Abstract
The efficacy of CS-834, a novel oral carbapenem, was assessed by using a murine model of pneumonia caused by penicillin-susceptible and penicillin-resistant Streptococcus pneumoniae and was compared with those of oral cephems, i.e., cefteram pivoxil, cefpodoxime proxetil, cefdinir, and cefditoren pivoxil. Intranasal inoculation of 106 CFU of penicillin-susceptible or penicillin-resistant S. pneumoniae in the exponential growth phase induced pneumonia and bacteremia in ddY mice within 48 h. For the treatment of infections caused by the penicillin-susceptible strain the antibiotics were administered orally at 0.4, 2, and 10 mg/kg of body weight twice daily for 2 days beginning at 24 h after bacterial inoculation, and for the treatment of infections caused by a penicillin-resistant strain the antibiotics were administered at 2, 10, and 50 mg/kg twice daily for 2 days beginning at 24 h after bacterial inoculation. Among the antibiotics tested, CS-834 exhibited the most potent efficacy against both types of strains. Against infections caused by penicillin-susceptible S. pneumoniae, CS-834 at all doses significantly reduced the numbers of viable cells in both the lungs and blood. Cefpodoxime proxetil at all doses and cefteram pivoxil and cefditoren pivoxil at doses of 2 and 10 mg/kg showed comparable efficacies. Against infections caused by penicillin-resistant S. pneumoniae, CS-834 at doses of 10 and 50 mg/kg showed the most potent efficacy among the antibiotics tested, resulting in the maximum decrease in the numbers of viable cells in the lungs. Comparable efficacies were observed with cefteram pivoxil and cefpodoxime proxetil at doses of 50 mg/kg each. The concentration of CS-834 in the lungs and blood was higher than that of cefdinir and was lower than those of the other antibiotics tested, suggesting that the potent therapeutic efficacy of CS-834 reflects its strong activity against S. pneumoniae.
Streptococcus pneumoniae is one of the most common pathogens, causing community-acquired pneumonia accompanied by high rates of morbidity and mortality, particularly in children, elderly people, and immunocompromised hosts (2, 9). The increase in the incidence of infections caused by penicillin-resistant S. pneumoniae has become a serious clinical problem in many countries (1, 4, 6, 8, 11, 15, 17). It has been reported that the frequency of isolation of penicillin-resistant S. pneumoniae strains was one-third among isolates from patients with severe infections, such as pneumonia and meningitis, in Japan (8).
Several new oral cephem antibiotics (cefdinir and the prodrugs, such as cefteram pivoxil, cefpodoxime proxetil, and cefditoren pivoxil) were reported to have very potent antibacterial activity against various species of gram-positive and gram-negative bacteria (12, 14, 18, 20). They showed only moderate activities against penicillin-resistant S. pneumoniae, however.
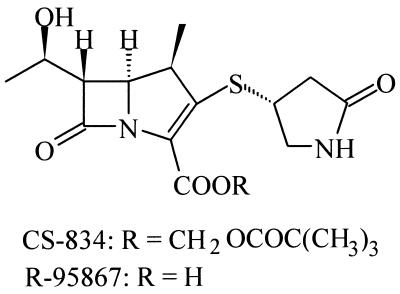
CS-834 is a novel orally absorbable carbapenem; its active metabolite is R-95867 (Fig. 1). This antibiotic showed a broad spectrum of activity, covering both gram-positive and gram-negative aerobes and anaerobes, including penicillin-susceptible and penicillin-resistant S. pneumoniae (21). In this study, we developed a murine pneumonia model of penicillin-susceptible and penicillin-resistant S. pneumoniae and investigated the therapeutic efficacy of CS-834 in this model.
FIG. 1.
Chemical structures of CS-834 and R-95867.
(This work was presented in part at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, La., 15 to 18 September 1996 [13].)
MATERIALS AND METHODS
Antibiotics.
The following compounds were synthesized at Sankyo Research Laboratories, Tokyo, Japan: CS-834, sodium salt of R-95867, cefpodoxime proxetil, cefpodoxime, cefteram, and cefditoren. The other compounds were obtained from the indicated sources: cefteram pivoxil, Toyama Chemical Co., Ltd., Tokyo, Japan; cefdinir, Fujisawa Pharmaceutical Co., Ltd., Osaka, Japan; and cefditoren pivoxil, Meiji Seika Kaisha, Ltd., Tokyo, Japan.
Bacterial strains.
Penicillin-susceptible strain 2132 (MIC of benzylpenicillin, 0.012 μg/ml) and penicillin-resistant strains 9605 and 9601 (MICs of benzylpenicillin, 1.56 μg/ml for both strains) of S. pneumoniae, originally isolated from patients with pneumonia in 1983 and 1993 in Japan, were used in this study.
Susceptibility testing.
MICs were determined by the usual twofold agar dilution method (5) with Pearlcore heart infusion agar (Eiken Chemical Co., Ltd., Tokyo, Japan) supplemented with 5% defibrinated horse blood. The inoculum size was 104 CFU per spot (5 μl). The inoculated plates were incubated at 37°C for 18 h.
Pneumonia caused by S. pneumoniae in mice.
After overnight incubation on brain heart infusion agar (Difco Laboratories, Detroit, Mich.) supplemented with 5% defibrinated horse blood (BHIA-B), freshly grown colonies were suspended in heart infusion broth (Difco Laboratories) supplemented with 10% heat-treated horse serum (HIB-S) at an optical density at 550 nm of 0.12 (JUNIOR II Spectrophotometer; Coleman Instruments Corp., Maywood, Ill.). The bacterial suspension was diluted 100-fold with fresh HIB-S and was incubated, with shaking, to yield an optical density at 550 nm of 0.12. This culture, which was in the exponential growth phase, was diluted 5-fold (for strain 9601), 10-fold (for strain 2132), and 25-fold (for strain 9605) with the same medium and was used for inoculation.
Experimental pneumonia was induced in male ddY mice weighing 19 to 21 g (SLC Japan Inc.) with penicillin-susceptible strain 2132 or penicillin-resistant strain 9605 or 9601. Mice were anesthetized lightly by intravenous injection of ketamine (Sankyo, Co., Ltd., Tokyo, Japan) at 1 mg/kg of body weight, and 50 μl (for strains 2132 and 9605) or 75 μl (for strain 9601) of a bacterial suspension (approximately 106 CFU) was inoculated through the nares into the lungs. To investigate the change in viable cell numbers in the lungs and blood, infected mice were killed at 24, 48, and 72 h after infection and their lungs and blood were removed. The lungs were homogenized in 2 ml of saline, and the homogenates and blood were serially diluted 10-fold with saline. One hundred microliters of the diluent was spread onto BHIA-B plates, and the plates were incubated at 37°C for 24 h. The viable cell numbers were determined by counting the numbers of colonies on the plates.
Histopathological examination.
The lung tissues were removed from unmedicated mice infected with strain 9605 at 24 h after infection and were immediately fixed in 10% neutral buffered formalin. The fixed tissues were embedded in paraffin wax, sectioned, and then stained with hematoxylin-eosin for histological examination or with methylene blue for the identification of bacteria.
Therapeutic efficacy.
Drugs were administered orally at 24, 32, 48, and 56 h after the bacterial challenge. Doses of 0.4, 2, and 10 mg/kg of body weight were used against infections caused by strain 2132; doses of 2, 10, and 50 mg/kg were used against infections caused by strain 9605; and a dose of 10 mg/kg was used against infections caused by strain 9601. At 72 h after infection the mice were killed, and their lungs and blood were removed. The lungs were homogenized in saline, and the homogenates and blood were serially diluted 10-fold with saline. One hundred microliters of the diluent was spread onto BHIA-B plates, and the plates were incubated at 37°C for 24 h. The viable cell numbers in the lungs and blood were determined by counting the numbers of colonies on the plates. The means and standard errors were calculated for each group. The detection limits for lungs infected with penicillin-susceptible and -resistant S. pneumoniae strains were 2.3 and 1.3 log10 CFU per lung, respectively, to avoid significant drug carryover. The detection limit for blood was 2.0 log10 CFU per ml for both infections caused by the penicillin-susceptible S. pneumoniae strain and infections caused by penicillin-resistant S. pneumoniae strains.
Pharmacokinetic studies.
Pharmacokinetic studies were done with mice of the same strain used for the pneumonia model, but the mice were not infected. At 15, 30, 60, 120, and 240 min after oral administration of antibiotics at 100 mg/kg of body weight, the mice were exsanguinated, and their blood and lungs were collected. Antibiotic concentrations in serum and lung homogenates were determined by the paper disc diffusion method by using Bacillus subtilis ATCC 6699 as the bioassay indicator (7). Four mice were used for each datum point.
Statistical analysis.
The statistical significance of the differences between the numbers of viable organisms recovered from the lungs and blood were evaluated by the Student t test. A difference between two groups was considered to be statistically significant if the P value was <0.05.
RESULTS
Antibacterial activity.
Table 1 presents the antibacterial activities of R-95867, cefteram, cefpodoxime, cefdinir, and cefditoren against the strains used in this study, penicillin-susceptible S. pneumoniae 2132 and penicillin-resistant S. pneumoniae 9605 and 9601. R-95867, as well as cefteram and cefditoren, showed potent activity against penicillin-susceptible strain 2132. The activity of R-95867 was stronger than those of cefpodoxime and cefdinir. Against penicillin-resistant strains 9605 and 9601, R-95867 exhibited the most potent activity, and it was 4- to 16-fold stronger than the reference antibiotics.
TABLE 1.
Antibacterial activities of R-95867 and reference antibiotics against penicillin-susceptible and -resistant S. pneumoniae strains used for experimental infections
| Strain | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| R-95867 | Cefteram | Cefpodoxime | Cefdinir | Cefditoren | |
| Penicillin-susceptible S. pneumoniae 2132a | ≤0.006 | ≤0.006 | 0.012 | 0.025 | ≤0.006 |
| Penicillin-resistant strains | |||||
| S. pneumoniae 9605b | 0.20 | 1.56 | 3.13 | 6.25 | 0.78 |
| S. pneumoniae 9601b | 0.20 | 1.56 | 3.13 | 6.25 | 0.78 |
MIC of benzylpenicillin, 0.012 μg/ml.
MIC of benzylpenicillin, 1.56 μg/ml.
Murine pneumonia model.
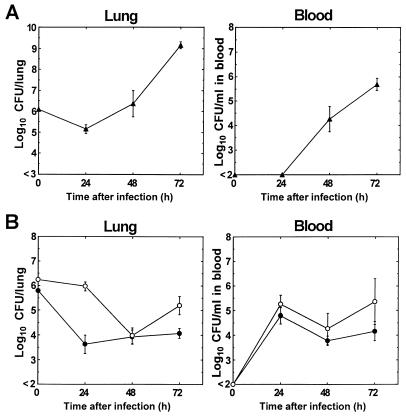
Figure 2 shows the changes in bacterial viability in the lungs and blood of mice after infection with penicillin-susceptible (Fig. 2A) and penicillin-resistant (Fig. 2B) strains. Both penicillin-susceptible and -resistant strains induced pneumonia and bacteremia in mice within 48 h after infection. The pneumonias were not fatal during a 72-h observation period after infection. The numbers of viable cells of penicillin-susceptible strain 2132 in the lungs and blood were approximately 105 CFU/lung and below 102 CFU/ml of blood, respectively, at 24 h after infection, and they increased to approximately 109 CFU/lung and approximately 106 CFU/ml of blood, respectively, at 72 h after infection. On the other hand, the numbers of viable cells of the penicillin-resistant strains in the lungs decreased to approximately 104 CFU/lung at 48 h after infection. The numbers of viable cells of strain 9601 increased to approximately 105 CFU/lung at 72 h, while the level of viable cells of strain 9605 remained almost unchanged until 72 h after infection. The numbers of viable cells of penicillin-resistant strains in blood increased to approximately 105 CFU/ml at 24 h, and the levels remained almost unchanged until 72 h after infection. Microscopic observation showed typical histopathological findings in the lung tissues of mice infected with penicillin-resistant strain 9605. The lung tissue was invaded by large numbers of inflammatory cells, mainly neutrophils, and cocci were present in alveolar spaces (data not shown).
FIG. 2.
Viable cell counts for penicillin-susceptible strain 2132 (A) and penicillin-resistant strains 9605 and 9601 (B) in the lungs and blood of mice at 24, 48, and 72 h after infection. Symbols: ▴, strain 2132; •, strain 9605; ○, strain 9601. Each point represents the mean ± standard error (n = 5 to 7).
Therapeutic efficacy against experimental pneumonia in mice.
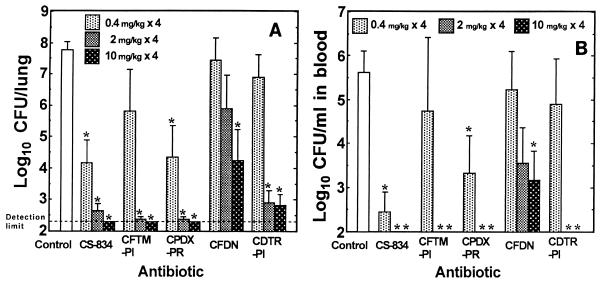
Figure 3 shows the therapeutic efficacies of CS-834 and the reference antibiotics against pneumonia caused by the penicillin-susceptible strain. At doses of 0.4, 2, and 10 mg/kg, all antibiotics exhibited dose-dependent efficacies against the infection. CS-834 was the most active among the antibiotics tested, and its use resulted in significant reductions in the numbers of viable cells in both the lungs and blood at all doses in comparison with the numbers of viable cells in the lungs and blood of the control group. Cefpodoxime proxetil at all doses and cefteram pivoxil and cefditoren pivoxil at doses of 2 and 10 mg/kg showed efficacies comparable to that of CS-834. The therapeutic efficacy of cefdinir was inferior to those of the other antibiotics tested, and its use resulted in significant reductions in bacterial numbers in the lungs and blood only when it was used at a dose of 10 mg/kg.
FIG. 3.
Efficacies of CS-834 and reference antibiotics against pneumonia caused by penicillin-susceptible strain 2132. Each column and bar represents the mean ± standard error, respectively (n = 4 to 6). The indicated doses of each drug were administered orally twice daily for 2 days beginning at 24 h after infection, and the numbers of viable cells in the lungs (A) and blood (B) of mice were determined at 72 h after infection. CFTM-PI, cefteram pivoxil; CPDX-PR, cefpodoxime proxetil; CFDN, cefdinir; CDTR-PI, cefditoren pivoxil. The asterisks indicate a significant difference (P < 0.05) compared with the results for the control group.
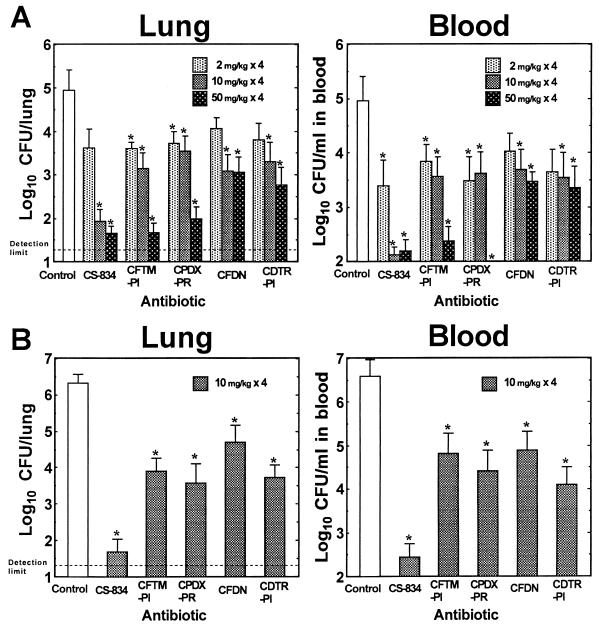
The efficacy of CS-834 was compared with those of the reference antibiotics against experimental pneumonia caused by penicillin-resistant strains 9605 (Fig. 4A) and 9601 (Fig. 4B). CS-834 at a dose of 2 mg/kg exhibited modest efficacy against infections caused by strain 9605, as did the other antibiotics tested. CS-834 at doses of 10 and 50 mg/kg, however, resulted in large reductions in the numbers of viable cells in both the lungs and blood. This effect was superior to the effects of the other antibiotics tested. Cefteram pivoxil and cefpodoxime proxetil showed potent efficacies at doses of 50 mg/kg, and their efficacies were comparable to the efficacy of CS-834. Cefditoren pivoxil and cefdinir exhibited modest efficacies at all doses, although their use at doses of 10 and 50 mg/kg resulted in significant reductions in the numbers of viable cells in the lungs and blood in comparison with the numbers of viable cells in the lungs and blood of the control group. At a dose of 10 mg/kg, CS-834 was the most active drug against pneumonia caused by strain 9601 when its activity was compared with those of the other antibiotics used at the same dose.
FIG. 4.
Efficacies of CS-834 and reference antibiotics against pneumonia caused by penicillin-resistant strains 9605 (A) and 9601 (B). Each column and bar represents the mean ± standard error (n = 8 to 10). The indicated doses of each drug were administered orally twice daily for 2 days beginning at 24 h after infection, and the numbers of viable cells in the lungs and blood of mice were determined at 72 h after infection. CFTM-PI, cefteram pivoxil; CPDX-PR, cefpodoxime proxetil; CFDN, cefdinir; CDTR-PI, cefditoren pivoxil. The asterisks indicate a significant difference (P < 0.05) compared with the results for the control group.
Pharmacokinetics of drugs in serum and lung.
Table 2 presents the values of the pharmacokinetic parameters for R-95867 and the reference antibiotics in the serum and lungs of mice after the administration of a single oral dose of 100 mg/kg of body weight. The concentrations of R-95867 in the serum and lungs were higher than those of cefdinir and lower than those of the other antibiotics tested. The mean ± standard error peak levels of R-95867 were 43.8 ± 5.4 μg/ml and 9.1 ± 0.75 μg/g in the serum and lungs, respectively, at 30 min after administration. At 120 min after administration the concentration of R-95867 in the lungs was below the detection limit (0.2 μg/ml). At all time points at which values were determined, the concentrations of cefdinir in the lungs were below the detection limit.
TABLE 2.
Pharmacokinetic parameters of the study antibiotics in mouse plasma and lunga
| Compartment and drug | Tmax (h) | Cmax (μg/ml or g) | t1/2 (h) | AUC0–6 (μg · h/ml or g) |
|---|---|---|---|---|
| Plasma | ||||
| R-95867 | 0.63 ± 0.13 | 43.8 ± 5.4 | 0.48 ± 0.06 | 55.8 ± 3.84 |
| CPDX | 0.50 ± 0 | 111 ± 6.8 | 1.52 ± 0.16 | 127 ± 6.52 |
| CFTM | 0.50 ± 0 | 88.7 ± 9.1 | 1.52 ± 0.16 | 149 ± 5.51 |
| CDTR | 0.69 ± 0.19 | 86.2 ± 1.3 | 1.54 ± 0.13 | 209 ± 14.1 |
| CFDN | 0.50 ± 0 | 15.2 ± 3.0 | 2.84 ± 0.30 | 37.8 ± 1.77 |
| Lung | ||||
| R-95867 | 0.63 ± 0.13 | 9.10 ± 0.75 | NC | 9.04 ± 0.67 |
| CPDX | 0.44 ± 0.06 | 38.6 ± 4.2 | 2.20 ± 0.63 | 49.2 ± 2.51 |
| CFTM | 0.50 ± 0 | 33.0 ± 6.7 | 0.41 ± 0.25 | 40.7 ± 7.61 |
| CDTR | 0.50 ± 0 | 29.8 ± 0.81 | 2.85 ± 0.23 | 77.5 ± 3.97 |
| CFDN | ND | ND | ND | ND |
Abbreviations: Tmax, time to maximum concentration of drug in serum or lungs; Cmax, maximum concentration of drug in serum or lungs; t1/2, half-life; AUC0–6, area under the concentration-time curve from time zero to 6 h; CPDX, cefpodoxime; CFTM, cefteram; CDTR, cefditoren; CFDN, cefdinir; NC, not calculated (insufficient datum points); ND, not detected.
DISCUSSION
It has been difficult to develop animal models of pneumonia caused by penicillin-resistant S. pneumoniae, since penicillin-resistant strains are less pathogenic than penicillin-susceptible ones for the induction of pneumonia in healthy animals (3, 10, 16, 19). Our model also showed that in the lungs the numbers of cells of the penicillin-resistant strain were lower than the numbers of cells of the penicillin-susceptible strain. For this reason, some investigators used models of pneumonia caused by penicillin-resistant pneumococci and induced in immunocompromised animals or by using foreign bodies, such as melted agar (3, 10, 16). We, however, successfully constructed with healthy mice a model of pneumonia caused by penicillin-resistant S. pneumoniae.
The reason that we used pathogens in the exponential growth phase is that they were more pathogenic than pathogens in other growth phases for the induction of pneumonia in mice (data not shown). In our pneumonia model, the numbers of viable cells of penicillin-resistant S. pneumoniae remained approximately 104 to 106 CFU in the lungs of mice with bacteremia from 24 to 72 h after infection, and this infection was not fatal until 72 h after infection. In addition, the histological changes, such as the inflammatory reaction, could be observed in infected lungs within 24 h, and higher doses of the antibiotics resulted in better therapeutic efficacy, suggesting that this experimental model of infection in ddY mice is reliable for evaluating the in vivo efficacies of antibiotics.
CS-834 showed the most potent activity among the antibiotics tested, in particular, against pneumonia caused by penicillin-resistant S. pneumoniae strains, although the concentrations of its active metabolite were lower than those of cefteram, cefpodoxime, and cefditoren in the lungs and blood of mice. These results suggest that the excellent therapeutic efficacy of CS-834 reflects the potent in vitro activity of R-95867, which was 4- to 16-fold greater than those of the other antibiotics against the penicillin-resistant strains. Against clinical isolates of penicillin-susceptible and -resistant S. pneumoniae, R-95867 also exhibited more potent activity than the cephem antibiotics: the MICs of R-95867 for 90% of strains of penicillin-susceptible and -resistant isolates were 0.05 and 0.39 μg/ml, respectively (21).
In conclusion, the new orally administered carbapenem CS-834 has been considered to be an extremely promising compound for further evaluation. Its antibacterial activity would cover not only pneumonia caused by penicillin-susceptible S. pneumoniae but also the pneumonia caused by penicillin-resistant S. pneumoniae encountered in general practice and could be used for the treatment of hospital outpatients. Clinical studies with CS-834 are in progress in Japan and the United States.
REFERENCES
- 1.Appelbaum P C. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Austrian R. Pneumococcal pneumonia. Diagnosis, epidemiologic, therapeutic and prophylactic considerations. Chest. 1986;90:738–743. doi: 10.1378/chest.90.5.738. [DOI] [PubMed] [Google Scholar]
- 3.Azoulay-Dupuis E, Vallée E, Veber B, Bédos J P, Bauchet J, Pocidalo J J. In vivo efficacy of a new fluoroquinolone, sparfloxacin, against penicillin-susceptible and -resistant and multiresistant strains of Streptococcus pneumoniae in a mouse model of pneumonia. Antimicrob Agents Chemother. 1992;36:2698–2703. doi: 10.1128/aac.36.12.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caputo G M, Appelbaum P C, Liu H H. Infections due to penicillin-resistant pneumococci. Clinical, epidemiologic, and microbiologic features. Arch Intern Med. 1993;153:1301–1310. [PubMed] [Google Scholar]
- 5.Committee for Revision of MIC Determination Method. Revision of minimal inhibitory concentration (MIC) determination method. Chemotherapy (Tokyo) 1981;29:76–79. [Google Scholar]
- 6.Feldman C, Kallenbach J M, Miller S D, Thorburn J R, Koornhof H J. Community-acquired pneumonia due to penicillin-resistant pneumococci. N Engl J Med. 1985;313:615–617. doi: 10.1056/NEJM198509053131006. [DOI] [PubMed] [Google Scholar]
- 7.Hisaoka, M., M. Ichikawa, and T. Terao. 1991. Microbiological assay method for the determination of panipenem concentrations in body fluids. Chemotherapy (Tokyo). 39(Suppl. 3):190–196.
- 8.Konno M Working Group for Penicillin-Resistant Streptococcus pneumoniae. An epidemiological study of penicillin-resistant Streptococcus pneumoniae in Japan. Jpn J Assoc Infect Dis. 1994;68:1338–1351. [PubMed] [Google Scholar]
- 9.MacFarlane J T, Finch R G, Ward M J, MacRae A D. Hospital study of adult community-acquired pneumonia. Lancet. 1982;ii:255–258. doi: 10.1016/s0140-6736(82)90334-8. [DOI] [PubMed] [Google Scholar]
- 10.Moine P, Vallée E, Azoulay-Dupuis E, Bourget P, Bédos J P, Bauchet J, Pocidalo J J. In vivo efficacy of a broad-spectrum cephalosporin, ceftriaxone, against penicillin-susceptible and -resistant strains of Streptococcus pneumoniae in a mouse pneumonia model. Antimicrob Agents Chemother. 1994;38:1953–1958. doi: 10.1128/aac.38.9.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neu H C. The crisis in antibiotic resistance. Science. 1992;257:1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 12.Neu H C, Saha G, Chin N-X. Comparative in vitro activity and β-lactamase stability of FK482, a new oral cephalosporin. Antimicrob Agents Chemother. 1989;33:1795–1800. doi: 10.1128/aac.33.10.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohya S, Fukuoka T, Koga T, Takenouchi T, Kawada H, Harasaki T, Hirasawa A, Ishii C, Sakagawa E, Kubota M, Yasuda H, Iwata M, Kuwahara S. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. CS-834, a new oral carbapenem. III. In vivo evaluation of CS-834, abstr. F107; p. 118. [Google Scholar]
- 14.Okamoto S, Hamana Y, Inoue M, Mitsuhashi S. In vitro and in vivo antibacterial activities of T-2588, a new oral cephalosporin, compared with those of other oral β-lactam antibiotics. Antimicrob Agents Chemother. 1987;31:1111–1116. doi: 10.1128/aac.31.7.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pallarés R, Liñares J, Vadillo M, Cabellos C, Manresa F, Viladrich P F, Martín R, Gudiol F. Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N Engl J Med. 1995;333:474–480. doi: 10.1056/NEJM199508243330802. [DOI] [PubMed] [Google Scholar]
- 16.Smith G M, Abbott K H. Development of experimental respiratory infections in neutropenic rats with either penicillin-resistant Streptococcus pneumoniae or β-lactamase-producing Haemophilus influenzae. Antimicrob Agents Chemother. 1994;38:608–610. doi: 10.1128/aac.38.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spika J S, Facklam R R, Plikaytis B D, Oxtoby M J the Pneumococcal Surveillance Working Group. Antimicrobial resistance of Streptococcus pneumoniae in the United States, 1979–1987. J Infect Dis. 1991;163:1273–1278. doi: 10.1093/infdis/163.6.1273. [DOI] [PubMed] [Google Scholar]
- 18.Tamura A, Okamoto R, Yoshida T, Yamamoto H, Kondo S, Inoue M, Mitsuhashi S. In vitro and in vivo antibacterial activities of ME1207, a new oral cephalosporin. Antimicrob Agents Chemother. 1988;32:1421–1426. doi: 10.1128/aac.32.9.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tateda K, Takashima K, Miyazaki H, Matsumoto T, Hatori T, Yamaguchi K. Noncompromised penicillin-resistant pneumococcal pneumonia CBA/J mouse model and comparative efficacies of antibiotics in this model. Antimicrob Agents Chemother. 1996;40:1520–1525. doi: 10.1128/aac.40.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Utsui Y, Inoue M, Mitsuhashi S. In vitro and in vivo antibacterial activities of CS-807, a new oral cephalosporin. Antimicrob Agents Chemother. 1987;31:1085–1092. doi: 10.1128/aac.31.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Utsui Y, Takenouchi T, Masuda N, Domon H, Kakuta M, Ishii C, Ishii C, Sakagawa E, Abe T, Ohya S, Yasuda H, Iwata M, Kuwahara S. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. CS-834, a new oral carbapenem. II. In vitro evaluation of R-95867, the active metabolite of CS-834, abstr. F106; p. 118. [Google Scholar]