Abstract
We report here a water-soluble metal cation sensor system based on the as-prepared or reduced form of an expanded porphyrin, texaphyrin. Upon metal complexation, a change in the redox state of the ligand occurs that is accompanied by a color change from red to green. Although long employed for synthesis in organic media, we have now found that this complexation-driven redox behavior may be used to achieve the naked eye detectable colorimetric sensing of several number of less-common metal ions in aqueous media. Exposure to In(III), Hg(II), Cd(II), Mn(II), Bi(III), Co(II), and Pb(II) cations leads to a colorimetric response within 10 min. This process is selective for Hg(II) under conditions of competitive analysis. Furthermore, among the subset of response-producing cations, In(III) proved unique in giving rise to a ratiometric change in the ligand-based fluorescence features, including an overall increase in intensity. The cation selectivity observed in aqueous media stands in contrast to what is seen in organic solvents, where a wide range of texaphyrin metal complexes may be prepared. The formation of metal cation complexes under the present aqueous conditions was confirmed by reversed phase high-performance liquid chromatography, ultra-violet-visible absorption and fluorescence spectroscopies, and high-resolution mass spectrometry.
Keywords: texaphyrin, fluorescent sensor, ion-sensing, indium, mercury
1. Introduction
The rapid and selective sensing of specific analytes remains a formidable challenge. This is particularly true for heavy metal cations under conditions of aqueous analysis. Precise methodologies, including liquid chromatography, atomic absorption, and mass spectrometry (MS), can provide quantitative analyses, but are not always amenable for field use. There is thus a need for new detection paradigms. Within this latter context, chemical-based approaches are particularly attractive. They offer the possibility of rapid analyses, often via the production of an easy-to-monitor fluoro- or colorimetric response. Chemical-based sensing systems are typically characterized by a high degree of portability. Traditional chemical sensor systems (referred to as “chemosensors”) involve a detection or so-called read-out element coupled to a recognition motif via one or more chemical linkers. Common sensing moieties (read-out elements) include stimuli responsive moieties, functional polymers, biomolecules, or metal complexes, and various dyes or fluorescent chromophores [1–6]. The diversity of sensing mechanisms this approach provides, coupled with the large number of linkage and recognition motifs currently known, affords ample opportunities for modification and, in favorable cases, can be used to tune both the analyte selectivity and the signal output to a given sensing need. However, as a general rule, each modification requires a new iterative round of synthesis. The use of an all-in-one system, where both complexation and signal readout are provided by the same chemical construct, could obviate this latter limitation. It may also offer advantages in terms of ease of use. Here, we show that an expanded porphyrin precursor, namely the reduced form of texaphyrin, may be exploited as an all-in-one optical sensor for certain heavy elements in aqueous solution, including the Hg(II) and In(III) cations.
To date, heavy metal ion detection has received considerable attention due to concerns associated with environmental contamination arising from industrial processes and the associated negative impacts on organismal health [5–15]. Significant progress has been made towards the rapid detection of mercury, cadmium, lead, aluminum, and arsenic, among other toxic metal species [15–17]. Sensors for Hg(II), in particular, have received considerable attention due to the ability of mercury (in several forms) to permeate membranes, accumulate, and persist in biological systems [18,19]. Indeed, the detection of this ion has been the subject of numerous research endeavors and reviews [20–22]. Ongoing efforts in the mercury sensor area are focused on improving selectivity and sensitivity and it is likely that further advances in the area will be forthcoming.
In contrast to what is true for mercury, little progress has been made towards the development of indium-targeted chemical sensor systems. Indium has a low natural abundance with concentrations in the earth’s crust below those of gold [23]. Nevertheless, indium is a key component in a number of semiconductor-based technologies (e.g., indium tin oxide ITO substrates). As 111In, this metal also plays a role in bioimaging [24–26]. However, it is not benign. In fact, it is subject to bioaccumulation [27]. Toxic effects (mainly nephrotoxicity) associated with indium contamination have been noted [28,29]. Simple detection of indium in waste streams is thus of paramount importance. However, there are very few reports of chemical-based indium sensors in the literature [30–32]. The majority of these do not function in aqueous media. In fact, to the best of our knowledge, the phosphoserine-based fluorescent probe designed by Lee and co-workers remains the only indium sensor that functions under aqueous conditions [30]. New In(III) detection systems that allow for the rapid, naked eye-detectable sensing of In(III) in water are thus needed. As detailed below, we believe that the reduced form of a specific porphyrin analogue, texaphyrin, may have a role to play in terms of meeting this need.
Porphyrins and its analogues (generally termed porphyr-inoids) are appreciated for their versatile coordination chemistry. Often metal insertion is accompanied by easy-to-visualize changes in the ultra-violet-visible spectroscopy (UV-Vis) and fluorescence profile of the core ligand. These species have thus found widespread use in both metal cation recognition and anion detection [33]. Attempts to fine-tune the selectivity of the parent porphyrin core have resulted in modification to the heterocyclic constituents and expansion of the internal lacuna. The larger cavity of these so-called expanded porphyrins has led to the discovery of chemical features not recapitulated in simple porphyrins. The penta-aza Schiff-base expanded porphyrin known as texaphyrin (2; Fig. 1) stands as a canonical example of this paradigm; it has allowed the synthesis and structural characterization of stable 1:1 complexes with a wide range of larger metal cations, including members of the trivalent lanthanide series, that are not effectively coordinated by simple porphyrins [34]. Metal complexation, typically carried out in organic media, is accompanied by a change in color from red to dark green as the precursor ligand (sp3-Tex, 1; Fig. 1) undergoes cation coordination-induced oxidation to the corresponding aromatic form (2; Fig. 1). This inherent color change has led us to explore whether the reduced texaphyrin might not act as a new scaffold for the fluorometric or colorimetric sensing of metal ions in aqueous media. As detailed below, these efforts have led to the discovery of what could emerge as a useful “all-in-one” optical sensor for certain heavy metal cations, in particular Hg(II) and In(III), that functions in acetate buffered water.
Fig. 1.
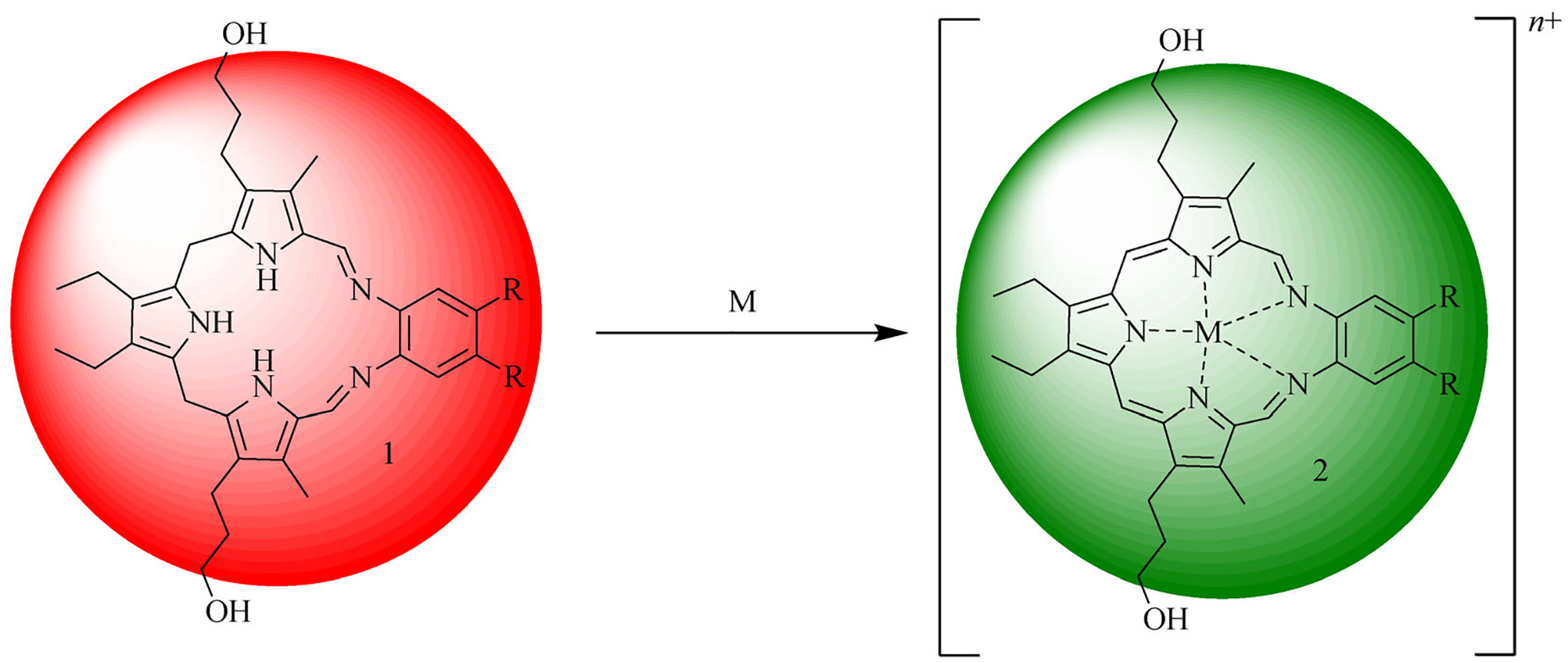
The reduced form of texaphyrin, referred to as sp3-Tex (1), is red. It turns green upon metalation and concomitant oxidation. This color change results from the aromatization of the initial ligand (left) to produce the corresponding metal complex 2 (right).
Texaphyrin has been extensively explored as a potential therapeutic, diagnostic, and theranostic agent [35–41]. Within this context, texaphyrin has most frequently been complexed (in methanol) with trivalent lanthanide cations and a small selection of other metals [34]. The Gd(III) and Lu(III) texaphyrin complexes were tested in clinical trials and were found to be tolerated at the administered doses. Ongoing efforts are exploring the use of texaphyrins as cancer-localizing drug delivery agents and as redox cycling triggers [42–46]. However, to date the redox features of texaphyrins and the electronic changes that accompany metal insertion have not been applied to the problem of metal ion sensing. A limitation is that the original cation coordination chemistry required the use of organic solvents (typically methanol) and was thus not amenable to ion sensing in aqueous environments. Recently, we reported that insertion of Bi(III) could be accomplished in water [36]. This has led to the present study.
The precursor to texaphyrin is a non-aromatic penta-aza tripyrrolic Schiff base (Fig. 1). Upon metal cation complexation this ligand undergoes oxidation (in the presence of O2). This oxidation serves to induce conjugation throughout the macrocycle and produce what is formally an aromatic system. This results in a change in color from red to green. Exploitation of this inherent property alleviates the need for an additional chromophore. The ability of texaphyrin to bind a metal cation and produce concurrently an observable spectral change makes this system attractive as an “all-in-one” sensor whose use would obviate some of the synthetic burden associated with more traditional multi-component chemical sensors. We have now found that texaphyrin is capable of stabilizing complexes with In(III), Hg(II), Cd(II), Mn(II), Bi(III), Co(II), and Pb(II) (studied as their respective acetate forms obtained by dissolving commercially available chloride, acetate, or nitrate salts in aqueous acetic acid/acetate buffer solution and exposing to sp3-Tex), with a marked color change accompanying complexation. When exposed to a mixture of metal ions, the formation of the Hg(II) texaphyrin complex was found to dominate. Exposure of sp3-Tex to In(III) under aqueous conditions led to an increase in the fluorescent signal that was visible by the naked eye. These findings are detailed further below.
2. Experimental
2.1. General procedures
Starting materials were purchased from Fisher Scientific, Acros, Alfa Aesar, Strem Chemicals or Sigma Aldrich and used without further purification unless otherwise noted. Solvents were purified using a solvent purifier system (vacuum atmospheres). Dichloromethane was freshly distilled after being dried over CaH2 under argon. Analytical and semi-preparative reversed phase high-performance liquid chromatography (RP-HPLC) analyses were performed on a Thermo scientific Dionex Ultimate 3000 instrument equipped with photo-diode array detector (set at 254, 400, 470 and 740 nm). The analytical column was a Syncronis C18 column, 5 μm, 4.6 × 250 mm (Thermo Scientific); the mobile phase consisted of an increasing gradient (from 10% to 99% in 10 min) of acetonitrile in water, both containing 0.1% acetic acid. In this case the flow rate was set to 1.2 mL·min−1. Low-resolution and high-resolution electrospray mass spectrometric (ESI-MS) analyses were carried out using Thermo Finnigan LTQ and Qq-FTICR (7 Tesla) instruments, respectively. Fluorescence spectra were recorded on an Agilent Technologies Cary Eclipse fluorescence spectrophotometer; excitation and emission slit widths were both 5 nm and the samples were excited at 470 nm. UV-visible spectra were recorded on a Varian Cary 5000 NiR-UV-Vis instrument.
Aqueous buffers were prepared using deionized water and NH4OAc (0.1 mol·L−1). The pH was adjusted to 7.44 using NaOHaq. as determined by a pH-meter.
2.2. Synthesis of sp3-Tex
The two key precursors tripyrrane (A) and the dinitro aromatic derivative (B) were obtained as gifts from Pharmacyclics, Inc. (now an Abbvie subsidiary). Compound B was reduced to the corresponding bis-amine product using hydrogen gas with the reaction carried out either at 100 psi in a sealed reactor or at 1 atmosphere using an H2-filled balloon in the presence of palladium on carbon (10%). After 15 h, the reaction mixture was filtered through a paper filter and the product was condensed with A in the presence of a catalytic amount of HCl in methanol. After stirring for 1 h at 50°C, the volatiles were removed under reduced pressure to give a dark red solid residue that was not purified and which was used directly for the complexation studies (Scheme 1).
Scheme 1.
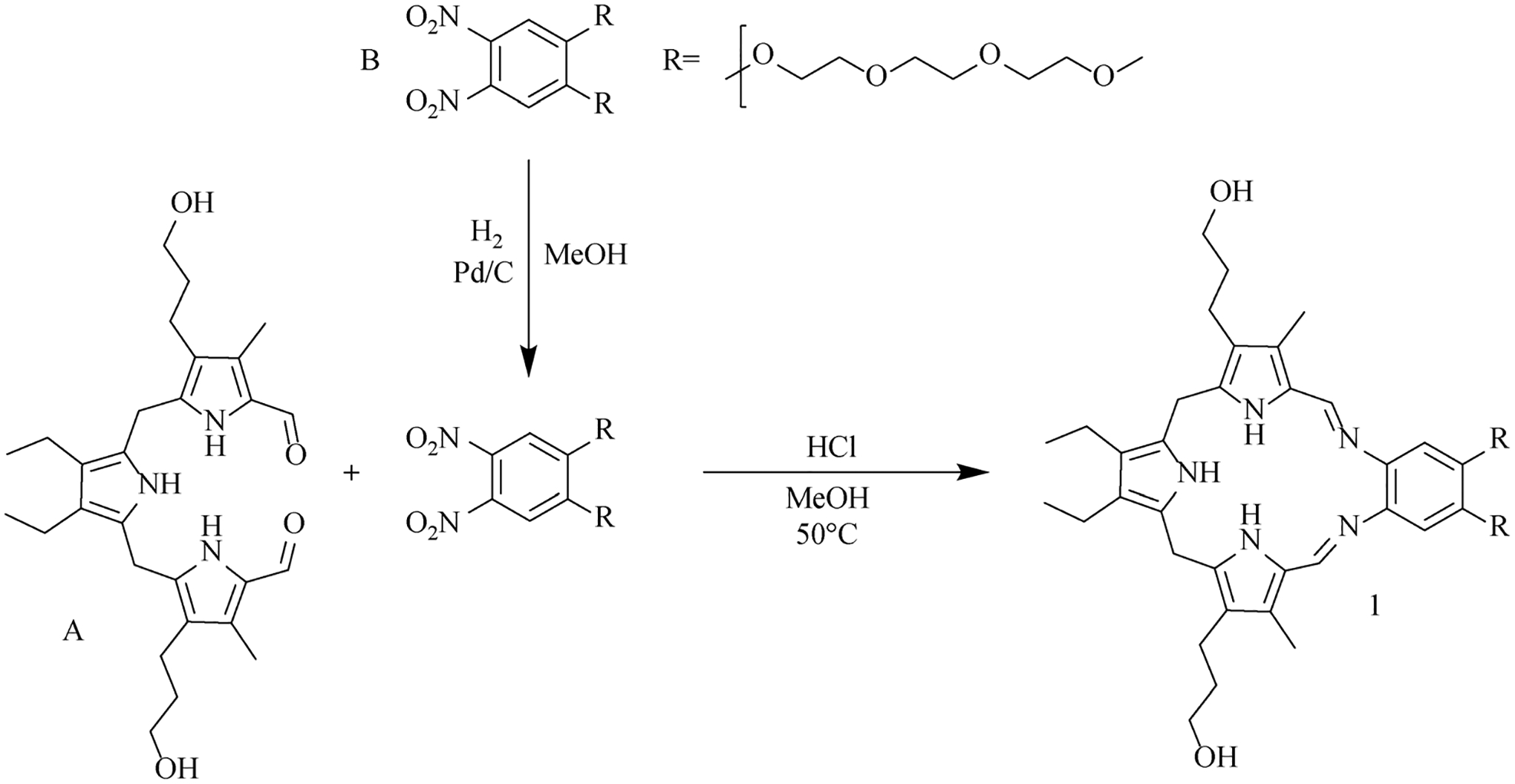
Synthetic procedure used to obtain sp3-Tex (1).
Stock solutions (100 mmol·L−1 in a 1: 1 mixture of acetic acid: aqueous NH4OAc buffer) of Nd(NO3)3, Lu(NO3)3, Eu(NO3)3, La(NO3)3, Y(NO3)3, Dy(NO3)3, Ho(NO3)3, Ga(NO3)3, Cd(NO3)3, Pb(NO3)2, Mn(OAc)2, Gd(OAc)3, Er(OAc)3, Hg(OAc)2, Zn(OAc)2, Bi(OAc)3, Ni(OAc)2, InCl3, SnCl4, AlCl3, FeCl3, MgCl2, CoCl3 and Co(NO3)3 were prepared. A 10 μL aliquot of a 10 mmol·L−1 stock solution of sp3-Tex in methanol(0.1 mmol·L−1) was added to 980 μL of NH4OAc(0.1 mol·L−1) in an Eppendorf tube. To each tube, 10 μL of a 100 mmol·L−1 stock solution of the metal salt under consideration in an aqueous buffer (1: 1 mixture of acetic acid and NH4OAc in water; pH ≈ 5‒6) were added to ligand 1 and the mixture was heated open to the air at 90°C for 10 min. The crude mixture was then directly analyzed by RP-HPLC, UV-visible spectroscopy, MS, and fluorescence emission spectroscopy. The acetate buffer described above was used so as to convert all metal cations to their acetate forms independent of the original salt form employed.
3. Results and discussion
Of the 23 metal salts screened, only 7 elicited a colorimetric response (Fig. 2). This selectivity for certain cations stands in stark contrast to the metalation protocols carried out in organic media, where a large number of metal complexes were readily formed (Fig. 1) [34]. The samples produced under the present aqueous conditions were subject to UV-Vis spectroscopic, RP-HPLC, and MS analyses to confirm the formation of the proposed metal complex.
Fig. 2.
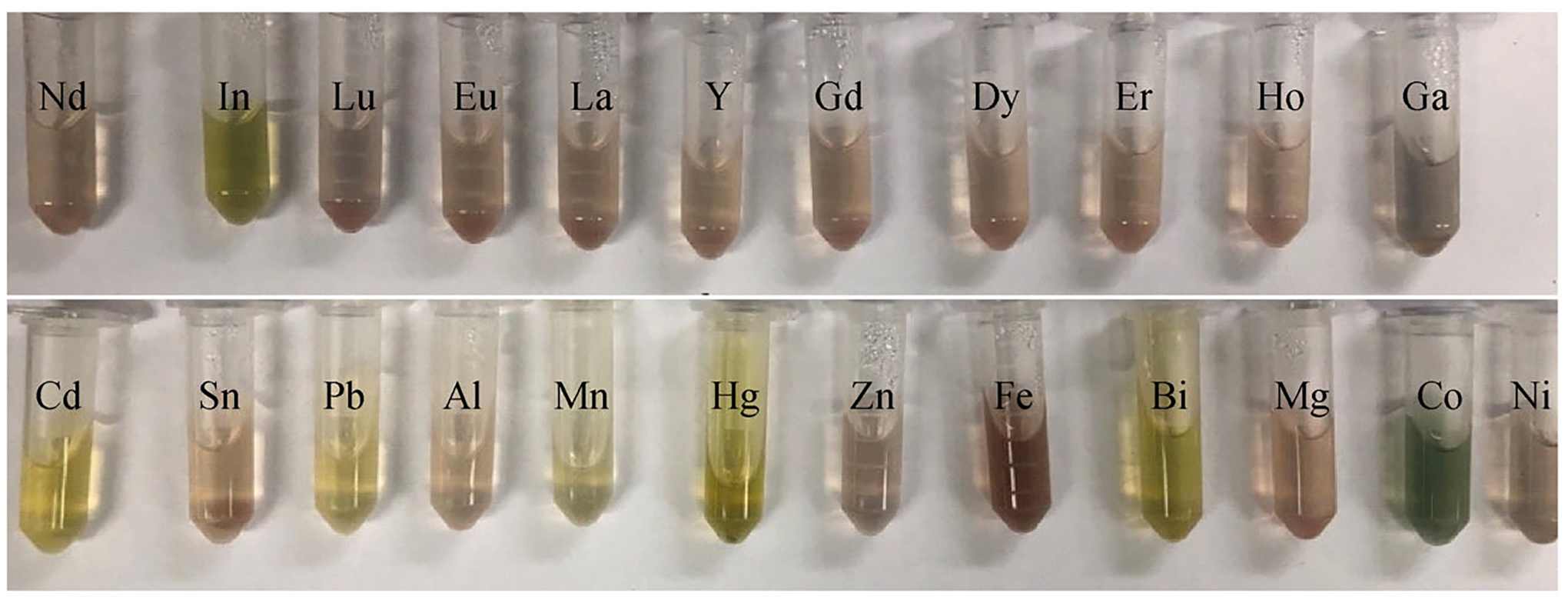
Photographs of solutions obtained when the indicated metal salts (10 equiv. from a 100 mmol·L−1 solution in an acetic acid: NH4OAc = 1: 1 aqueous buffer) are mixed with sp3-Tex (0.1 mmol·L−1 in 0.1 mol·L−1 NH4OAc buffer) and heated at 90°C for 10 min (pH ≈ 5‒6). Cations that were found to provide a response were Mn(OAc)2, Co(NO3)3, CoCl3, Cd(NO3)3, InCl3, Hg(OAc)2, Pb(NO3)2, and Bi(OAc)3. The acetic acid: NH4OAc = 1: 1 aqueous buffer was used to convert all cations to their respective acetate derivatives and to control for putative anion effects.
The solutions giving rise to a colorimetric response were further tested by fluorescence emission spectroscopy. Only the sample produced by exposing sp3-Tex to In(III) produced a fluorescence signature that was readily apparent to the naked eye when exposed to a small laboratory UV light (i.e., a TLC lamp). The only other metal cation that produced a fluorescence increase as measured by a fluorimeter was Cd(II) (cf. Electronic Supplemental Information (ESM), S3); however, this fluorescence increase was undetectable to the naked eye under a UV lamp. This selectivity led us to test sp3-Tex as a fluorometric sensor for In(III).
When a solution of sp3-Tex (0.1 mmol·L−1 in aqueous acetate buffer) is treated with In(III) (1 mmol·L−1 in aqueous acetate buffer) a marked increase in the intensity of the fluorescence signal centered at around 752 nm is seen (Fig. 3). This increase is ascribed to formation of the In(III) texaphyrin complex (In(III)-Tex). To provide support for this conclusion, the fluorescence spectrum of In(III)-Tex (synthesized according to a literature procedure[34]) was also recorded in methanol (Fig. 3, right). In aqueous media, texaphyrin molecules are known to aggregate. Aggregation has been shown to quench fluorescence in some systems, whereas in methanol, the extent of aggregation of texaphyrins is reduced [47,48]. Likely as a consequence of such aggregation effects, the fluorescence of the complex in methanol is far more intense than in aqueous media. Additionally, there is a shift in the λmax from 748 nm in methanol to 752 nm in water. This red shift in the emission is thought to be caused by aggregation with the higher energy band being ascribed to the less aggregated species present in methanol [49].
Fig. 3.
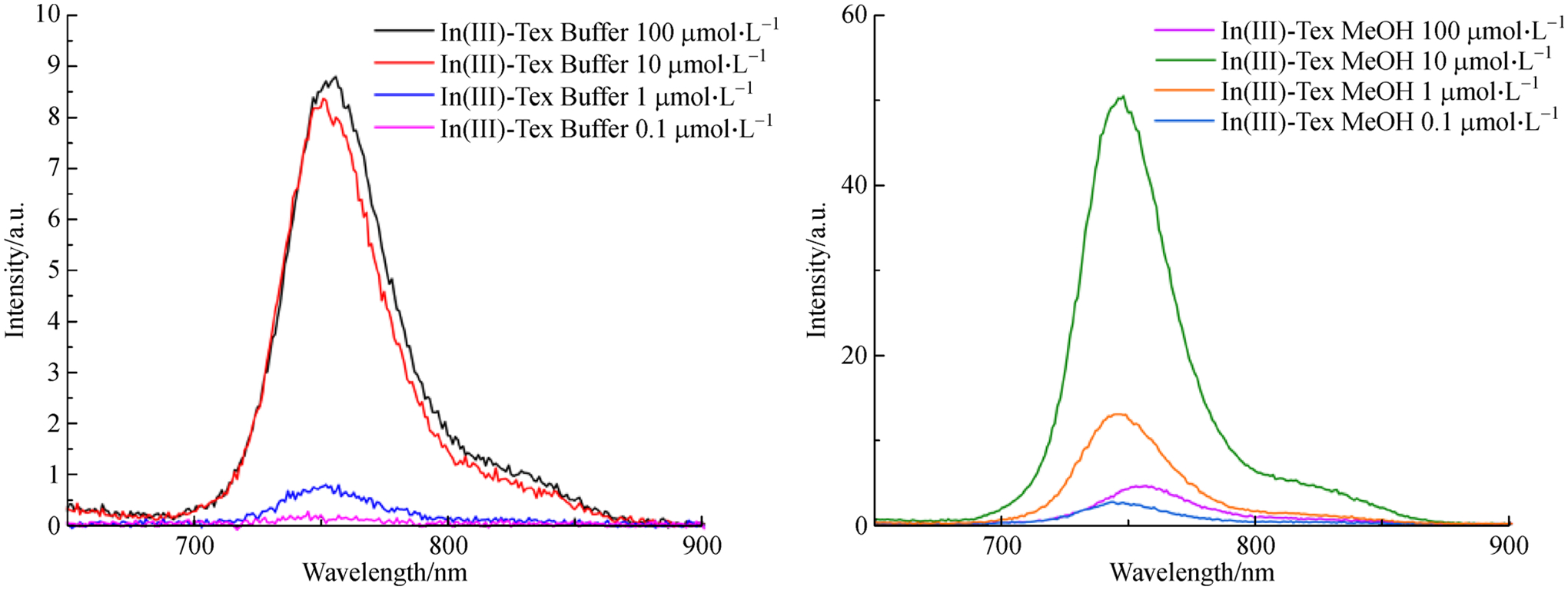
Fluorescence spectra at different concentrations of a solution of sp3-Tex (0.1 mmol·L−1 in aqueous acetate buffer) and InCl3 (1 mmol·L−1 in aqueous acetate buffer), then diluted to the indicated concentrations (λmax = 752 nm, left) and fluorescence of pre-formed In(III)-texaphyrin complex (In(III)-Tex) in methanol (λmax = 748 nm, right).
Varying the concentration of InCl3 under the conditions where sp3-Tex was being tested as a possible sensor revealed a ratiometric fluorescence response (Fig. 4). Initially, a fluorescence signal at ca. 525 nm can be observed when sp3-Tex is subjected to testing (90°C for 10 min). Upon increasing the concentration of InCl3 in the system, the fluorescence signal at 525 nm is seen to decrease as the peak attributed to the In-texaphyrin complex (752 nm) increases. This response reaches saturation at around 2000 μmol·L−1 (20 equivalents) of InCl3. At saturation, the signal at 752 nm is roughly 12 times the signal at 525 nm.
Fig. 4.
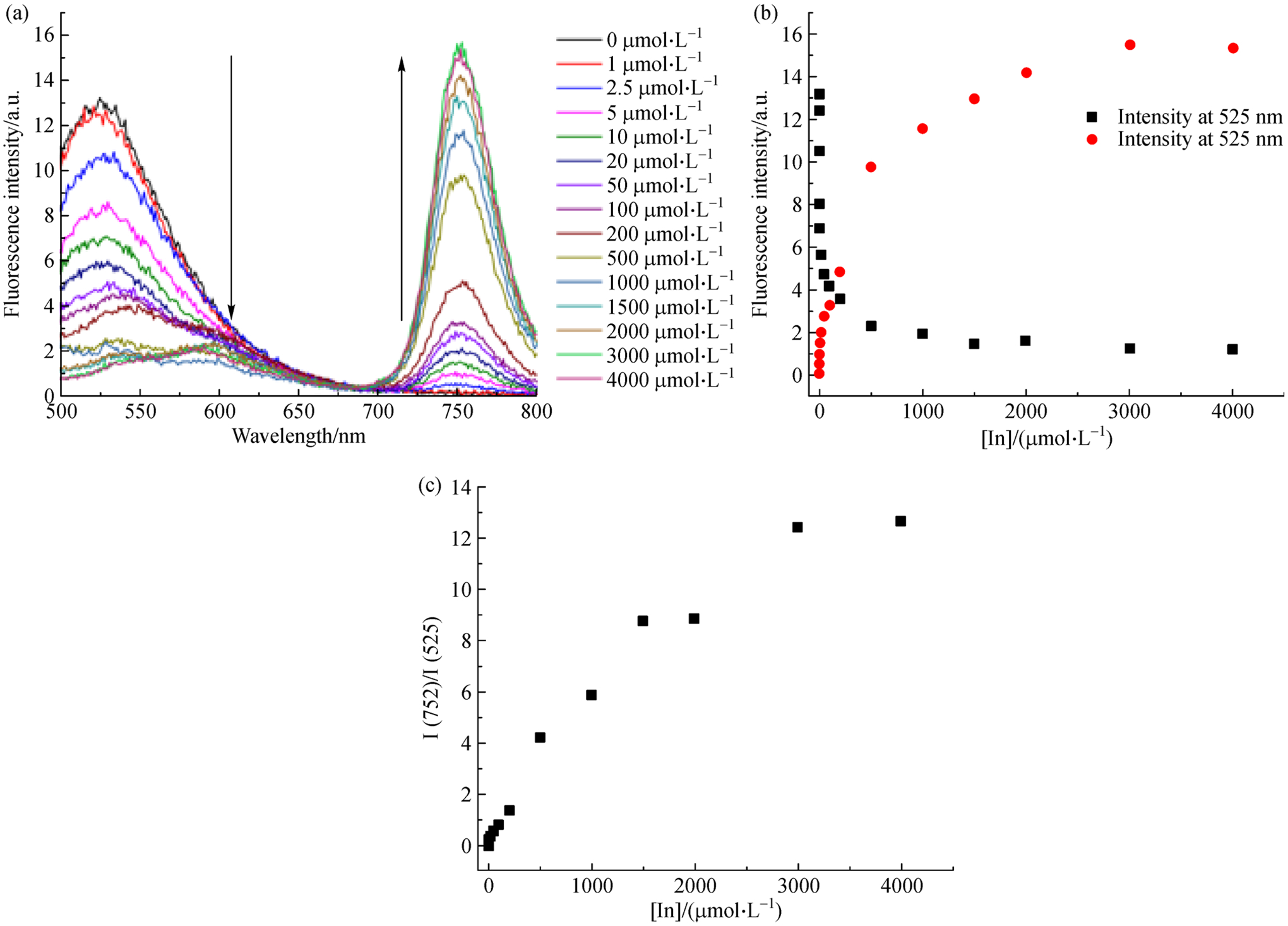
(a) Fluorescence spectra of sp3-Tex recorded in the presence of increasing concentrations of In(III); (b) Change in fluorescence intensity at 525 nm and 752 nm seen in the presence of increasing concentrations of In(III); (c) Ratio of fluorescence signal at 752 nm and 525 nm as a function of increasing In(III) concentration.
The fluorescence signal was used to estimate the limit of detection (LOD). For a [sp3-Tex] = 0.1 mmol·L−1 the In(III)-texaphyrin fluorescence signature was observable at concentrations as low as 2.5 μmol·L−1 (288 ppb) and 0.1 μmol·L−1 (11.5 ppb) in water and methanol, respectively. Visually, the fluorescence signal produced by In(III)-texaphyrin is easily observed in methanol at concentrations as low as 10 μmol·L−1 when the solutions are subject to irradiation with a handheld UV lamp (Fig. 5). Introducing methanol into aqueous solutions containing sp3-Tex prior to treatment with In(III) led to an increase in the fluorescence intensity; however, the complexities associated with adding methanol and the accompanying dilutions led us to conclude that such a strategy was likely to be ineffective in terms of producing an optimized system suitable for field use (cf. ESM). Although further study is warranted, we suggest that simple buffered aqueous solutions of sp3-Tex may prove adequate for this latter purpose.
Fig. 5.
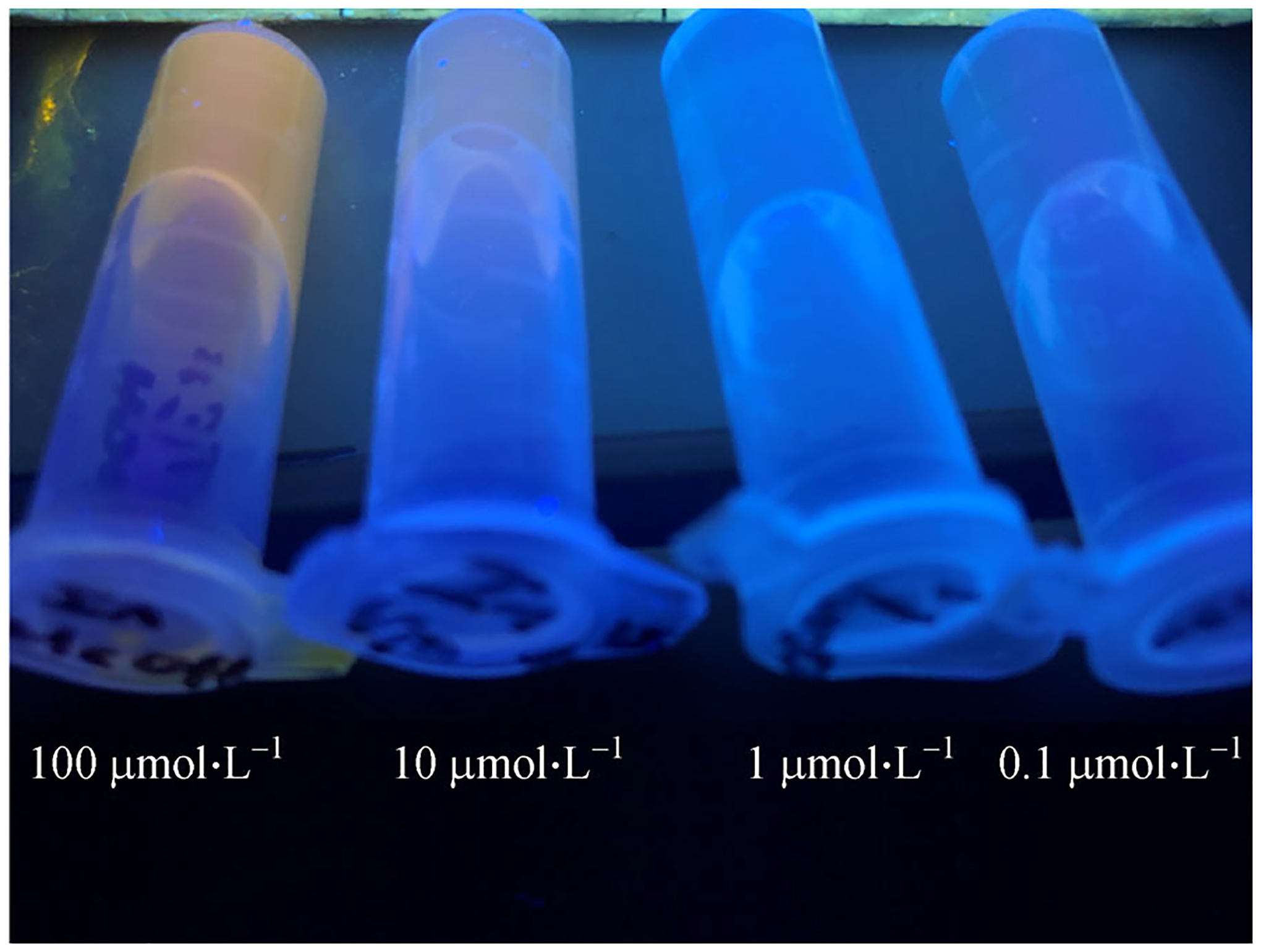
The fluorescence emitted by In(III)-texaphyrin can be observed by the naked eye down to a concentration of roughly 10 μmol·L−1 in methanol when solutions containing low concentrations of sp3-Tex and an In(III) source are subject to illumination using a simple handheld UV lamp.
The reaction of InCl3 with sp3-Tex (1) and spectra of the as-prepared solution were shown in Fig. 6. When monitored by UV-vis spectroscopy, the complexation of In(III) showed a shift in the Soret band from 365 nm to 467 nm, as well as the emergence of a Q-type band at 735 nm. Other metal cations that provided for an observable color change upon exposure to sp3-Tex gave rise to similar spectral features. Evidence of complexation came from RP-HPLC analyses in conjunction with MS studies. The RP-HPLC trace of the In(III)-texaphyrin complex was characterized by a single peak with a retention time of 9 min. Additionally, high resolution MS revealed a parent peak consistent with complex formation. Specifically, the In(III)-texaphyrin complex was observed as a 2+ species, which is expected upon loss of the two labile axial anionic ligands (Fig. 6(c)). Other metallotexaphyrin derivatives eluted at similar, but distinctly different, retention times when subject to analogous RP-HPLC analysis. The formation of the other metal complexes produced under aqueous metal insertion conditions was confirmed via low-resolution MS (cf. Figs. S6–S14, ESM). It is worth mentioning that each of the complexes prepared in this study proved stable for several days at room temperature when kept as aqueous solutions under slightly acidic conditions (i.e., at pH = ca. 5).
Fig. 6.
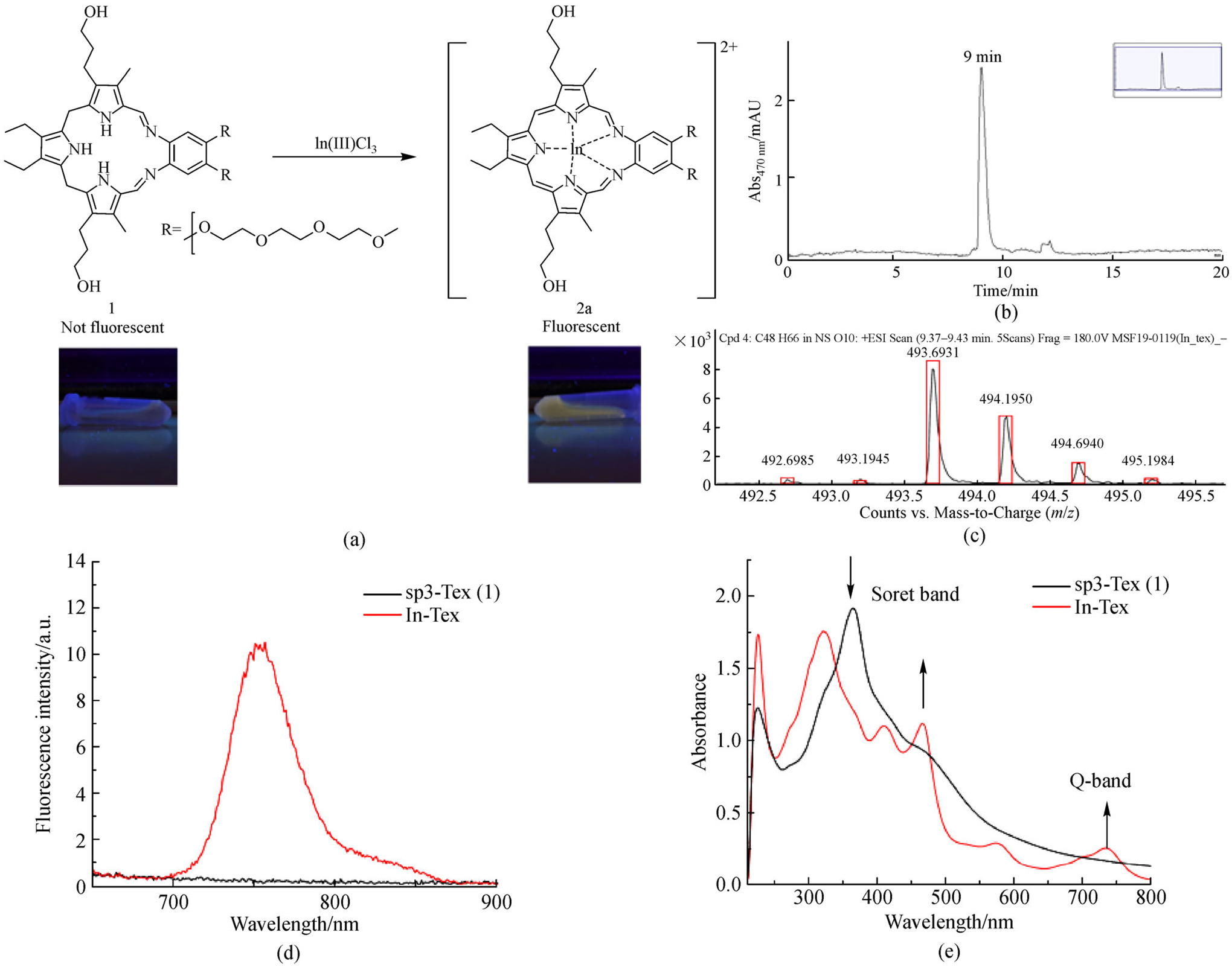
(a) Reaction scheme, (b) HPLC trace λdetector = 470 nm, (c) high-resolution mass spectrum (d) fluorescence change λex = 470 nm, and (e) UV-Vis absorption for solutions prepared by mixing InCl3 with sp3-Tex (1) under the indicated experimental conditions.
Further studies were carried out to determine the selectivity of the sensor system. Upon exposure of sp3-Tex to a mixture of metals (InCl3, Hg(OAc)2, Mn(OAc)2, Cd(NO3)3, and Pb(NO3)2) in the same aqueous acetate buffer as used above, the only ion seen to complex was Hg(II) (as confirmed via UV-Vis spectroscopic, HPLC, and MS analyses; ESM). This selectivity is believed to be a consequence of the kinetics of the complexation reaction. Hg(II) is a rather small cation and thus coordinates to the texaphyrin rapidly (i.e., more quickly than either degradation of sp3-Tex or complexation of other metal cations). The selectivity for Hg(II) over lanthanides is thought to be more thermodynamic in origin. The hydration energies of the trivalent lanthanide cations are relatively high and complexation by sp3-Tex cannot outcompete the stabilization provided by the aqueous environment, at least under the conditions of the present experiments.
Transmetalation, where a Cd(II)-texaphyrin complex was tested as a precursor to produce a lanthanide texaphyrin in aqueous solution, was attempted. Here, 10 equivalents of gadolinium(III) acetate were added to a preformed solution of the Cd(II)-texaphyrin complex. The resulting solution was heated for 10 min. No Gd-Tex was observed via mass spec analysis. In contrast, an analogous experiment carried out using Hg(OAc)2 led to formation of Hg(II)-Tex (cf. Figs. S13 and S14, ESM). This was taken as further evidence that the hydration energies of the trivalent lanthanide cations are simply too high to allow for effective metal insertion under aqueous conditions.
Of note is that exposure of sp3-Tex to Hg(II) per the above protocols complex does not lead to a discernible change in the fluorescence of the system (cf. Fig. S3, ESM). This may reflect a heavy metal quenching effect or vibronic relaxation pathways favored in the case of a metal cation that is not bound in a fully rigid fashion. In the event, this finding, which stands in contrast to what was seen for In(III), leads for a sensing selectivity: Depending on the measurement modality employed, sp3-Tex is a selective sensor for either Hg(II) or In(III). Moreover, since the Hg(II) and In(III) texaphyrin complexes have very different retention times via HPLC analysis, it is possible to differentiate between the two metalated species with analytical precision if desired. We thus propose that our system (i.e., texaphyrin precursor sp3-Tex) can act as a fluorometric probe in waste water streams where In(III) is suspected of being present and as a colorimetric probe for detection of mercury(II) in aqueous mixtures where various metal cation contaminants are present. As needed, RP-HPLC analyses can provide an unambiguous confirmation of the results obtained from such qualitative screening studies.
4. Conclusions
In conclusion, we have developed an optical chemical sensor system based on the reduced texaphyrin scaffold that may be used to sense both the In(III) and Hg(II) ions by the naked eye. Upon exposure of the texaphyrin precursor, sp3-Tex, to an indium(III) cation source in aqueous media, a ratiometric fluorescence response, including an overall “turn-on” in the overall intensity and color, was seen under conditions of simple UV illumination. The detection limits of these metals were found to be as low as 288 ppb in water, mimicking real-world analysis situations, and even lower in the presence of methanol. Competition and transmetalation studies demonstrated a preference for the mercury(II) cation with attendant colorimetric sensing via UV-vis spectroscopic analysis. These studies demonstrate what is to our knowledge the first instance of using the easy-to-visualize color change associated with oxidation-metalation of the texaphyrin core to detect preferentially certain heavy metal ions in aqueous buffer. The rich chemistry of porphyrinoid species, coupled with the inherent color change seen in many instances upon metalation, might render this broad class of compounds useful as field-compatible sensors for toxic and hazardous metal ions.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (Grants CA068682 to J.L.S.) and the Robert A. Welch Foundation (F-0018). HDR would like to thank UT Austin for a Scientist in Residence Fellowship and the Los Alamos National Lab for a Seaborg Fellowship.
Footnotes
Electronic Supplementary Material Supplementary material is available in the online version of this article at https://doi.org/10.1007/s11705-019-1888-y and is accessible for authorized users.
References
- 1.Wu D, Sedgwick AC, Gunnlaugsson T, Akkaya EU, Yoon J, James TD. Fluorescent chemosensors: The past, present, and future. Chemical Society Review, 2017, 46(23): 7105–7123 [DOI] [PubMed] [Google Scholar]
- 2.Li Z, Askim JR, Suslick KS. The optoelectronic nose: Colorimetric and fluorometric sensor arrays. Chemical Reviews, 2019, 119(1): 231–292 [DOI] [PubMed] [Google Scholar]
- 3.Kaur B, Kaur N, Kumar S. Colorimetric metal ion sensors—a comprehensive review of the years 2011‒2016. Coordination Chemistry Reviews, 2018, 358: 13–69 [Google Scholar]
- 4.Piriya A, Joseph P, Daniel K, Lakshmanan S, Kinoshita T, Muthusamy S. Colorimetric sensors for rapid detection of various analytes. Materials Science and Engineering C, 2017, 78: 1231–1245 [DOI] [PubMed] [Google Scholar]
- 5.Long F, Zhu A, Shi H, Wang H, Liu J. Rapid on-site/in-situ detection of heavy metal ions in environmental water using a structure-switching DNA optical biosensor. Scientific Reports, 2013, 3(1): 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou W, Saran R, Liu J. Metal sensing by DNA. Chemical Reviews, 2017, 117(12): 8272–8325 [DOI] [PubMed] [Google Scholar]
- 7.Nolan EM, Lippard SJ. Turn-on and ratiometric mercury sensing in water with a red-emitting probe. Journal of the American Chemical Society, 2007, 129(18): 5910–5918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azmi NA, Ahmad SH, Low SC. Detection of mercury ions in water using a membrane-based colorimetric sensor. RSC Advances, 2018, 8(1): 251–261 [Google Scholar]
- 9.Chang J, Zhou G, Gao X, Mao S, Cui S, Ocola LE, Yuan C, Chen J. Real-time detection of mercury ions in water using a reduced graphene oxide/DNA field-effect transistor with assistance of a passivation layer. Sensing and Bio-Sensing Research, 2015, 5: 97–104 [Google Scholar]
- 10.Karthikeyan K, Sujatha L. Fluorometric sensor for mercury ion detection in a fluidic MEMS device. IEEE Sensors Journal, 2018, 18(13): 5225–5231 [Google Scholar]
- 11.Maher S, Bastani B, Smith B, Jjunju Z, Taylor S, Young IS. Portable fluorescent sensing array for monitoring heavy metals in water. IEEE Sensors, 2016: 1–3 [Google Scholar]
- 12.He W, Luo L, Liu Q, Chen Z. Colorimetric sensor array for discrimination of heavy metal ions in aqueous solution based on three kinds of thiols as receptors. Analytical Chemistry, 2018, 90(7): 4770–4775 [DOI] [PubMed] [Google Scholar]
- 13.Niu L, Li H, Feng L, Guan Y, Chen Y, Duan C, Wu L, Guan Y, Tung C, Yang Q. BODIPY-based fluorometric sensor array for the highly sensitive identification of heavy-metal ions. Analytica Chimica Acta, 2013, 775: 93–99 [DOI] [PubMed] [Google Scholar]
- 14.Singh RK, Mishra S, Jena S, Panigrahi B, Das B, Jayabalan R, Parhi PK, Mandal D. Rapid colorimetric sensing of gadolinium by EGCG-derived AgNPs: The development of a nanohybrid bioimaging probe. Chemical Communications, 2018, 54(32): 3981–3984 [DOI] [PubMed] [Google Scholar]
- 15.Denis M, Pancholi J, Jobe K, Watkinson M, Goldup SM. Chelating rotaxane ligands as fluorescent sensors for metal ions. Angewandte Chemie International Edition, 2018, 57(19): 5310–5314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong W, Li W, Hu X, Zhao B, Zhang F, Zhang D. Highly sensitive colorimetric sensing for heavy metal ions by strong polyelectrolyte photonic hydrogels. Journal of Materials Chemistry, 2011, 21(43): 17193–17201 [Google Scholar]
- 17.Moghaddam MR, Carrara S, Hogan CF. Multi-colour bipolar electrochemiluminescence for heavy metal ion detection. Chemical Communications, 2018, 55(8): 3–6 [DOI] [PubMed] [Google Scholar]
- 18.Boening DW. Ecological effects, transport, and fate of Mercury: A general review. Chemosphere, 2000, 40(12): 1335–1351 [DOI] [PubMed] [Google Scholar]
- 19.Zheng W, Aschner M, Ghersi-egea J. Brain barrier systems: A new frontier in metal neurotoxicological research. Toxicology and Applied Pharmacology, 2003, 192(1): 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selid PD, Xu H, Collins EM, Face-Collins MS, Zhao JX. Sensing mercury for biomedical and environmental monitoring. Sensors (Basel), 2009, 9(7): 5446–5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu J, Wu T, Zhang G, Liu S. Highly selective fluorescence sensing of mercury ions over a broad concentration range based on mixed polymeric micelles. Macromolecules, 2012, 45(9): 3939–3947 [Google Scholar]
- 22.Nolan EM, Lippard SJ. Tools and tactics for the optical detection of mercuric ion. Chemical Reviews, 2008, 108(9): 3443–3480 [DOI] [PubMed] [Google Scholar]
- 23.Zhang K, Wu Y, Wang W, Li B, Zhang Y, Zuo T. Resources, conservation and recycling indium from waste LCDs: A review. Resources, Conservation and Recycling, 2015, 104: 276–290 [Google Scholar]
- 24.Thakur ML, Welch MJ, Joist JH, Coleman RE. Indium-III labeled platelets: Studies on preparation and evaluation of in vitro and in vivo functions. Thrombosis Research, 1976, 9(4): 345–357 [DOI] [PubMed] [Google Scholar]
- 25.Thakur M, Lavender JP, Arnot R, Silvester DJ, Segal AW. Indium-III-labeled autologous leukocytes in man. Journal of Nuclear Medicine, 1977, 18(10): 1014–1021 [PubMed] [Google Scholar]
- 26.Zolata H, Abbasi F, Afarideh H. Synthesis, characterization and theranostic evaluation of indium-III labeled multifunctional super-paramagnetic iron oxide nanoparticles. Nuclear Medicine and Biology, 2015, 42(2): 164–170 [DOI] [PubMed] [Google Scholar]
- 27.Alfantazi AM, Moskalyk RR. Processing of indium: A review. Materials & Design, 2003, 16(8): 687–694 [Google Scholar]
- 28.Lim CH, Han J, Cho H, Kang M. Studies on the toxicity and distribution of indium compounds according to particle size in sprague-dawley rats. Toxicological Research, 2014, 30(1): 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka A, Hirata M, Kiyohara Y, Nakano M, Omae K, Shiratani M, Koga K. Review of pulmonary toxicity of indium compounds to animals and humans. Thin Solid Films, 2010, 518(11): 2934–2936 [Google Scholar]
- 30.Mehta PK, Hwang GW, Park J, Lee K. Highly sensitive ratiometric fluorescent detection of indium(III) using fluorescent probe based on phosphoserine as a receptor. Analytical Chemistry, 2018, 90(19): 11256–11264 [DOI] [PubMed] [Google Scholar]
- 31.Wu YC, Li H, Yang H. A sensitive and highly selective fluorescent sensor for In3+. Organic & Biomolecular Chemistry, 2010, 8(15): 3394–3397 [DOI] [PubMed] [Google Scholar]
- 32.Kim SK, Kim SH, Kim HJ, Lee SH, Lee SW, Ko J, Bartsch RA, Kim JS. Indium(III)-induced fluorescent excimer formation and extinction in calix[4]arene—fluoroionophores. Inorganic Chemistry, 2005, 44(22): 7866–7875 [DOI] [PubMed] [Google Scholar]
- 33.Ding Y, Zhu W, Xie Y. Development of ion chemosensors based on porphyrin analogues. Chemical Reviews, 2017, 117(4): 2203–2256 [DOI] [PubMed] [Google Scholar]
- 34.Sessler JL, Mody TD, Hemmi GW, Lynch V. Synthesis and structural characterization of lanthanide(III) texaphyrins. Inorganic Chemistry, 1993, 32(14): 3175–3187 [Google Scholar]
- 35.Preihs C, Arambula JF, Lynch VM, Siddik H, Sessler JL. Bismuth- and lead-texaphyrin complexes: Towards potential α-core emitters for radiotherapy. Chemical Communications, 2010, 46(42): 7900–7902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thiabaud G, Radchenko V, Wilson JJ, John KD, Birnbaum ER, Sessler JL. Rapid insertion of bismuth radioactive isotopes into texaphyrin in aqueous media. Journal of Porphyrins and Phthalo-cyanines, 2017, 21(12): 882–886 [Google Scholar]
- 37.Maiya BG, Harriman A, Sessler JL, Hemmi G, Murai T, Mallouk TE. Ground- and excited-state spectral and redox properties of cadmium(II) texaphyrin. Journal of Physical Chemistry, 1989, 93(24): 8111–8115 [Google Scholar]
- 38.Sessler JL, Murai T, Lynch V, Cyr M. An “expanded porphyrin”: The synthesis and structure of a new aromatic pentadentate ligand of chemistry. Journal of the American Chemical Society, 1988, 110(16): 5586–5588 [Google Scholar]
- 39.Sessler JL, Dow WC, Connor DO, Harriman A, Hemmi G, Mody TD, Miller RA, Qing F, Springs S, Woodburn K, et al. Biomedical applications of lanthanide(III) texaphyrins lutetium(III) texaphyrins as potential photodynamic therapy photosensitizers. Journal of Alloys and Compounds, 1997, 249(1–2): 146–152 [Google Scholar]
- 40.Magda D, Miller RA. Motexafin gadolinium: A novel redox active drug for cancer therapy. Seminars in Cancer Biology, 2006, 16(6): 466–476 [DOI] [PubMed] [Google Scholar]
- 41.Hannah S, Lynch V, Guldi DM, Gerasimchuk N, Macdonald CLB, Magda D, Sessler JL. Late first-row transition-metal complexes of texaphyrin. Journal of the American Chemical Society, 2002, 124(28): 8416–8427 [DOI] [PubMed] [Google Scholar]
- 42.Thiabaud G, Arambula JF, Siddik ZH, Sessler JL. Photoinduced reduction of Pt IV within an anti-proliferative Pt IV-texaphyrin conjugate. Chemistry (Weinheim an der Bergstrasse, Germany), 2014, 20(29): 8942–8947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thiabaud G, Mccall R, He G, Arambula JF, Siddik ZH, Sessler JL. Activation of platinum(IV) prodrugs by motexafin gadolinium as a redox mediator. Angewandte Chemie International Edition, 2016, 55(41): 12626–12631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arambula JF, Sessler JL, Siddik ZH. Overcoming biochemical pharmacologic mechanisms of platinum resistance with a texaphyrin-platinum conjugate. Bioorganic & Medicinal Chemistry Letters, 2011, 21(6): 1701–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arambula JF, Sessler JL, Siddik ZH. A texaphyrin-oxaliplatin conjugate that overcomes both pharmacologic and molecular mechanisms of cisplatin resistance in cancer cells. MedChem-Comm, 2012, 3(10): 1275–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee MH, Kim EJ, Park SY, Hong KS, Kim S, Sessler JL. Acid-triggered release of doxorubicin from a hydrazone-linked Gd3+-texaphyrin conjugate. Chemical Communications, 2016, 52(69): 10551–10554 [DOI] [PubMed] [Google Scholar]
- 47.Blesic M, Melo E, Petrovski Z, Plechkova NV, Lopes NC, Seddon KR, Rebelo PN. On the self-aggregation and fluorescence quenching aptitude of surfactant ionic liquids. Journal of Physical Chemistry B, 2008, 112(29): 8645–8650 [DOI] [PubMed] [Google Scholar]
- 48.Mei J, Leung NLC, Kwok RTK, Lam JWY, Tang BZ. Aggregation-induced emission: Together we shine, united we soar! Chemical Reviews, 2015, 115(21): 11718–11940 [DOI] [PubMed] [Google Scholar]
- 49.Quinn SD, Magennis SW. Optical detection of gadolinium(III) ions via quantum dot aggregation. RSC Advances, 2017, 7(40): 24730–24735 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


