INTRODUCTION
Today, few Americans may remember the devastating implications and fear caused by poliomyelitis outbreaks given that there has not been an outbreak of paralytic polio in the United States since 1979. Before the development of effective vaccines, poliomyelitis was a seasonal epidemic disease in the United States. The number of cases peaked in 1952 when more than 20,000 cases of paralytic polio were reported.1 Although some people recovered from paralytic polio, many suffered permanent paralysis and even death. Hospital wards filled with iron lungs and permanently disabled children served as painful visible reminders to society of the toll this debilitating disease took on young lives. With an estimated 350,000 polio cases worldwide and endemic disease in 125 countries the World Health Assembly launched the Global Polio Eradication Initiative (GPEI) in 1988, targeting the disease for eradication.
POLIO AS A CANDIDATE FOR ERADICATION
Global eradication is currently defined as, “the worldwide absence of a specific disease agent in nature as a result of deliberate control efforts that may be discontinued where the agent is judged no longer to present a significant risk from extrinsic sources.”2 Determining if a disease is a good candidate for eradication depends on whether or not it meets 4 key criteria. These criteria include the following:
Humans are required to maintain the pathogen.
Sensitive and specific diagnostic tools are available.
There is an effective intervention to terminate human-to-human transmission.
There is proof of principle (ie, elimination of transmission in a large geographic area).
Poliovirus requires a specific cell receptor (PVR or CD155) for infection that is expressed only on human and simian cells.3 Therefore, humans are the only host for sustained poliovirus transmission as primate population sizes are too small to maintain sustained transmission. Breaking the chains of human-to-human transmission can eradicate the virus. Although asymptomatic cases present a challenge to surveillance, AFP reporting and virologic testing of stool are reliable ways to detect polio cases in populations. Although not without certain limitations, oral poliovirus vaccine (OPV) and inactivated poliovirus vaccine (IPV) are effective tools in preventing infection in individuals and reducing circulation of poliovirus within communities. With its low cost, ease of administration, and ability to induce mucosal immunity, OPV has been a particularly useful tool in interruption of transmission in populations. Lastly, early elimination of polio in the Western hemisphere served as proof of principle that eradication was possible throughout the world.
CLINICAL ASPECTS OF POLIO
Polioviruses, consisting of 3 antigenic types (serotypes 1, 2, and 3), are positive-sense single-stranded RNA Enteroviruses belonging to the Picornaviridae family.3 Most poliovirus infections occur after oral ingestion of the virus followed by replication in the oral and intestinal mucosa, and most infections are asymptomatic.3 In less than 1% of infections, the virus attacks the motor neurons of the anterior horn cells in the spinal cord, leading to destruction of those cells resulting in permanent paralysis of muscles. The most common cause of death from polio is respiratory insufficiency, when the infection affects respiratory muscles, occurring in about 5% to 10% of cases.3,4 There is absence of sensory abnormalities, although deep tendon reflexes may be absent as a result of the impact of infection on muscle function. Nerve conduction and electromyographic studies have determined destruction of the anterior horn cells of the spinal cord to be the anatomic location of paralysis.5 The potential clinical course of poliovirus infections is summarized in Table 1.3
Table 1.
Clinical description of poliomyelitis
| Consequence | Symptoms | Infections (%) |
|---|---|---|
| Inapparent infection without symptoms | None | 72 |
| Minor illness | Transient illness; 1–3 d fever, malaise, drowsiness, headache, nausea, vomiting, constipation, or sore throat, in various combinations | 24 |
| Nonparalytic poliomyelitis (aseptic meningitis) | Minor illness characterized as fever, sore throat, vomiting, malaise; 1–2 d later stiffness of neck or back; vomiting, severe headache, pain in limbs, back neck (lasts 2–10 d, recovery is usually rapid and complete) | 4 |
| Paralytic poliomyelitis | Minor illness for several days, symptom-free period of 1–3 d, followed by rapid onset of flaccid paralysis with fever and progression to maximum extent of paralysis within a few days | <1 |
Data from Sutter RW, Kew OM, Cochi SL, et al. 28 - Poliovirus vaccine—live. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines (sixth edition). London: W.B. Saunders; 2013. p. 598–645.
Paralytic polio is estimated to occur in 1 in 100 to 1 in 2000 infections (average, 1 in 200). Type 1 is the most neurovirulent virus, whereas type 2 is least neurovirulent. AFP is not specific for polio, and there are many other causes of AFP.6 Features of the 4 most common diagnoses to consider in the differential diagnosis of AFP can be seen in Table 2.
Table 2.
Distinguishing features of 4 common diagnoses of AFP
| Poliomyelitis | Guillain-Barré Syndrome | Traumatic Neuritis (After Injection) | Transverse Myelitis | |
|---|---|---|---|---|
| Paralysis | Development of paralysis 24- to 48-h onset to full paralysis; descending; reduced or absent muscle tone in affected limbs; decreased or absent deep tendon reflexes; cranial nerve involvement and respiratory insufficiency only when bulbar involvement present | Development of paralysis from hours to 10 d; ascending; global hypotonia; globally absent deep tendon reflexes; cramps; tingling; hypoesthesia of palms and soles; cranial nerve involvement often present, affecting nerves VII, IX, X, XI, XII | Development of paralysis from hours to 4 d; reduced or absent muscle tone in affected limb; decreased or absent deep tendon reflexes; hypothermia in affected limb | Development of paralysis from hours to 4 d; hypotonia in affected limbs; deep tendon reflexes absent in lower limbs early; hyperreflexia late; anesthesia of lower limbs with sensory level |
| Clinical features | High fever at onset; always present at onset of flaccid paralysis; gone when progression of paralysis stops; severe myalgia; backache | Respiratory insufficiency in severe cases, enhanced by bacterial pneumonia; frequent blood pressure alterations; sweating; blushing; body temperature fluctuations; transient bladder dysfunction | Fever common before, during, and after flaccid paralysis; pain in gluteus | Occasional respiratory insufficiency; autonomic signs and symptoms present; bladder dysfunction |
| Diagnostic tests | Inflammatory cerebrospinal fluid; abnormal nerve conduction velocity, third week: anterior horn cell disease (normal during first 2 wk); abnormal electromyography at 3 wk | Albumin-cytologic dissociation of cerebrospinal fluid; abnormal nerve conduction velocity; third week: slowed conduction; decreased motor amplitudes | Abnormal nerve conduction velocity, third week: axonal damage | Mild elevation in cells of cerebrospinal fluid |
| Sequelae at 3 mo and up to 1 y | Severe, asymmetric atrophy; skeletal deformities developing later | Symmetric atrophy of distal muscles | Moderate atrophy; only in affected limbs | Flaccid diplegia; atrophy after years |
Adapted from Sutter RW, Kew OM, Cochi SL, et al. 28-Poliovirus vaccine—live, Table 28–3: distinguishing features of four common diagnoses of acute flaccid paralysis. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines (sixth edition). London: W.B. Saunders; 2013. p. 604; with permission.
EPIDEMIOLOGY OF POLIO
Wild poliovirus (WPV) is transmitted through person-to-person contact through the fecal-oral or oral-oral routes.3 Fecal-oral spread is most common in developing countries, whereas oral-oral transmission is probably the major mode of transmission in industrialized countries, with high standards of hygiene.4 The incubation period is 3 to 6 days between infection and first symptoms (minor illness) and from 7 to 21 days from infection to onset of paralytic disease.3 Individuals are most infectious immediately before and 1 to 2 weeks after the onset of paralytic disease.3 Poliovirus is excreted for approximately 2 weeks in saliva and longer (3–6 weeks) in stool.3 In immunodeficient persons, prolonged shedding (more than 6 months) or chronic excretion (more than 5 years) rarely can occur, although only 45 prolonged excretors have been identified to date, largely confined to high- and middle-upper-income countries.3 Poliovirus transmission typically peaks in the warm, summer months in temperate climates, which is why eradication efforts attempt to boost population immunity through vaccination campaigns during the cooler low season to interrupt transmission.
Molecular methods such as genomic sequencing have further contributed to the understanding of poliovirus transmission and epidemiology. As the WPV genotype naturally evolves at a rate of around 1% nucleotide substitutions per site per year, lineages can be tracked to further map geographic transmission and identify gaps in surveillance.3,7 Orphan viruses, denoted by more than 1.5% difference in nucleotide sequence in the VP1 region compared with other known viruses, can identify areas where viruses have been transmitting over time without being detected.3
PROGRESS TOWARD GLOBAL ERADICATION OF POLIO
In the last several years, WPV case counts have been at record lows, and Africa seems to be polio-free for the first time in history—as of July 28, 2015, the last case detected in Africa was in Somalia in August 2014.8 Although hundreds of thousands of WPV cases were found globally 30 years ago, only 359 WPV cases were seen in 9 countries in 2014, and 34 cases as of July 28, 2015.8 In 1988, 125 countries were considered endemic (never having interrupted poliovirus transmission), although only 3 are classified as such today (Fig. 1). All of the recent WPV cases reported are type 1. The last case of type 3 WPV was seen in November 2012, whereas the last naturally occurring case of type 2 WPV was found in 1999.8 Of 6 World Health Organization (WHO) regions, 4 encompassing greater than 80% of the world’s population have been certified polio free, meaning at least 3 years have passed in the presence of high-quality surveillance with no WPV cases occurring.
Fig. 1.
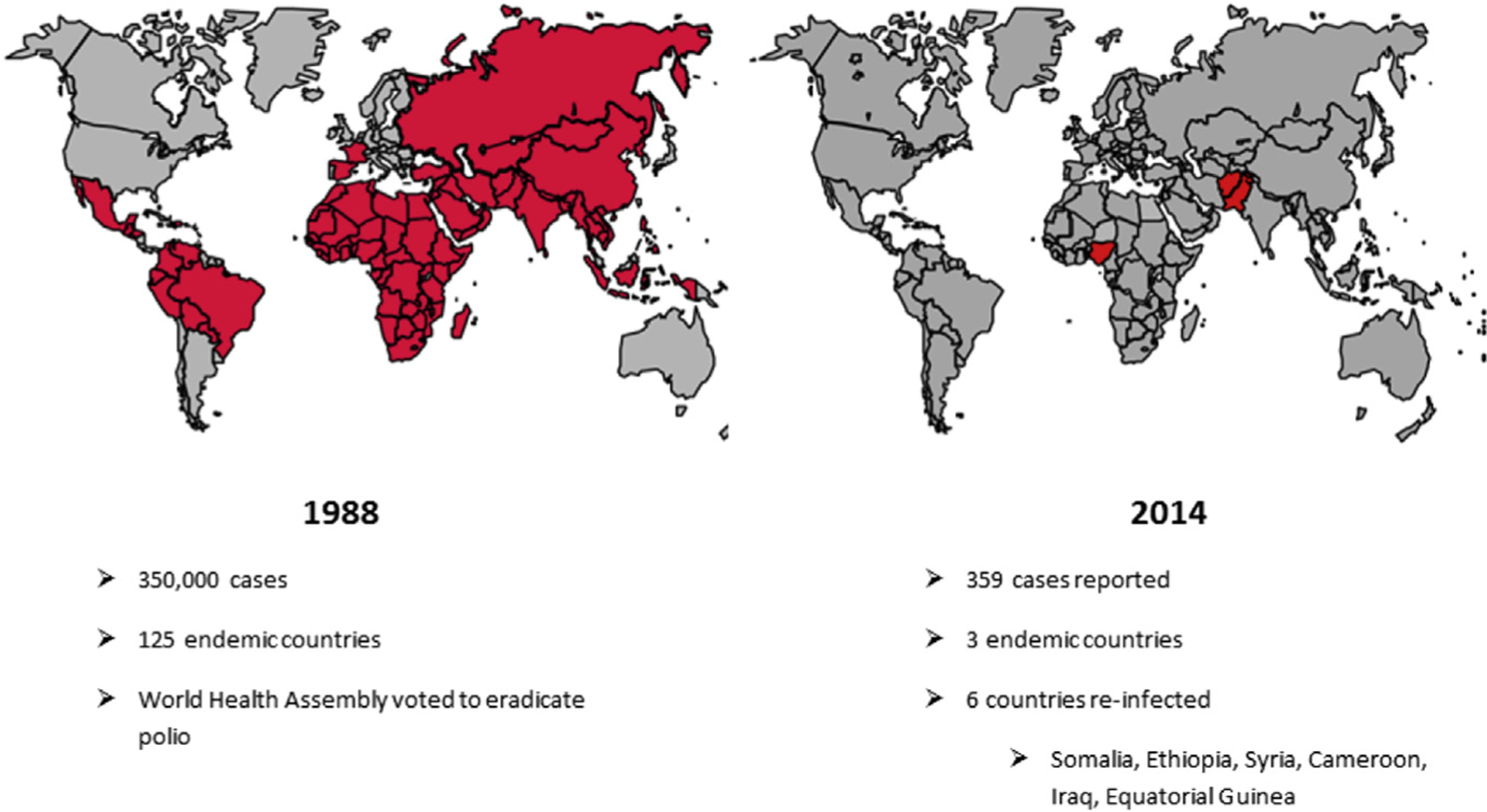
Progress in polio eradication from 1988 to 2014. (Data from The global polio eradication initiative. Data and Monitoring. 2015. Available at: http://www.polioeradication.org/Dataandmonitoring.aspx. Accessed March 27, 2015.)
Although 2012 saw near cessation of international spread of WPV, 2013 brought an increase in cases with evidence that adult travelers were contributing. During the low season of 2014, WPV exportation occurred in several countries, leading the International Health Regulations Emergency Committee to declare the situation a “Public Health Emergency of International Concern” in May 20149; this led to additional travel recommendations and increased vigilance among the global community.10
ERADICATION STRATEGIES
The main strategies for eradication up until this point have been ongoing strengthening of routine immunization coverage, continued supplementary immunization activities (SIAs), extensive surveillance to find the virus, and mopping up efforts in areas with continued transmission. SIAs are mass vaccination campaigns conducted within a few days, multiple times a year, vaccinating all children less than 5 years regardless of vaccination history. Strategies such as finger and house marking can allow independent monitors to survey the area after the campaign to identify missed children and assess the quality of the campaign. Satellite and geographic information system technology have also been used to track vaccinators and further improve campaign quality.
AFP surveillance is the gold standard for detecting cases of polio. The Global Polio Laboratory Network consists of 146 WHO-accredited laboratories following standardized protocols for the following analyses: poliovirus detection by polymerase chain reaction and/or viral isolation, serotype, determination if the virus is wild or vaccine type (ie, wild or Sabin-like or vaccine-derived poliovirus [VDPV]), and genomic sequencing.11 Sequencing results are closely analyzed by comparing nucleotide sequences in the VP1 coding region of isolates to track pathways of poliovirus transmission and guide vaccine choices and strategies through the last stages of the eradication program.11 In addition to AFP surveillance, environmental surveillance is being increasingly used to monitor for the presence of poliovirus in pooled sewage or other environmental samples and to provide insight into international spread of the virus, particularly in areas in which termination of transmission has been difficult.
AVAILABLE VACCINES
Oral Poliovirus Vaccine
OPV consists of live attenuated polioviruses, originally developed by Albert Sabin. Multiple presentations of OPV are available, including trivalent OPV (tOPV) containing types 1, 2, and 3; bivalent OPV (bOPV) containing types 1 and 3; monovalent OPV (mOPV) for types 1 and 3. Type 2 mOPV has also been developed recently and is important for use in stockpiles available for potential outbreak control in the posteradication era.
Although seroconversion rates to tOPV approach 100% in developed countries against all 3 serotypes, developing countries generally have much lower rates of detectable antibodies after vaccination with 3 doses of tOPV particularly to types 1 and 3. An analysis of seroconversion studies found an average rate of only 73% to type 1 and 70% to type 3.12 Data suggest that the type 2 component in OPV as well as ubiquitous enteric viruses present in developing countries interfere with responses to types 1 and 3.12 The inferior immunogenicity in developing countries can be overcome by additional doses (sometimes more than 10) of OPV, which lead to population immunity levels exceeding herd immunity thresholds for stopping transmission. In addition, OPV is sensitive to heat and must be transported in an intact cold chain, which proves difficult in rural and hard-to-reach areas, locations where the last reservoirs of WPV remain.
Thus far, OPV has been the mainstay of polio eradication. The live vaccine is inexpensive (less than 15¢ per dose through the United Nations Children’s Fund), simple to administer (does not require a trained health worker or produce sharps waste), and induces intestinal mucosal immunity in reducing transmission in settings in which fecal-oral transmission predominates. A systematic review of mucosal immunity of polio vaccines found that individuals vaccinated with OPV were protected against shedding of poliovirus in stool samples collected after challenge (an indicator of induction of intestinal mucosal immunity) compared with unvaccinated individuals (odds ratio, 0.13; 95% confidence interval, 0.08–0.24).13 In contrast, the proportion of IPV recipients who shed after an OPV challenge is not different than naive persons receiving OPV for the first time (see later for further discussion of IPV).
Risks of Oral Poliovirus Vaccine
Vaccine-associated paralytic polio During the replication process, the Sabin strains of OPV can mutate and rarely revert to neurovirulent variants causing paralysis clinically indistinguishable from that caused by WPV.14 Vaccine-associated paralytic polio (VAPP) causes paralysis in OPV recipients and close contacts. In a review of epidemiology and burden, the global risk of VAPP was determined to be 4.7 cases per million births or approximately 500 cases of VAPP estimated globally, per year, with 90% estimated to occur in low- and lower-middle-income countries.15 About 26% to 31% of VAPP cases were associated with the type 2 component of tOPV.15
Circulating vaccine-derived poliovirus Vaccine virus can rarely mutate and regain both the neurovirulence and transmissibility properties of WPVs, causing outbreaks of polio. When prolonged replication of the vaccine virus takes place (isolates having >1% divergence [0.6% for type 2] from the original OPV strain), the virus is considered a VDPV.3 VDPVs are grouped into 1 of 3 categories: (1) immunodeficient VDPVs isolated from individuals with B-cell immunodeficiency who maintain chronic infection after vaccination with OPV, (2) cVDPVs requiring evidence of person-to-person transmission in the community (eg, cluster of ≥2 AFP cases), and (3) ambiguous VDPVs (aVDPVs), which do not belong to the previous categories.3
The largest risk factor for generation of cVDPVs is low overall population immunity allowing vaccine viruses to mutate and spread within a susceptible population.16,17 cVDPV outbreaks have occurred in areas with complex challenges, such as those with insecurity, poor infrastructure, and low immunization coverage, and can easily spread beyond borders, causing outbreaks and sporadic cases elsewhere. Outbreaks in Pakistan, Afghanistan, Nigeria, and Somalia have occurred in conjunction with WPV outbreaks in areas where groups of children remain unvaccinated because of conflict and poor access. Repeated SIAs using OPV containing the parent Sabin strain of the strain causing the outbreak have been shown to stop cVDPV outbreaks.18
Inactivated Poliovirus Vaccine
The current IPV formulation contains 40-8-32 D-Antigen units for poliovirus types 1, 2, and 3, respectively, and is available in stand-alone vaccine or as multivalent presentations.19 Made from selected WPV strains grown in Vero cell culture or human diploid cells, IPV establishes a strong immune response in recipients. Nearly 100% seroconversion rates and high antibody titers to all 3 serotypes are seen after 3 doses and greater than 90% seroconversion rates after 2 doses when administered after 8 weeks of age.20 Immunogenicity depends on the number of doses as well as the age of administration because of interference of maternal antibodies in infants. In a study in Puerto Rico comparing US schedules (2, 4, and 6 months) with WHO’s Expanded Programme on Immunization (EPI) schedules (6, 10, and 14 weeks), seroconversion rates were lower for types 1 and 2 in the EPI schedule study arm, whereby maternal antibody levels were higher.21
In another study in Cuba, 63% of infants seroconverted to type 2 after a single dose of IPV when administered at 4 months of age.22 Among those who did not seroconvert after 1 dose of IPV, 98% had a priming response to a subsequent dose of IPV, that is, they developed significant antibody responses within 7 days of subsequent exposure to IPV.22 Although seroconversion is associated with protection from paralysis, questions remain as to whether persons who are primed are protected from disease (ie, can antibody be made quickly enough after exposure to provide protection). Experience in Hungary, which had a major problem with VAPP, showed that VAPP was eliminated when the country chose a sequential IPV/OPV schedule with 1 dose of IPV followed by OPV, suggesting priming is protective.23 Conversely, an outbreak of WPV1 in Senegal was associated with 36% effectiveness after 1 dose and 89% effectiveness after 2 doses, a finding more compatible with the need to seroconvert to assure protection.24
Studies have shown neutralizing antibodies against poliovirus to persist for at least 5 years in all vaccine recipients after a primary immunization series of 3 to 4 doses.25,26 Antibody concentration may decline with time in some people, although no association has been seen with antibody less than detectable levels and increased susceptibility to paralytic disease.27 As all high-income countries administer 3 or more doses, the precise duration of immunity for 1 or 2 doses of IPV remains less understood.27
Concerns raised with Inactivated Poliovirus Vaccine
IPV, whether give alone or with other vaccines, is considered to be safe and has not been causally associated with any serious adverse events.27 The high immunogenicity provided by IPV leads to high levels of individual protection against paralysis in the recipient. However, IPV is less effective in inducing intestinal mucosal immunity than OPV among previously unvaccinated individuals, although quantity and duration of shedding may be reduced.13 For this reason, IPV does not provide the same amount of community protection against transmission that OPV does. In addition, IPV is more expensive than OPV, with negotiated costs for developing countries of $1 or more per dose when compared with 15¢ for a dose of OPV. IPV, a vaccine administered intramuscularly, requires trained health workers and produces sharps waste. As IPV is produced from WPV strains, there is a risk of accidental release during production, as has happened in The Netherlands in 1992 and Belgium in 2014.28,29 Because a release in a developing country with crowding, poor hygiene, and sanitation is more risky than in an industrialized country, IPV manufacture from WPV strains is limited to a few developed countries, limiting global production capacity.
Israel (and the WHO European Region) was certified polio free in 2002. Israel initiated an IPV-only schedule in 2005 and also has an extensive environmental surveillance system to detect viruses. In early February 2013, WPV1 was isolated indicating an introduction into the Southern districts, although no paralytic polio cases were detected.30 Officials responded with intensified environmental surveillance, a bOPV SIA, and an addition of 1 dose of bOPV back into the routine immunization schedule.30 WPV circulation took place for more than 1 year, with termination of transmission in March of 2014. The experience alerted the public health community to the continued need for OPV in areas where fecal-oral spread of wild viruses is predominant, the value of environmental surveillance in addition to AFP surveillance in countries at particular risk, and the need for mOPV stockpiles in the posteradication era in case wild viruses are reintroduced.31
In contrast, Yogyakarta, Indonesia, switched from OPV to only IPV in 2007. In this experience, no cVDPVs emerged. Thus, IPV may be more effective in preventing the emergence of cVDPVs because of the lower force of infection of polio vaccine viruses than wild viruses versus curtailing transmission of WPVs or existing cVDPVs.32 In addition, IPV alone has eliminated polio in Nordic countries and other industrialized countries that have switched to only IPV, where oral-to-oral transmission is thought to be the predominant mode of transmission. With the exception of Israel, all these countries have maintained a WPV-free status.33
USING ORAL AND INACTIVATED POLIOVIRUS VACCINES TOGETHER
Administration of IPV before OPV has been shown to prevent the incidence of VAPP in some areas, a schedule that theoretically uses the humoral and mucosal strengths of both vaccines.27 Effective reduction in VAPP after introduction of IPV/OPV sequential schedules has been seen in Denmark34 (3 doses of IPV followed by 3 doses of OPV), Hungary23 (1 dose of IPV followed by 3 doses of OPV), and the United States35 (2 doses of IPV followed by 2 doses of OPV).
Studies examining IPV use in children previously vaccinated with tOPV have suggested that IPV is effective at closing immunity gaps, especially for type 2 poliovirus.36,37 In fact, a single dose of IPV in infants and children previously vaccinated with multiple doses of OPV reduced the prevalence of shedding by 39% to 76% and boosted mucosal immunity for types 1 and 3 after OPV challenge compared with no vaccination.38,39 A birth dose of OPV allows for induction of mucosal immunity before infants are exposed to enteric pathogens and increases seroconversion rates.27
Simultaneous use of OPV and IPV in developing countries has shown induction of high antibody responses to all 3 poliovirus types.27 In a 3-country study, concomitant administration of OPV and IPV at 6, 10, and 14 weeks after a birth dose of OPV yielded the highest seroconversion rates in Oman and The Gambia when compared with OPV only given at the same schedule. The third country, Thailand, illustrated similar seroconversion rates in both groups.40
ENDGAME STRATEGY AND TIMELINE
Despite the significant gains made in reduction of the number of polio cases due to OPV, the rare adverse events including VAPP and VDPVs require the phased withdrawal of all OPV use in the final stage of the eradication process.41 The 2013–18 Eradication and Endgame Strategic Plan41 describes complete interruption of transmission and elimination of all polio disease, which includes WPV, VDPVs, and VAPP, using the available tools of OPV and IPV followed by withdrawal of OPV use.
In recent years, the serotype profile of cVDPVs has shifted, with type 2 related cVDPVs representing a larger proportion of all cVDPV cases (97% of the 628 cases of cVDPV from 2006–2013).18 This trend can be seen in Fig. 2 and is thought to be due to increased use of bOPV in campaigns in recent years, leading to reduced population immunity to type 2, a risk factor for cVDPV emergence.42,43 In addition, type 2 WPV seems to have been eradicated with the last naturally occurring case detected in 1999. Because type 2 also causes a proportion of VAPP cases, type 2 vaccine virus is causing harm and potentially doing little good. Thus, the strategic plan calls for global, synchronized replacement of tOPV with bOPV after prior introduction of at least 1 dose of IPV into routine immunization schedules in all 125 countries using OPV only.41 Introduction of IPV will ensure that a proportion of the population is protected against type 2 polio, boost immunity to types 1 and 3, and mitigate other risks associated with the switch.
Fig. 2.
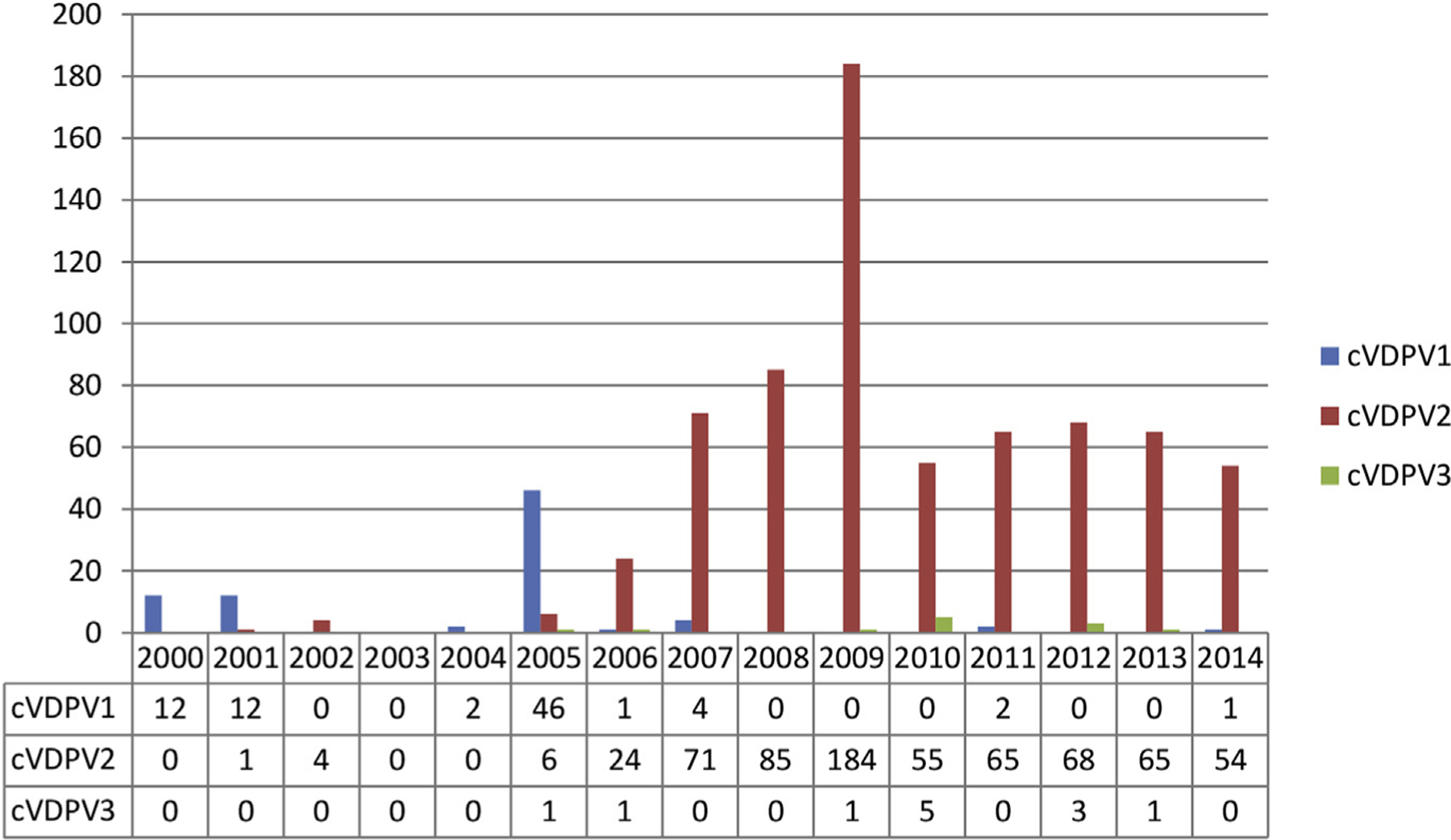
Reported paralytic cases of cVDPV by type worldwide, 2000–14 (as of July 28, 2015). (Adapted from Patel M, Zipursky S, Orenstein W, et al. Polio endgame: the global introduction of inactivated polio vaccine. Expert Rev Vaccines 2015;14(5):752; with permission; and Data from http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Circulatingvaccinederivedpoliovirus.aspx. Accessed July 30, 2015.)
After IPV introduction, the globally synchronized switch is scheduled to occur in April 2016. WHO will assess global readiness and monitor the absence of all persistent cVDPV2s globally as a precondition for implementing the switch. These events represent a public health effort unprecedented in time and scope and will require coordination and partnership across many global, national, and local organizations as well as public-private partnership.
CHALLENGES AND OPPORTUNITIES
India, one of the last countries to eradicate smallpox, was believed by some to be a near-impossible location for the eradication of polio because of high population density, poor sanitation, weak immunization systems, remote villages, and massive migrant populations. But as a result of strong ownership and resource investments from the local to national level, accountability, and international partnerships, India (and the entire South-East Asian region) was certified polio free by WHO in March 2014.44,45 Important lessons learned from India’s exceptional polio eradication effort include development of detailed plans to maintain high coverage in SIAs, close monitoring and supervision, robust communication strategies, and research and innovation to overcome operational barriers to ensure every child is vaccinated.46
Pakistan, on the other hand, remains the engine of WPV transmission. In 2014, 85% of WPV cases seen worldwide were detected in Pakistan and many cases in neighboring Afghanistan were imported across the border from Pakistan.8 For years after mid-2012 when local authorities banned polio vaccination, nearly 350,000 children in some districts of the Federally Administered Tribal Areas had not received polio vaccine during SIAs.47 That number has since decreased to less than 50,000.48 In other areas of Pakistan, polio workers (often women) have been attacked and killed. Although great strides have been made in the last year, until all children in conflict-affected areas can be reached with vaccine, Pakistan remains a major challenge for interruption of WPV. Substantial progress has been made in the African region as Nigeria should soon be removed from the list of endemic countries (ie, countries which have never interrupted transmission of indigenous strains of poliovirus).
POSTERADICATION RISKS AND MITIGATION
As WPV cases continue to decrease and the world inches closer to polio eradication, considerations of late-stage risks are essential. Reintroduction of WPV and release from manufacturers or laboratories are of ultimate concern. The endgame plan calls for appropriate handling and containment of all infectious poliovirus stocks from biomedical facilities to ensure that WPV cannot be transmitted to an increasingly susceptible community.41 Shedding of VDPV among adults and children with primary B-cell immunodeficiency constitutes a long-term risk, as those individuals have been shown to shed virus for up to several years after administration of OPV, a consideration as long as OPV vaccination continues.49
The cost for maintenance of current levels of vaccination, program implementation, surveillance and laboratory capacity, and outbreak response are estimated to be $5.5 billion dollars for current global strategic plan.41 The cost of abandoning global eradication efforts completely is substantial in terms of program costs, treatment costs, and human lives lost. Estimated incremental net benefits of the GPEI between 1988 and 2035 amount to approximately $40 billion to $50 billion saved through eradication efforts.50 Letting the pressure off now is expected to result in skyrocketing numbers of cases. GPEI is the single largest internationally coordinated public health effort in history and there is strong economic justification for finishing the job completely.50
RESEARCH NEEDS
These final stages of the polio eradication endgame have revealed unique challenges to eliminating the disease from areas with the last reservoirs of virus, leading to innovation in vaccine technology and delivery. At this stage in the polio endgame, focus is being placed on new innovations such as technology to help achieve lower-cost IPV, strategies to make vaccine manufacture safer, and operational advances to make vaccine administration simpler.43 Specifics on areas of research on OPV and IPV can be seen in Table 3.51
Table 3.
Ongoing research on current and future tools for polio eradication
| Research Question/Tool | Importance | Status |
|---|---|---|
| Current tools | ||
| Combination schedules of IPV and OPV | Understand role of IPV and bOPV when used together in recommended schedules in developing countries | Immunogenicity (humoral and intestinal) of ≥1 IPV dose combined with bOPV in 6–10–14 wk mixed schedules (Colombia-Dominican Republic-Guatemala-Panama, India, Bangladesh) Immunogenicity (humoral and intestinal) of IPV / bOPV in 2-4-6 mo sequential regimen (Chile) Duration of protection of boosting of intestinal immunity induced by IPV in OPV primed populations (India) |
| Fractional doses of IPV | Reduced cost | Immunogenicity with fractional IPV doses via ID route (The Gambia, Bangladesh) |
| New tools | ||
| Immunogenicity of monovalent IPV-2 (m-IPV2) | Improved humoral and intestinal immunogenicity Reduced cost with 1 dose of m-IPV2 |
Phase I (Belgium) study on safety completed Phase II (Panama) study underway to evaluate safety and immunogenicity |
| Immunogenicity of IPV adjuvants | Reduced cost Increased supply Potential for enhanced mucosal immunity |
Clinical studies being planned to evaluate potential for aluminum salts adjuvants and for dmLT (double-mutant heat-labile enterotoxin) adjuvants for IPV |
| Feasibility of novel routes of administration | Operational advantages Potential for use in SIAs Concomitant use with other vaccines |
Studies in humans being planned or implemented to evaluate impact of IM (Intramuscular) and ID (Intradermal) delivery of IPV through use of disposable jet injectors, microneedle patch, and other novel delivery techniques |
| Development of genetically stable OPV and attenuated IPV seed strains | Potential use in outbreak control (reduced risk of VDPVs and VAPP) or in routine immunization if IPV is considered inadequate in reducing transmission risk Further attenuated and less infectious seed strains for safe IPV manufacture |
In preclinical development or planning phase |
| Immunogenicity with Sabin IPV | Minimize risk of reintroduction of WPV from IPV manufacturing facilities | Sabin IPV has been licensed in Japan Efficacy and feasibility of large-scale production are currently being evaluated |
Adapted from Bandyopadhyay AS, Garon J, Seib K, et al. Polio vaccination: past, present and future. Future Microbiol 2015;801–2. http://dx.doi.org/10.2217/fmb.15.19.
ROLE OF NORTH AMERICAN PHYSICIANS
Even though naturally occurring paralytic poliomyelitis has not been seen in the United States since 1979, physicians play an important role in the global polio eradication effort. Current Advisory Committee on Immunization Practices recommendations for routine polio immunization include 4 doses of IPV to be given at ages 2, 4, and 6 to 18 months and 4 to 6 years.52 Physicians should ensure that all patients receive routine childhood polio immunization and that travelers are vaccinated with an additional dose of IPV if they will be visiting a country with polio infection.10 New International Health Regulations require proof of vaccination of a dose of polio vaccine when leaving a country with polio infection (long-term travelers staying for >4 weeks). The dose should be given at least 4 weeks and no more than 12 months before departure.9
Although diagnosis of poliomyelitis in the United States is rare, physicians should suspect polio in a patient who presents with a clinically compatible case. There may not be a history of travel to a polio endemic or epidemic country as the transmitting infection could be subclinical. At least 2 stool samples should be collected, 24 hours apart, ideally within 14 days of the onset of paralysis. A single case of suspected paralytic polio demands immediate attention. Timely notification of state health department and CDC is critical. Finally, US physicians should advocate for and support introduction of IPV in developing countries as well as US government support for the overall initiative. As long as WPV exists anywhere, risk of importation exists everywhere.53
KEY POINTS.
More than 99% of poliomyelitis cases are asymptomatic or consist of mild illness without paralysis. Acute Flaccid Paralysis (AFP), a sign of polio, is also a sign of many other diseases.
Physicians should suspect polio in a clinically compatible case. There may not be a history of travel to a polio endemic/epidemic country as the person transmitting virus to the paralytic case could have had an asymptomatic infection or nonparalytic illness.
A single case of paralytic polio demands immediate attention, including notification of the state health department and Centers for Disease Control and Prevention (CDC) and collection of stool samples for laboratory confirmation.
Since 1988, the number of poliomyelitis cases has been reduced by more than 99%, yet reservoirs of disease remain. Barriers to eradication include insecurity, political commitment, and immunization system quality.
US physicians play an important role in surveillance, vaccination, advocacy, and financial support for global eradication of polio.
Footnotes
Disclosure Statement: W.A. Orenstein and J.R. Garon are supported by the Bill & Melinda Gates Foundation under Work Order 23848 awarded to the Task Force for Global Health. Apart from those disclosed, the authors do not have any conflicts of interest to report and do not have relevant affiliations, relationships, or financial involvement with organizations with a financial interest in materials disclosed in the article. No writing assistance was used in the production of this article.
REFERENCES
- 1.Hinman AR, Koplan JP, Orenstein WA, et al. Live or inactivated poliomyelitis vaccine: an analysis of benefits and risks. Am J Public Health 1988;78(3):291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cochi SL, Dowdle WR. Disease eradication in the 21st century: implications for global health Cambridge (MA): MIT Press Books; 2011. Available at: http://mitpress.mit.edu/books/disease-eradication-21st-century. Accessed May 10, 2015. [Google Scholar]
- 3.Poliomyelitis (Polio) In World Health Organization, International travel and health. 2014. Available at http://www.who.int/ith/diseases/polio/en/. Accessed December 22, 2014. [Google Scholar]
- 4.WHO | Poliomyelitis (Polio). WHO Available at: http://www.who.int/ith/diseases/polio/en/. Accessed December 22, 2014.
- 5.Wiechers D Electrophysiology of acute polio revisited. Ann N Y Acad Sci 1995; 753(1):111–9. [DOI] [PubMed] [Google Scholar]
- 6.Sutter RW, Kew OM, Cochi SL, et al. 28-Poliovirus vaccine—live, Table 28–2: causes and differential diagnosis of acute flaccid paralysis. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines (sixth edition). London: W.B. Saunders; 2013. p. 603. Available at: http://www.sciencedirect.com/science/article/pii/B9781455700905000355. [Google Scholar]
- 7.Jorba J, Campagnoli R, De L, et al. Calibration of multiple poliovirus molecular clocks covering an extended evolutionary range. J Virol 2008;82(9):4429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Global Polio Eradication Initiative. Data Monit 2015. Available at: http://www.polioeradication.org/Dataandmonitoring.aspx. Accessed March 27, 2015.
- 9.WHO | WHO statement on the meeting of the International Health Regulations Emergency Committee concerning the international spread of wild poliovirus. WHO Available at: http://www.who.int/mediacentre/news/statements/2014/polio-20140505/en/. Accessed May 14, 2014.
- 10.Wallace GS, Seward JF, Pallansch MA, et al. , Centers for Disease Control and Prevention. Interim CDC guidance for polio vaccination for travel to and from countries affected by wild poliovirus. MMWR Morb Mortal Wkly Rep 2014;63(27): 591–4. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6327a4.htm?s_cid5mm6327a4_w. Accessed January 8, 2015. [PMC free article] [PubMed] [Google Scholar]
- 11.Porter KA, Diop OM, Burns CC, et al. Tracking progress toward polio eradication—worldwide, 2013–2014. MMWR Morb Mortal Wkly Rep 2015;64(15): 415–20. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6415a4.htm?s_cid5mm6415a4_w. Accessed April 23, 2015. [PMC free article] [PubMed] [Google Scholar]
- 12.Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis 1991;13(5):926–39. [DOI] [PubMed] [Google Scholar]
- 13.Hird TR, Grassly NC. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog 2012;8(4):e1002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowdle WR, De Gourville E, Kew OM, et al. Polio eradication: the OPV paradox. Rev Med Virol 2003;13(5):277–91. [DOI] [PubMed] [Google Scholar]
- 15.Platt LR, Estívariz CF, Sutter RW. Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden. J Infect Dis 2014; 210(Suppl 1):S380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kew O, Morris-Glasgow V, Landaverde M, et al. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science 2002;296(5566):356–9. [DOI] [PubMed] [Google Scholar]
- 17.Estívariz CF, Watkins MA, Handoko D, et al. A large vaccine-derived poliovirus outbreak on Madura Island—Indonesia, 2005. J Infect Dis 2008;197(3):347–54. [DOI] [PubMed] [Google Scholar]
- 18.Update on vaccine-derived polioviruses—worldwide, July 2012–December 2013. Centers for Disease Control and Prevention Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6311a5.htm. Accessed February 3, 2015.
- 19.Vidor E 27-Poliovirus vaccine-inactivated. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines (sixth edition). Philadelphia: Elsevier/Saunders; 2013. p. 573–97. [Google Scholar]
- 20.Estivariz CF, Pallansch MA, Anand A, et al. Poliovirus vaccination options for achieving eradication and securing the endgame. Curr Opin Virol 2013;3(3): 309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dayan GH, Thorley M, Yamamura Y, et al. Serologic response to inactivated poliovirus vaccine: a randomized clinical trial comparing 2 vaccination schedules in Puerto Rico. J Infect Dis 2007;195(1):12–20. [DOI] [PubMed] [Google Scholar]
- 22.Resik S, Tejeda A, Sutter RW, et al. Priming after a fractional dose of inactivated poliovirus vaccine. N Engl J Med 2013;368(5):416–24. [DOI] [PubMed] [Google Scholar]
- 23.Dömö k I Experiences associated with the use of live poliovirus vaccine in Hungary, 1959–1982. Rev Infect Dis 1984;6(Suppl 2):S413–8. [DOI] [PubMed] [Google Scholar]
- 24.Robertson SE, Traverso HP, Drucker JA, et al. Clinical efficacy of a new, enhanced-potency, inactivated poliovirus vaccine. Lancet 1988;1(8591):897–9. [DOI] [PubMed] [Google Scholar]
- 25.Carlsson R-M, Claesson BA, Fagerlund E, et al. Antibody persistence in five-year-old children who received a pentavalent combination vaccine in infancy. Pediatr Infect Dis J 2002;21(6):535–41. [DOI] [PubMed] [Google Scholar]
- 26.Langue J, Matisse N, Pacoret P, et al. Persistence of antibodies at 5–6 years of age for children who had received a primary series vaccination with a pentavalent whole-cell pertussis vaccine and a first booster with a pentavalent acellular pertussis vaccine: immunogenicity and tolerance of second booster with a tetravalent acellular vaccine at 5–6 years of age. Vaccine 2004;22(11–12):1406–14. [DOI] [PubMed] [Google Scholar]
- 27.Weekly Epidemiological Record. Polio vaccines: who position paper, January 2014. World Health Organization; 2014. p. 73–92. Available at: http://www.who.int/wer/2014/wer8909.pdf?ua51. Accessed December 22, 2014.
- 28.Mulders MN, Reimerink JHJ, Koopmans MPG, et al. Genetic analysis of wild-type poliovirus importation into the Netherlands (1979–1995). J Infect Dis 1997;176(3): 617–24. [DOI] [PubMed] [Google Scholar]
- 29.Accidental release of 45 litres of concentrated live polio virus solution into the environment - Belgium. European Centre for Disease Prevention and Control 2014. Available at: http://ecdc.europa.eu/en/publications/Publications/communicable-disease-threats-report-13-sep-2014.pdf. Accessed May 9, 2015.
- 30.Anis E, Kopel E, Singer S, et al. Insidious reintroduction of wild poliovirus into Israel, 2013. Euro Surveill 2013;18(38):1–5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24084337. [DOI] [PubMed] [Google Scholar]
- 31.Kopel E, Kaliner E, Grotto I. Lessons from a public health emergency—importation of wild poliovirus to Israel. N Engl J Med 2014;371(11):981–3. [DOI] [PubMed] [Google Scholar]
- 32.Wahjuhono G, Revolusiana Widhiastuti D, et al. Switch from oral to inactivated poliovirus vaccine in Yogyakarta province, Indonesia: summary of coverage, immunity, and environmental surveillance. J Infect Dis 2014;210(Suppl 1):S347–52. [DOI] [PubMed] [Google Scholar]
- 33.Bonnet M-C, Dutta A Worldwide experience with inactivated poliovirus vaccine. Vaccine 2008;26(39):4978–83. [DOI] [PubMed] [Google Scholar]
- 34.Von Magnus H, Petersen I. Vaccination with inactivated poliovirus vaccine and oral poliovirus vaccine in Denmark. Rev Infect Dis 1984;6(Suppl 2):S471–4. [DOI] [PubMed] [Google Scholar]
- 35.Alexander L, Seward JF, Santibanez TA, et al. Vaccine policy changes and epidemiology of poliomyelitis in the united states. JAMA 2004;292(14):1696–701. [DOI] [PubMed] [Google Scholar]
- 36.Hanlon P, Hanlon L, Marsh V, et al. Serological comparisons of approaches to polio vaccination in The Gambia. Lancet 1987;1(8536):800–1. [DOI] [PubMed] [Google Scholar]
- 37.Moriniere BJ, Van Loon FPL, Rhodes PH, et al. Immunogenicity of a supplemental dose of oral versus inactivated poliovirus vaccine. The Lancet 1993;341(8860): 1545–50. [DOI] [PubMed] [Google Scholar]
- 38.John J, Giri S, Karthikeyan AS, et al. Effect of a single inactivated poliovirus vaccine dose on intestinal immunity against poliovirus in children previously given oral vaccine: an open-label, randomised controlled trial. The Lancet 2014; 384(9953):1505–12. [DOI] [PubMed] [Google Scholar]
- 39.Jafari H, Deshpande JM, Sutter RW, et al. Efficacy of inactivated poliovirus vaccine in India. Science 2014;345(6199):922–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Combined immunization of infants with oral and inactivated poliovirus vaccines: results of a randomized trial in The Gambia, Oman, and Thailand. WHO Collaborative Study Group on Oral and Inactivated Poliovirus Vaccines. Bull World Health Organ 1996;74(3):253–68. [PMC free article] [PubMed] [Google Scholar]
- 41.Global Polio Eradication Initiative. Polio Eradication & Endgame Strategic Plan 2013–2018 2013. Available at: http://www.polioeradication.org/Portals/0/Document/Resources/StrategyWork/PEESP_EN_US.pdf. Accessed May 16, 2015.
- 42.Global Polio Eradication Initiative. Circulating vaccine-derived poliovirus 2000–2013 2013. Available at: http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Circulatingvaccinederivedpoliovirus.aspx. Accessed March 5, 2015.
- 43.Patel M, Zipursky S, Orenstein W, et al. Polio endgame: the global introduction of inactivated polio vaccine. Expert Rev Vaccines 2015;14(5):749–62. [DOI] [PubMed] [Google Scholar]
- 44.SEARO | India three years polio-free. SEARO Available at: http://www.searo.who.int/mediacentre/features/2014/sea-polio/en/. Accessed February 26, 2015.
- 45.SEARO | WHO South-East Asia Region certified polio-free. SEARO Available at: http://www.searo.who.int/mediacentre/releases/2014/pr1569/en/. Accessed February 26, 2015.
- 46.Polio-free certification and lessons learned—South-East Asia region, March 2014 Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6342a2.htm. Accessed February 26, 2015. [PMC free article] [PubMed]
- 47.Alexander JP, Zubair M, Khan M, et al. Progress and peril: poliomyelitis eradication efforts in Pakistan, 1994–2013. J Infect Dis 2014;210(Suppl 1):S152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.WHO | Statement on the 5th IHR Emergency Committee meeting regarding the international spread of wild poliovirus. WHO Available at: http://who.int/mediacentre/news/statements/2015/polio-5th-statement/en/. Accessed May 15, 2015.
- 49.Grassly NC. The final stages of the global eradication of poliomyelitis. Philos Trans R Soc Lond B Biol Sci 2013;368(1623):20120140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duintjer Tebbens RJ, Pallansch MA, Cochi SL, et al. Economic analysis of the global polio eradication initiative. Vaccine 2010;29(2):334–43. [DOI] [PubMed] [Google Scholar]
- 51.Bandyopadhyay AS, Garon J, Seib K, et al. Polio vaccination: past, present and future. Future Microbiol 2015;10:791–808. [DOI] [PubMed] [Google Scholar]
- 52.Vaccines: ACIP vaccine recommendations - polio - CDC Available at: http://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/polio.html. Accessed October 14, 2014.
- 53.Mundel T, Orenstein WA. No country is safe without global eradication of poliomyelitis. N Engl J Med 2013;369(21):2045–6. [DOI] [PubMed] [Google Scholar]


