dalmaroni A, Racine V, Bornens M. & Julicher F. Experimental and theoretical study of mitotic spindle orientation. Nature 447, 493–496, doi: 10.1038/nature05786 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Dinarina A. et al. Chromatin shapes the mitotic spindle. Cell 138, 502–513, doi: 10.1016/j.cell.2009.05.027 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Dimitracopoulos A. et al. Mechanochemical Crosstalk Produces Cell-Intrinsic Patterning of the Cortex to Orient the Mitotic Spindle. Curr Biol 30, 3687–3696 e3684, doi: 10.1016/j.cub.2020.06.098 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.di Pietro F, Echard A. & Morin X. Regulation of mitotic spindle orientation: an integrated view. EMBO Rep 17, 1106–1130, doi: 10.15252/embr.201642292 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knoblich JA Mechanisms of asymmetric stem cell division. Cell 132, 583–597, doi:S0092-8674(08)00208-0 [pii] 10.1016/j.cell.2008.02.007 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Knoblich JA Asymmetric cell division: recent developments and their implications for tumour biology. Nat Rev Mol Cell Biol 11, 849–860, doi:nrm3010 [pii] 10.1038/nrm3010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonczy P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nat Rev Mol Cell Biol 9, 355–366, doi:nrm2388 [pii] 10.1038/nrm2388 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Dwivedi D, Kumari A, Rathi S, Mylavarapu SVS & Sharma M. The dynein adaptor Hook2 plays essential roles in mitotic progression and cytokinesis. J Cell Biol 218, 871–894, doi: 10.1083/jcb.201804183 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanafusa H. et al. PLK1-dependent activation of LRRK1 regulates spindle orientation by phosphorylating CDK5RAP2. Nat Cell Biol 17, 1024–1035, doi: 10.1038/ncb3204 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Toyoshima F. & Nishida E. Integrin-mediated adhesion orients the spindle parallel to the substratum in an EB1- and myosin X-dependent manner. EMBO J 26, 1487–1498, doi:7601599 [pii] 10.1038/sj.emboj.7601599 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouissou A. et al. gamma-Tubulin Ring Complexes and EB1 play antagonistic roles in microtubule dynamics and spindle positioning. EMBO J 33, 114–128, doi: 10.1002/embj.201385967 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polverino F. et al. The Aurora-A/TPX2 Axis Directs Spindle Orientation in Adherent Human Cells by Regulating NuMA and Microtubule Stability. Curr Biol 31, 658–667 e655, doi: 10.1016/j.cub.2020.10.096 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Mora-Bermudez F, Matsuzaki F. & Huttner WB Specific polar subpopulations of astral microtubules control spindle orientation and symmetric neural stem cell division. Elife 3, doi: 10.7554/eLife.02875 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodakuntla S, Jijumon AS, Villablanca C, Gonzalez-Billault C. & Janke C. Microtubule-Associated Proteins: Structuring the Cytoskeleton. Trends Cell Biol 29, 804–819, doi: 10.1016/j.tcb.2019.07.004 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Nguyen-Ngoc T, Afshar K. & Gonczy P. Coupling of cortical dynein and G alpha proteins mediates spindle positioning in Caenorhabditis elegans. Nat Cell Biol 9, 1294–1302, doi: 10.1038/ncb1649 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Singh D, Schmidt N, Muller F, Bange T. & Bird AW Destabilization of Long Astral Microtubules via Cdk1-Dependent Removal of GTSE1 from Their Plus Ends Facilitates Prometaphase Spindle Orientation. Curr Biol, doi: 10.1016/j.cub.2020.11.040 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Gupta ML Jr., Carvalho P, Roof DM & Pellman D. Plus end-specific depolymerase activity of Kip3, a kinesin-8 protein, explains its role in positioning the yeast mitotic spindle. Nat Cell Biol 8, 913–923, doi: 10.1038/ncb1457 (2006). [DOI] [PubMed] [Google Scholar]
- 21.McHugh T, Gluszek AA & Welburn JPI Microtubule end tethering of a processive kinesin-8 motor Kif18b is required for spindle positioning. J Cell Biol 217, 2403–2416, doi: 10.1083/jcb.201705209 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janke C. & Magiera MM The tubulin code and its role in controlling microtubule properties and functions. Nat Rev Mol Cell Biol 21, 307–326, doi: 10.1038/s41580-020-0214-3 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Gundersen GG & Bulinski JC Distribution of tyrosinated and nontyrosinated alpha-tubulin during mitosis. J Cell Biol 102, 1118–1126, doi: 10.1083/jcb.102.3.1118 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szyk A, Deaconescu AM, Piszczek G. & Roll-Mecak A. Tubulin tyrosine ligase structure reveals adaptation of an ancient fold to bind and modify tubulin. Nat Struct Mol Biol 18, 1250–1258, doi: 10.1038/nsmb.2148 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peris L. et al. Tubulin tyrosination is a major factor affecting the recruitment of CAP-Gly proteins at microtubule plus ends. J Cell Biol 174, 839–849, doi: 10.1083/jcb.200512058 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bieling P. et al. CLIP-170 tracks growing microtubule ends by dynamically recognizing composite EB1/tubulin-binding sites. J Cell Biol 183, 1223–1233, doi: 10.1083/jcb.200809190 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erck C. et al. A vital role of tubulin-tyrosine-ligase for neuronal organization. Proc Natl Acad Sci U S A 102, 7853–7858, doi: 10.1073/pnas.0409626102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh D, Schmidt N, Müller F, Bange T. & Bird AW Destabilization of Long Astral Microtubules via Cdk1-Dependent Removal of GTSE1 from Their Plus Ends Facilitates Prometaphase Spindle Orientation. Curr Biol 31, 766–781.e768, doi: 10.1016/j.cub.2020.11.040 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Poulson ND & Lechler T. Robust control of mitotic spindle orientation in the developing epidermis. J Cell Biol 191, 915–922, doi: 10.1083/jcb.201008001 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laan L. et al. Cortical dynein controls microtubule dynamics to generate pulling forces that position microtubule asters. Cell 148, 502–514, doi: 10.1016/j.cell.2012.01.007 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotak S, Busso C. & Gonczy P. Cortical dynein is critical for proper spindle positioning in human cells. J Cell Biol 199, 97–110, doi: 10.1083/jcb.201203166 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elias S. et al. Huntingtin regulates mammary stem cell division and differentiation. Stem Cell Reports 2, 491–506, doi: 10.1016/j.stemcr.2014.02.011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du Q. & Macara IG Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell 119, 503–516 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Morin X. & Bellaiche Y. Mitotic Spindle Orientation in Asymmetric and Symmetric Cell Divisions during Animal Development. Dev Cell 21, 102–119, doi:S1534–5807(11)00246-2 [pii] 10.1016/j.devcel.2011.06.012 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Gallini S. et al. NuMA Phosphorylation by Aurora-A Orchestrates Spindle Orientation. Curr Biol, doi: 10.1016/j.cub.2015.12.051 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Pan Z. et al. An autoinhibited conformation of LGN reveals a distinct interaction mode between GoLoco motifs and TPR motifs. Structure 21, 1007–1017, doi: 10.1016/j.str.2013.04.005 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Zhu J. et al. LGN/mInsc and LGN/NuMA complex structures suggest distinct functions in asymmetric cell division for the Par3/mInsc/LGN and Galphai/LGN/NuMA pathways. Mol Cell 43, 418–431, doi:S1097–2765(11)00533–8 [pii] 10.1016/j.molcel.2011.07.011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saadaoui M. et al. Loss of the canonical spindle orientation function in the Pins/LGN homolog AGS3. EMBO Rep 18, 1509–1520, doi: 10.15252/embr.201643048 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanada K. & Tsai LH G protein betagamma subunits and AGS3 control spindle orientation and asymmetric cell fate of cerebral cortical progenitors. Cell 122, 119–131 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Yoshiura S, Ohta N. & Matsuzaki F. Tre1 GPCR signaling orients stem cell divisions in the Drosophila central nervous system. Dev Cell 22, 79–91, doi: 10.1016/j.devcel.2011.10.027 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Cazorla-Vazquez S. & Engel FB Adhesion GPCRs in Kidney Development and Disease. Front Cell Dev Biol 6, 9, doi: 10.3389/fcell.2018.00009 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonald WS et al. Altered cleavage plane orientation with increased genomic aneuploidy produced by receptor-mediated lysophosphatidic acid (LPA) signaling in mouse cerebral cortical neural progenitor cells. Mol Brain 13, 169, doi: 10.1186/s13041-020-00709-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Afshar K. et al. RIC-8 is required for GPR-1/2-dependent Galpha function during asymmetric division of C. elegans embryos. Cell 119, 219–230, doi: 10.1016/j.cell.2004.09.026 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Hess HA, Roper JC, Grill SW & Koelle MR RGS-7 completes a receptor-independent heterotrimeric G protein cycle to asymmetrically regulate mitotic spindle positioning in C. elegans. Cell 119, 209–218, doi: 10.1016/j.cell.2004.09.025 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Wang H. et al. Ric-8 controls Drosophila neural progenitor asymmetric division by regulating heterotrimeric G proteins. Nat Cell Biol 7, 1091–1098, doi: 10.1038/ncb1317 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Woodard GE et al. Ric-8A and Gi alpha recruit LGN, NuMA, and dynein to the cell cortex to help orient the mitotic spindle. Mol Cell Biol 30, 3519–3530, doi:MCB.00394-10 [pii] 10.1128/MCB.00394-10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chishiki K, Kamakura S, Hayase J. & Sumimoto H. Ric-8A, an activator protein of Galphai, controls mammalian epithelial cell polarity for tight junction assembly and cystogenesis. Genes Cells 22, 293–309, doi: 10.1111/gtc.12477 (2017). [DOI] [PubMed] [Google Scholar]
- 48.McClelland LJ et al. Structure of the G protein chaperone and guanine nucleotide exchange factor Ric-8A bound to Galphai1. Nat Commun 11, 1077, doi: 10.1038/s41467-020-14943-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng B. et al. Structure, Function, and Dynamics of the Galpha Binding Domain of Ric-8A. Structure 27, 1137–1147 e1135, doi: 10.1016/j.str.2019.04.013 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kant R, Zeng B, Thomas CJ, Bothner B. & Sprang SR Ric-8A, a G protein chaperone with nucleotide exchange activity induces long-range secondary structure changes in Galpha. Elife 5, doi: 10.7554/eLife.19238 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papasergi-Scott MM et al. Dual phosphorylation of Ric-8A enhances its ability to mediate G protein alpha subunit folding and to stimulate guanine nucleotide exchange. Sci Signal 11, doi: 10.1126/scisignal.aap8113 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seven AB et al. Structures of Galpha Proteins in Complex with Their Chaperone Reveal Quality Control Mechanisms. Cell Rep 30, 3699–3709 e3696, doi: 10.1016/j.celrep.2020.02.086 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urnavicius L. et al. The structure of the dynactin complex and its interaction with dynein. Science 347, 1441–1446, doi: 10.1126/science.aaa4080 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reck-Peterson SL, Redwine WB, Vale RD & Carter AP The cytoplasmic dynein transport machinery and its many cargoes. Nat Rev Mol Cell Biol 19, 382–398, doi: 10.1038/s41580-018-0004-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okumura M, Natsume T, Kanemaki MT & Kiyomitsu T. Dynein-Dynactin-NuMA clusters generate cortical spindle-pulling forces as a multi-arm ensemble. Elife 7, doi: 10.7554/eLife.36559 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seldin L, Poulson ND, Foote HP & Lechler T. NuMA localization, stability, and function in spindle orientation involve 4.1 and Cdk1 interactions. Mol Biol Cell 24, 3651–3662, doi: 10.1091/mbc.E13-05-0277 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kotak S, Busso C. & Gonczy P. NuMA interacts with phosphoinositides and links the mitotic spindle with the plasma membrane. EMBO J 33, 1815–1830, doi: 10.15252/embj.201488147 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng Z, Wan Q, Meixiong G. & Du Q. Cell cycle-regulated membrane binding of NuMA contributes to efficient anaphase chromosome separation. Mol Biol Cell 25, 606–619, doi: 10.1091/mbc.E13-08-0474 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kiyomitsu T. & Cheeseman IM Cortical dynein and asymmetric membrane elongation coordinately position the spindle in anaphase. Cell 154, 391–402, doi: 10.1016/j.cell.2013.06.010 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Du Q, Stukenberg PT & Macara IG A mammalian Partner of inscuteable binds NuMA and regulates mitotic spindle organization. Nat Cell Biol 3, 1069–1075 (2001). [DOI] [PubMed] [Google Scholar]
- 61.Culurgioni S, Alfieri A, Pendolino V, Laddomada F. & Mapelli M. Inscuteable and NuMA proteins bind competitively to Leu-Gly-Asn repeat-enriched protein (LGN) during asymmetric cell divisions. Proc Natl Acad Sci U S A 108, 20998–21003, doi: 10.1073/pnas.1113077108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Du Q, Taylor L, Compton DA & Macara IG LGN blocks the ability of NuMA to bind and stabilize microtubules. A mechanism for mitotic spindle assembly regulation. Curr Biol 12, 1928–1933 (2002). [DOI] [PubMed] [Google Scholar]
- 63.Seldin L, Muroyama A. & Lechler T. NuMA-microtubule interactions are critical for spindle orientation and the morphogenesis of diverse epidermal structures. Elife 5, doi: 10.7554/eLife.12504 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pirovano L. et al. Hexameric NuMA:LGN structures promote multivalent interactions required for planar epithelial divisions. Nat Commun 10, 2208, doi: 10.1038/s41467-019-09999-w (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Renna C. et al. Organizational Principles of the NuMA-Dynein Interaction Interface and Implications for Mitotic Spindle Functions. Structure 28, 820–829 e826, doi: 10.1016/j.str.2020.04.017 (2020). [DOI] [PubMed] [Google Scholar]
- 66.Urnavicius L. et al. Cryo-EM shows how dynactin recruits two dyneins for faster movement. Nature 554, 202–206, doi: 10.1038/nature25462 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McKenney RJ, Huynh W, Tanenbaum ME, Bhabha G. & Vale RD Activation of cytoplasmic dynein motility by dynactin-cargo adapter complexes. Science 345, 337–341, doi: 10.1126/science.1254198 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kotak S, Afshar K, Busso C. & Gonczy P. Aurora A kinase regulates proper spindle positioning in C. elegans and in human cells. J Cell Sci 129, 3015–3025, doi: 10.1242/jcs.184416 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kotak S, Busso C. & Gonczy P. NuMA phosphorylation by CDK1 couples mitotic progression with cortical dynein function. Embo J 32, 2517–2529, doi: 10.1038/emboj.2013.172 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee BH, Schwager F, Meraldi P. & Gotta M. p37/UBXN2B regulates spindle orientation by limiting cortical NuMA recruitment via PP1/Repo-Man. J Cell Biol 217, 483–493, doi: 10.1083/jcb.201707050 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keshri R, Rajeevan A. & Kotak S. PP2A--B55gamma counteracts Cdk1 and regulates proper spindle orientation through the cortical dynein adaptor NuMA. J Cell Sci 133, doi: 10.1242/jcs.243857 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kiyomitsu T. & Cheeseman IM Chromosome- and spindle-pole-derived signals generate an intrinsic code for spindle position and orientation. Nat Cell Biol, doi:ncb2440 [pii] 10.1038/ncb2440 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sana S, Keshri R, Rajeevan A, Kapoor S. & Kotak S. Plk1 regulates spindle orientation by phosphorylating NuMA in human cells. Life Sci Alliance 1, e201800223, doi: 10.26508/lsa.201800223 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tame MA, Raaijmakers JA, Afanasyev P. & Medema RH Chromosome misalignments induce spindle-positioning defects. EMBO Rep 17, 317–325, doi: 10.15252/embr.201541143 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodrigues NT et al. Kinetochore-localized PP1-Sds22 couples chromosome segregation to polar relaxation. Nature 524, 489–492, doi: 10.1038/nature14496 (2015). [DOI] [PubMed] [Google Scholar]
- 76.Chang CC, Huang TL, Shimamoto Y, Tsai SY & Hsia KC Regulation of mitotic spindle assembly factor NuMA by Importin-beta. J Cell Biol 216, 3453–3462, doi: 10.1083/jcb.201705168 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnston CA, Hirono K, Prehoda KE & Doe CQ Identification of an Aurora-A/PinsLINKER/Dlg spindle orientation pathway using induced cell polarity in S2 cells. Cell 138, 1150–1163, doi:S0092–8674(09)00978–7 [pii] 10.1016/j.cell.2009.07.041 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnston CA et al. Formin-mediated actin polymerization cooperates with Mushroom body defect (Mud)-Dynein during Frizzled-Dishevelled spindle orientation. J Cell Sci 126, 4436–4444, doi: 10.1242/jcs.129544 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Segalen M. et al. The Fz-Dsh planar cell polarity pathway induces oriented cell division via Mud/NuMA in Drosophila and zebrafish. Dev Cell 19, 740–752, doi:S1534-5807(10)00457-0 [pii] 10.1016/j.devcel.2010.10.004 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gong Y, Mo C. & Fraser SE Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature 430, 689–693, doi: 10.1038/nature02796 (2004). [DOI] [PubMed] [Google Scholar]
- 81.Yang Y. et al. CYLD regulates spindle orientation by stabilizing astral microtubules and promoting dishevelled-NuMA-dynein/dynactin complex formation. Proc Natl Acad Sci U S A 111, 2158–2163, doi:1319341111 [pii] 10.1073/pnas.1319341111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Habib SJ et al. A localized Wnt signal orients asymmetric stem cell division in vitro. Science 339, 1445–1448, doi: 10.1126/science.1231077 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Junyent S. et al. Specialized cytonemes induce self-organization of stem cells. Proc Natl Acad Sci U S A 117, 7236–7244, doi: 10.1073/pnas.1920837117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Okuchi Y. et al. Wnt-modified materials mediate asymmetric stem cell division to direct human osteogenic tissue formation for bone repair. Nat Mater, doi: 10.1038/s41563-020-0786-5 (2020). [DOI] [PubMed] [Google Scholar]
- 85.Ramkumar N. & Baum B. Coupling changes in cell shape to chromosome segregation. Nat Rev Mol Cell Biol 17, 511–521, doi: 10.1038/nrm.2016.75 (2016). [DOI] [PubMed] [Google Scholar]
- 86.Redemann S. et al. Membrane invaginations reveal cortical sites that pull on mitotic spindles in one-cell C. elegans embryos. PLoS One 5, e12301, doi: 10.1371/journal.pone.0012301 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fehon RG, McClatchey AI & Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol 11, 276–287, doi: 10.1038/nrm2866 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Machicoane M. et al. SLK-dependent activation of ERMs controls LGN-NuMA localization and spindle orientation. J Cell Biol 205, 791–799, doi: 10.1083/jcb.201401049 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carminati M. et al. Concomitant binding of Afadin to LGN and F-actin directs planar spindle orientation. Nat Struct Mol Biol 23, 155–163, doi: 10.1038/nsmb.3152 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Zhu M. et al. MISP is a novel Plk1 substrate required for proper spindle orientation and mitotic progression. J Cell Biol 200, 773–787, doi: 10.1083/jcb.201207050 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maier B, Kirsch M, Anderhub S, Zentgraf H. & Krämer A. The novel actin/focal adhesion-associated protein MISP is involved in mitotic spindle positioning in human cells. Cell Cycle 12, 1457–1471, doi: 10.4161/cc.24602 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kwon M, Bagonis M, Danuser G. & Pellman D. Direct Microtubule Binding by Myosin-10 Orients Centrosomes toward Retraction Fibers and Subcortical Actin Clouds. Dev Cell 34, 323–337, doi: 10.1016/j.devcel.2015.06.013 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matsumura S. et al. Interphase adhesion geometry is transmitted to an internal regulator for spindle orientation via caveolin-1. Nat Commun 7, ncomms11858, doi: 10.1038/ncomms11858 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Farina F. et al. The centrosome is an actin-organizing centre. Nat Cell Biol 18, 65–75, doi: 10.1038/ncb3285 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Inoue D. et al. Actin filaments regulate microtubule growth at the centrosome. EMBO J 38, doi: 10.15252/embj.201899630 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dogterom M. & Koenderink GH Actin-microtubule crosstalk in cell biology. Nat Rev Mol Cell Biol 20, 38–54, doi: 10.1038/s41580-018-0067-1 (2019). [DOI] [PubMed] [Google Scholar]
- 97.Jaffe AB, Kaji N, Durgan J. & Hall A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol 183, 625–633, doi:jcb.200807121 [pii] 10.1083/jcb.200807121 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fink J. et al. External forces control mitotic spindle positioning. Nat Cell Biol 13, 771–778, doi:ncb2269 [pii] 10.1038/ncb2269 (2011). [DOI] [PubMed] [Google Scholar]
- 99.Kanchanawong P. et al. Nanoscale architecture of integrin-based cell adhesions. Nature 468, 580–584, doi: 10.1038/nature09621 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Taneja N. et al. Focal adhesions control cleavage furrow shape and spindle tilt during mitosis. Sci Rep 6, 29846, doi: 10.1038/srep29846 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lechler T. & Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437, 275–280, doi:http://www.nature.com/nature/journal/v437/n7056/suppinfo/nature03922_S1.html (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rodriguez-Boulan E. & Macara IG Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol 15, 225–242, doi: 10.1038/nrm3775 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Williams SE, Beronja S, Pasolli HA & Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature 470, 353–358, doi: 10.1038/nature09793 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gloerich M, Bianchini JM, Siemers KA, Cohen DJ & Nelson WJ Cell division orientation is coupled to cell-cell adhesion by the E-cadherin/LGN complex. Nat Commun 8, 13996, doi: 10.1038/ncomms13996 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Takeichi M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol 15, 397–410, doi: 10.1038/nrm3802 (2014). [DOI] [PubMed] [Google Scholar]
- 106.Hart KC et al. E-cadherin and LGN align epithelial cell divisions with tissue tension independently of cell shape. Proc Natl Acad Sci U S A 114, E5845–E5853, doi: 10.1073/pnas.1701703114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nestor-Bergmann A. et al. Decoupling the Roles of Cell Shape and Mechanical Stress in Orienting and Cueing Epithelial Mitosis. Cell Rep 26, 2088–2100 e2084, doi: 10.1016/j.celrep.2019.01.102 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bosveld F. et al. Epithelial tricellular junctions act as interphase cell shape sensors to orient mitosis. Nature, doi: 10.1038/nature16970 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peyre E. et al. A lateral belt of cortical LGN and NuMA guides mitotic spindle movements and planar division in neuroepithelial cells. J Cell Biol 193, 141–154, doi:jcb.201101039 [pii] 10.1083/jcb.201101039 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saadaoui M. et al. Dlg1 controls planar spindle orientation in the neuroepithelium through direct interaction with LGN. J Cell Biol 206, 707–717, doi:jcb.201405060 [pii] 10.1083/jcb.201405060(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hertwig WAO, Hertwig & Hertwig R. Untersuchungen zur Morphologie und Physiologie der Zelle. (Jena, 1884). [Google Scholar]
- 112.Campinho P. et al. Tension-oriented cell divisions limit anisotropic tissue tension in epithelial spreading during zebrafish epiboly. Nat Cell Biol 15, 1405–1414, doi: 10.1038/ncb2869 (2013). [DOI] [PubMed] [Google Scholar]
- 113.Wyatt TP et al. Emergence of homeostatic epithelial packing and stress dissipation through divisions oriented along the long cell axis. Proc Natl Acad Sci U S A 112, 5726–5731, doi: 10.1073/pnas.1420585112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carlton JG, Jones H. & Eggert US Membrane and organelle dynamics during cell division. Nat Rev Mol Cell Biol 21, 151–166, doi: 10.1038/s41580-019-0208-1 (2020). [DOI] [PubMed] [Google Scholar]
- 115.Sunchu B. & Cabernard C. Principles and mechanisms of asymmetric cell division. Development 147, doi: 10.1242/dev.167650 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smart IH Variation in the plane of cell cleavage during the process of stratification in the mouse epidermis. Br J Dermatol 82, 276–282, doi: 10.1111/j.1365-2133.1970.tb12437.x (1970). [DOI] [PubMed] [Google Scholar]
- 117.Box K, Joyce BW & Devenport D. Epithelial geometry regulates spindle orientation and progenitor fate during formation of the mammalian epidermis. Elife 8, doi: 10.7554/eLife.47102 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ouspenskaia T, Matos I, Mertz AF, Fiore VF & Fuchs E. WNT SHH Antagonism Specifies and Expands Stem Cells prior to Niche Formation. Cell 164, 156–169, doi: 10.1016/j.cell.2015.11.058 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Byrd KM et al. LGN plays distinct roles in oral epithelial stratification, filiform papilla morphogenesis and hair follicle development. Development 143, 2803–2817, doi: 10.1242/dev.136010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tang N, Marshall WF, McMahon M, Metzger RJ & Martin GR Control of mitotic spindle angle by the RAS-regulated ERK1/2 pathway determines lung tube shape. Science 333, 342–345, doi: 10.1126/science.1204831 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tang Z. et al. Mechanical Forces Program the Orientation of Cell Division during Airway Tube Morphogenesis. Dev Cell 44, 313–325 e315, doi: 10.1016/j.devcel.2017.12.013 (2018). [DOI] [PubMed] [Google Scholar]
- 122.Costa G. et al. Asymmetric division coordinates collective cell migration in angiogenesis. Nat Cell Biol 18, 1292–1301, doi: 10.1038/ncb3443 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Casaletto JB, Saotome I, Curto M. & McClatchey AI Ezrin-mediated apical integrity is required for intestinal homeostasis. Proc Natl Acad Sci U S A 108, 11924–11929, doi: 10.1073/pnas.1103418108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen J. & Krasnow MA Integrin Beta 1 suppresses multilayering of a simple epithelium. PLoS One 7, e52886, doi: 10.1371/journal.pone.0052886 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Strzyz PJ et al. Interkinetic nuclear migration is centrosome independent and ensures apical cell division to maintain tissue integrity. Dev Cell 32, 203–219, doi: 10.1016/j.devcel.2014.12.001 (2015). [DOI] [PubMed] [Google Scholar]
- 126.Das RM & Storey KG Mitotic spindle orientation can direct cell fate and bias Notch activity in chick neural tube. EMBO Rep 13, 448–454, doi: 10.1038/embor.2012.42 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Konno D. et al. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat Cell Biol 10, 93–101, doi: 10.1038/ncb1673 (2008). [DOI] [PubMed] [Google Scholar]
- 128.Morin X, Jaouen F. & Durbec P. Control of planar divisions by the G-protein regulator LGN maintains progenitors in the chick neuroepithelium. Nat Neurosci 10, 1440–1448, doi: 10.1038/nn1984 (2007). [DOI] [PubMed] [Google Scholar]
- 129.Carroll TD et al. Interkinetic nuclear migration and basal tethering facilitates post-mitotic daughter separation in intestinal organoids. J Cell Sci 130, 3862–3877, doi: 10.1242/jcs.211656 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Quyn AJ et al. Spindle orientation bias in gut epithelial stem cell compartments is lost in precancerous tissue. Cell Stem Cell 6, 175–181, doi: 10.1016/j.stem.2009.12.007 (2010). [DOI] [PubMed] [Google Scholar]
- 131.Chang JT et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science 315, 1687–1691, doi: 10.1126/science.1139393 (2007). [DOI] [PubMed] [Google Scholar]
- 132.Chang JT et al. Asymmetric proteasome segregation as a mechanism for unequal partitioning of the transcription factor T-bet during T lymphocyte division. Immunity 34, 492–504, doi: 10.1016/j.immuni.2011.03.017 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Barnett BE et al. Asymmetric B cell division in the germinal center reaction. Science 335, 342–344, doi: 10.1126/science.1213495 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lim HYG et al. Keratins are asymmetrically inherited fate determinants in the mammalian embryo. Nature 585, 404–409, doi: 10.1038/s41586-020-2647-4 (2020). [DOI] [PubMed] [Google Scholar]
- 135.Paridaen JT & Huttner WB Neurogenesis during development of the vertebrate central nervous system. EMBO Rep 15, 351–364, doi: 10.1002/embr.201438447 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Raghavan S, Bauer C, Mundschau G, Li Q. & Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol 150, 1149–1160, doi: 10.1083/jcb.150.5.1149 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Carroll JM, Romero MR & Watt FM Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell 83, 957–968, doi: 10.1016/0092-8674(95)90211-2 (1995). [DOI] [PubMed] [Google Scholar]
- 138.Bultje RS et al. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron 63, 189–202, doi: 10.1016/j.neuron.2009.07.004 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dong Z, Yang N, Yeo SY, Chitnis A. & Guo S. Intralineage directional Notch signaling regulates self-renewal and differentiation of asymmetrically dividing radial glia. Neuron 74, 65–78, doi: 10.1016/j.neuron.2012.01.031 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kawaguchi D, Furutachi S, Kawai H, Hozumi K. & Gotoh Y. Dll1 maintains quiescence of adult neural stem cells and segregates asymmetrically during mitosis. Nat Commun 4, 1880, doi: 10.1038/ncomms2895 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Matos I. et al. Progenitors oppositely polarize WNT activators and inhibitors to orchestrate tissue development. Elife 9, doi: 10.7554/eLife.54304 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mogessie B, Scheffler K. & Schuh M. Assembly and Positioning of the Oocyte Meiotic Spindle. Annu Rev Cell Dev Biol 34, 381–403, doi: 10.1146/annurev-cellbio-100616-060553 (2018). [DOI] [PubMed] [Google Scholar]
- 143.Pfender S, Kuznetsov V, Pleiser S, Kerkhoff E. & Schuh M. Spire type actin nucleators cooperate with Formin-2 to drive asymmetric oocyte division. Curr Biol 21, 955–960, doi: 10.1016/j.cub.2011.04.029 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Caussinus E. & Gonzalez C. Induction of tumor growth by altered stem-cell asymmetric division in Drosophila melanogaster. Nat Genet 37, 1125–1129, doi: 10.1038/ng1632 (2005). [DOI] [PubMed] [Google Scholar]
- 145.Nakajima Y, Meyer EJ, Kroesen A, McKinney SA & Gibson MC Epithelial junctions maintain tissue architecture by directing planar spindle orientation. Nature 500, 359–362, doi: 10.1038/nature12335 (2013). [DOI] [PubMed] [Google Scholar]
- 146.Boumahdi S. et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature 511, 246–250, doi: 10.1038/nature13305 (2014). [DOI] [PubMed] [Google Scholar]
- 147.Siegle JM et al. SOX2 is a cancer-specific regulator of tumour initiating potential in cutaneous squamous cell carcinoma. Nat Commun 5, 4511, doi: 10.1038/ncomms5511 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Beck B. et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature 478, 399–403, doi: 10.1038/nature10525 (2011). [DOI] [PubMed] [Google Scholar]
- 149.Morrow A, Underwood J, Seldin L, Hinnant T. & Lechler T. Regulated spindle orientation buffers tissue growth in the epidermis. Elife 8, doi: 10.7554/eLife.48482 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zimdahl B. et al. Lis1 regulates asymmetric division in hematopoietic stem cells and in leukemia. Nat Genet 46, 245–252, doi: 10.1038/ng.2889 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Diaz-Horta O. et al. GPSM2 mutations in Chudley-McCullough syndrome. Am J Med Genet A 158A, 2972–2973, doi: 10.1002/ajmg.a.35636 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Doherty D. et al. GPSM2 mutations cause the brain malformations and hearing loss in Chudley-McCullough syndrome. Am J Hum Genet 90, 1088–1093, doi: 10.1016/j.ajhg.2012.04.008 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mauriac SA et al. Defective Gpsm2/Galphai3 signalling disrupts stereocilia development and growth cone actin dynamics in Chudley-McCullough syndrome. Nat Commun 8, 14907, doi: 10.1038/ncomms14907 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fischer E. et al. Defective planar cell polarity in polycystic kidney disease. Nat Genet 38, 21–23, doi: 10.1038/ng1701 (2006). [DOI] [PubMed] [Google Scholar]
- 155.Jayaraman D, Bae BI & Walsh CA The Genetics of Primary Microcephaly. Annu Rev Genomics Hum Genet 19, 177–200, doi: 10.1146/annurev-genom-083117-021441 (2018). [DOI] [PubMed] [Google Scholar]
- 156.Pilaz LJ et al. Prolonged Mitosis of Neural Progenitors Alters Cell Fate in the Developing Brain. Neuron 89, 83–99, doi: 10.1016/j.neuron.2015.12.007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Siegrist SE & Doe CQ Microtubule-induced Pins/Galphai cortical polarity in Drosophila neuroblasts. Cell 123, 1323–1335, doi: 10.1016/j.cell.2005.09.043 (2005). [DOI] [PubMed] [Google Scholar]
- 158.Lough KJ et al. Telophase correction refines division orientation in stratified epithelia. Elife 8, doi: 10.7554/eLife.49249 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bergstralh DT, Lovegrove HE & St Johnston D. Lateral adhesion drives reintegration of misplaced cells into epithelial monolayers. Nat Cell Biol 17, 1497–1503, doi: 10.1038/ncb3248 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cammarota C, Finegan TM, Wilson TJ, Yang S. & Bergstralh DT An Axon-Pathfinding Mechanism Preserves Epithelial Tissue Integrity. Curr Biol, doi: 10.1016/j.cub.2020.09.061 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang S, Cebrian C, Schnell S. & Gumucio DL Radial WNT5A-Guided Post-mitotic Filopodial Pathfinding Is Critical for Midgut Tube Elongation. Dev Cell 46, 173–188 e173, doi: 10.1016/j.devcel.2018.06.011 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang S. et al. RYK-mediated filopodial pathfinding facilitates midgut elongation. Development 147, doi: 10.1242/dev.195388 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Packard A. et al. Luminal mitosis drives epithelial cell dispersal within the branching ureteric bud. Dev Cell 27, 319–330, doi: 10.1016/j.devcel.2013.09.001 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Matsuzaki F. & Shitamukai A. Cell Division Modes and Cleavage Planes of Neural Progenitors during Mammalian Cortical Development. Cold Spring Harb Perspect Biol 7, a015719, doi: 10.1101/cshperspect.a015719 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Seery JP & Watt FM Asymmetric stem-cell divisions define the architecture of human oesophageal epithelium. Curr Biol 10, 1447–1450, doi: 10.1016/s0960-9822(00)00803-4 (2000). [DOI] [PubMed] [Google Scholar]
- 166.Wu M. et al. Epicardial spindle orientation controls cell entry into the myocardium. Dev Cell 19, 114–125, doi: 10.1016/j.devcel.2010.06.011 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Loeffler D. et al. Asymmetric lysosome inheritance predicts activation of haematopoietic stem cells. Nature 573, 426–429, doi: 10.1038/s41586-019-1531-6 (2019). [DOI] [PubMed] [Google Scholar]
- 168.Xie Y, Juschke C, Esk C, Hirotsune S. & Knoblich JA The phosphatase PP4c controls spindle orientation to maintain proliferative symmetric divisions in the developing neocortex. Neuron 79, 254–265, doi: 10.1016/j.neuron.2013.05.027 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Postiglione MP et al. Mouse inscuteable induces apical-basal spindle orientation to facilitate intermediate progenitor generation in the developing neocortex. Neuron 72, 269–284, doi: 10.1016/j.neuron.2011.09.022 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jonassen JA, SanAgustin J, Baker SP & Pazour GJ Disruption of IFT complex A causes cystic kidneys without mitotic spindle misorientation. J Am Soc Nephrol 23, 641–651, doi: 10.1681/ASN.2011080829 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jonassen JA, San Agustin J, Follit JA & Pazour GJ Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J Cell Biol 183, 377–384, doi: 10.1083/jcb.200808137 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bonucci M. et al. mTOR and S6K1 drive polycystic kidney by the control of Afadin-dependent oriented cell division. Nat Commun 11, 3200, doi: 10.1038/s41467-020-16978-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dumont NA et al. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat Med 21, 1455–1463, doi: 10.1038/nm.3990 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Villegas E. et al. Plk2 regulates mitotic spindle orientation and mammary gland development. Development 141, 1562–1571, doi: 10.1242/dev.108258 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang X. et al. E-cadherin bridges cell polarity and spindle orientation to ensure prostate epithelial integrity and prevent carcinogenesis in vivo. PLoS Genet 14, e1007609, doi: 10.1371/journal.pgen.1007609 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shafer MER et al. Lineage Specification from Prostate Progenitor Cells Requires Gata3-Dependent Mitotic Spindle Orientation. Stem Cell Reports 8, 1018–1031, doi: 10.1016/j.stemcr.2017.02.004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li H. et al. Impaired Planar Germ Cell Division in the Testis, Caused by Dissociation of RHAMM from the Spindle, Results in Hypofertility and Seminoma. Cancer Res 76, 6382–6395, doi: 10.1158/0008-5472.CAN-16-0179 (2016). [DOI] [PubMed] [Google Scholar]
- 178.Hehnly H. et al. A mitotic kinase scaffold depleted in testicular seminomas impacts spindle orientation in germ line stem cells. Elife 4, e09384, doi: 10.7554/eLife.09384 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zigman M. et al. Mammalian inscuteable regulates spindle orientation and cell fate in the developing retina. Neuron 48, 539–545, doi: 10.1016/j.neuron.2005.09.030 (2005). [DOI] [PubMed] [Google Scholar]
- 180.Cayouette M. & Raff M. The orientation of cell division influences cell-fate choice in the developing mammalian retina. Development 130, 2329–2339, doi: 10.1242/dev.00446 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.