Abstract
Background:
Sodium-glucose co-transporter 2 (SGLT2) inhibitors have been recommended in the practice guidelines for the treatment of patients with heart failure with reduced ejection fraction; however, their effects among patients with preserved ejection fraction have been debatable.
Objective:
We aim to evaluate the SGLT2 inhibitor effect among patients with heart failure with reduced ejection fraction, including DELIVER and EMPEROR-Preserved trials.
Methods:
We performed a systematic literature search using the PubMed, Embase, Scopus, and Cochrane libraries for relevant articles from inception until August 30th, 2022. Statistical analysis was performed by calculating hazard ratio (HR) using the random effect model with a 95% confidence interval (CI) and probability value (P). Statistical significance was met if 95% CI does not cross numeric “1” and P < .05.
Results:
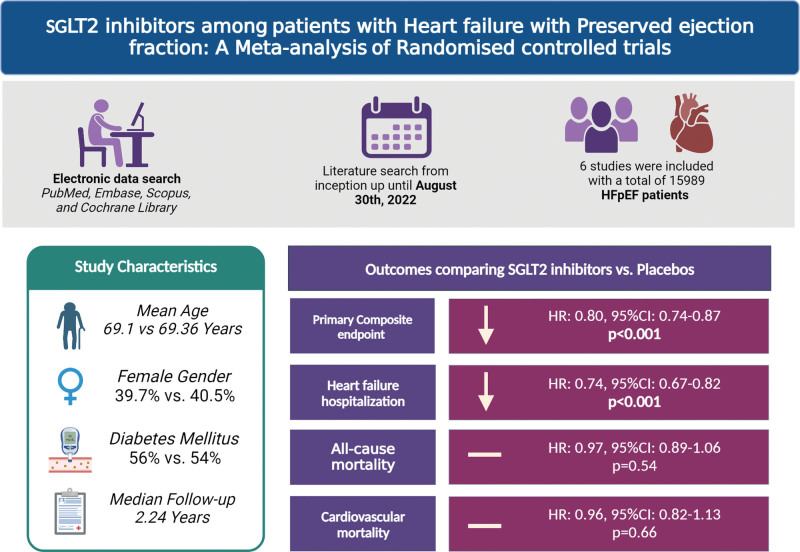
Six studies with a total of 15,989 total patients were included in the final analysis. The mean age of patients enrolled in SGLT2 inhibitors and placebo was 69.13 and 69.37 years, respectively. The median follow-up duration was 2.24 years. SGLT2 inhibitors reduced composite cardiovascular mortality or first hospitalization for heart failure (HR, 0.80 [95% CI: 0.74–0.87], P < .001, I2 = 0%), heart failure hospitalization (HR, 0.74 [95% CI: 0.67–0.82], P < .001, I2 = 0%) compared with placebo. However, all-cause mortality (HR, 0.97 [95% CI: 0.89–1.06], P = .54, I2 = 0%) and cardiovascular mortality (HR, 0.96 [95% CI: 0.82–1.13), P = .66, I2 = 35.09%] were comparable between both groups.
Conclusion:
Our study finding shows that SGLT2 inhibitors significantly reduced the risk of first HF hospitalization or cardiovascular death and HF hospitalization; however, all-cause mortality was comparable between the groups.
Keywords: cardiovascular outcomes, heart failure, SGLT2 inhibitors
Key clinical message.
What is already known on this topic: SGLT2 inhibitors have shown promising results among patients with HFrEF, however till date, only a few trials are available on HFpEF patients.
What this study adds: Among patients with HFpEF, SGLT2 inhibitors significantly reduced primary composite endpoints and heart failure hospitalization with the largest sample size thus far.
How this study might affect research, practice, or policy: SGLT2 inhibitors are an optimal drug class among patients with heart failure with preserved ejection fraction. This drug has shown better outcomes and can be very helpful in reducing morbidity and mortality among heart failure patients.
1. Introduction
Heart failure (HF) has conventionally been divided based on ejection fraction. Those with a reduced ejection fraction (40% or less) have HF because of loss of cardiomyocytes accompanied by ventricular dilatation.[1] Patients with a preserved ejection fraction (50% or more) have HF that is due to various reasons such as myocardial hypertrophy and fibrosis,[2] impaired diastolic compliance and relaxation,[3] subclinical systolic dysfunction,[4] renal dysfunction leading to elevated intracardiac filling pressures, fluid retention, and exercise intolerance.[5] It is accompanied by comorbidities (such as obesity) that do not lead to marked ventricular enlargement.[1] Patients with an ejection fraction between 40% and 50% are classified as mildly reduced and resemble reduced ejection fraction HF patients with respect to their response to treatment.[6]
While HF with reduced ejection fraction (HFrEF) can be treated with drugs that act to attenuate the overactivation of endogenous neurohormonal systems,[7] HF with preserved ejection fraction (HFpEF) is associated with substantial mortality and morbidity,[8] yet, no therapy has adequately improved outcomes.[5,9–11]
Sodium-glucose cotransporter 2 (SGLT2) inhibitors were initially developed for lowering blood glucose in type 2 diabetes mellitus (T2DM). Still, they have been shown to have cardioprotective and renoprotective effects in various diseases like chronic HF and chronic kidney disease (CKD) regardless of T2DM status.[11–16] Guidelines strongly recommend using SGLT2 inhibitors for chronic HFrEF.[17,18] In a small subgroup of diabetic patients with HF with ejection fraction > 50%, sotagliflozin showed decreased risk of HF hospitalization.[19,20] Two trials, EMPEROR-preserved[11] and DELIVER,[13] studied patients with HF with mildly reduced or preserved ejection fraction and showed reductions in composite cardiovascular death or HF events.
Despite these trials, the clinical recommendations for using SGLT2 in HFpEF still need to be clarified. This could be due to uncertainty around clinical benefits and outcomes of cardiovascular death that the trials were not designed to examine.[12] Another question still needs to be addressed whether the drug benefits patients on the highest end of the ejection fraction spectrum.[12,13]
Thus, this meta-analysis was performed to increase the power of clinical endpoints and help conclude the clinical benefits and mortality outcomes of SGLT2 inhibitors in HFpEF patients.
2. Methods
This meta-analysis was conducted and reported following the Cochrane and preferred reporting items for systematic review and meta-analysis 2020 guidelines and performed according to established methods, as described previously.[21–23] The pre-specified study protocol has been registered in the PROSPERO (CRD42023388472).
2.1. Search strategy
We conducted a systematic literature search in PubMed, Embase, and Cochrane Library using predefined MESH terms by using “AND” and “OR.” The following search terms were used: ((((((((((((heart failure[MeSH Terms]) OR (diastolic heart failure[MeSH Terms])) OR (Heart failure)) OR (preserved ejection fraction)) OR (HFpEF)) OR (Cardiac Failure)) AND (SGLT2 Inhibitor[MeSH Terms])) OR (Sodium-glucose co-transporter 2 inhibitors [Other Term])) OR (Empagliflozin[Other Term])) OR (Dapagliflozin[Other Term])) OR (Sotagliflozin[Other Term])) AND (randomized controlled trial [Other Term])) AND (cardiovascular outcomes[Other Term]). The search was performed from inception up until 30 August 2022 without any restrictions on the language of the studies. All the studies were carefully screened and exported to the endnote reference manager used to handle searched citations. A manual check was carried through to crosscheck for any remaining duplicates. Two reviewers (V.J. and A.V.) reviewed the papers based on the title and abstract. Discrepancies regarding the inclusion of studies were arbitrated by the senior author (A.J.).
2.2. Eligibility criteria
We included studies with patients of age ≥ 18 years of age without any restrictions of the language barrier. All randomized controlled trials with data on HFpEF and desired outcomes of interest were sought to be eligible for inclusion into the study. It was decided to include studies with 2 arms with an SGLT2 inhibitor as the intervention arm and a placebo as a comparator. Any study tested on animals, review, case reports, case series, studies on patients < 18 years, abstract, studies with a single arm or without HF patients, and studies without outcomes of interest were excluded from the review.
2.3. Outcomes
The primary outcome for this meta-analysis was a composite of first HF hospitalization or cardiovascular death. The secondary outcomes were HF hospitalization, all-cause mortality, and cardiovascular mortality.
2.4. Data extraction and quality assessment
The following data were extracted from all the included studies: Study type, author, year of publication, number of patients in both groups, age, sex, follow-up period, comorbidities, and primary and secondary outcomes. Two investigators (A.I. and J.C.) independently appraised the potential risk of bias for a randomized controlled trial using Cochrane’s risk of bias 2 tool.[24] We then classified studies as having a high risk of bias, some concerns, or low risk of bias based on the scores as determined by following the risk of bias 2 tool handbook.
2.5. Statistical analysis
Statistical analysis was performed by calculating hazard ratio (HR) using the DerSimonian and Laird random effect model,[25] with a test for overall effect reported as Z value, 95% confidence interval (CI), and probability value (P). Statistical significance was met if 95% CI does not cross numeric “1” and P < .05. The heterogeneity among studies was assessed by Higgins I-squared (I2) statistical model with I2 values. As a guide, I2 < 25% indicated low, 25–50% moderate, and >50% high heterogeneity.[26] Publication bias was assessed using the graphical presentation of funnel plot asymmetry.[24] All statistical analyses were performed using S.T.A.T.A. version 17.1 (StataCorp, College Station, TX).
3. Results
3.1. Study selection
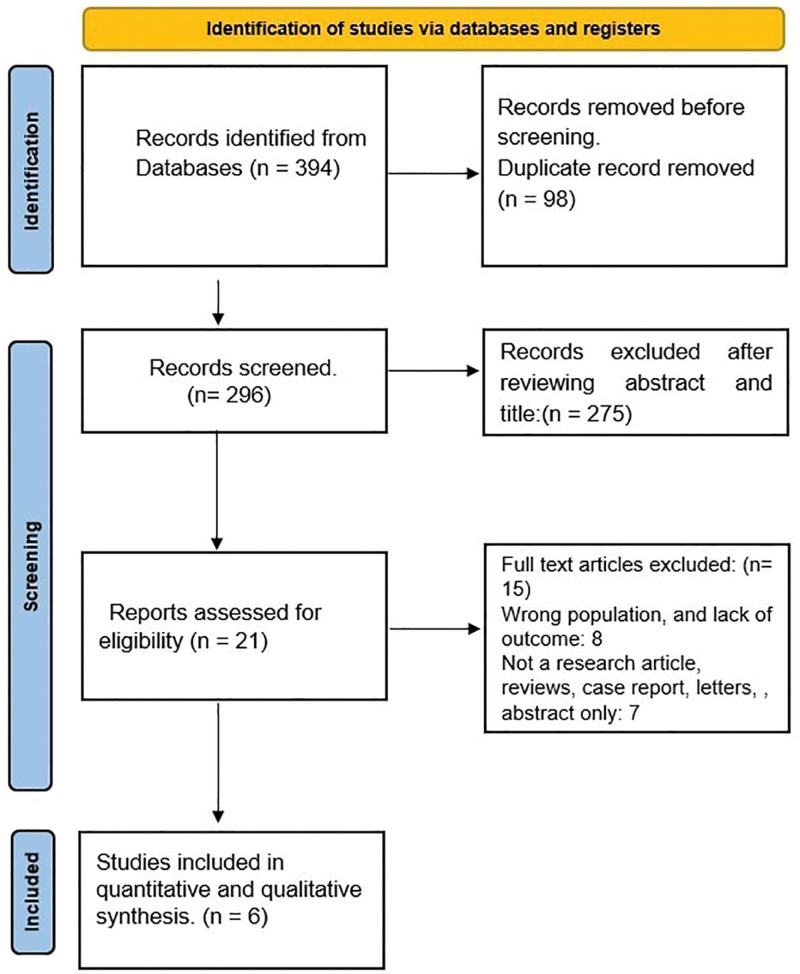
The preliminary database search using the pre-specified keywords yielded 394 articles, of which 98 duplicate studies were excluded. Two hundred seventy-five were further excluded from the initial post-title and abstract screening based on the inclusion and exclusion criteria and comparison arm. The full-text review was conducted for the remaining 21 studies identified during the search period in which 2 articles were not retrieved. Fifteen studies were excluded as they either had unmatching target populations, were not primary research articles or case reports, or lacked a comparison arm. Hence, 6 studies that met the eligibility criteria were included in our study (Table 1). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram is depicted in Figure 1.
Table 1.
Baseline demographic, comorbidity, and study characteristics of included studies.
| Author | Study design | Sample size | Intervention | LVEF inclusion criteria | Age, yr | Male, % | DM, % | BMI, kg/m2 | Follow up, yr |
|---|---|---|---|---|---|---|---|---|---|
| EMPEROR Preserved, 2020 | RCT | 2997/2991 | Empagliflozin | LVEF ≥ 40% | 71.8/71.9 | 55.4/55.3 | 48.9/49.2 | 29.7/29.9 | 2.18 |
| VERTIS CV, 2020 | RCT | 680/327 | Ertugliflozin | LVEF ≥ 45% | 63.8/64.7 | 65.6/63.3 | 100/100 | 32.6/32.9 | 3.5 |
| DECLARE-TIMI 58, 2019 | RCT | 399/409 | Dapagliflozin | LVEF ≥ 45% | NA | NA | 100/100 | NA | 4.2 |
| SCORED, 2021 | RCT | 1667 | Sotagliflozin | LVEF ≥ 50 | NA | NA | 1667 | NA | 1.3 |
| SOLOIST-WHF, 2021 | RCT | 127/129 | Sotagliflozin | LVEF ≥ 50 | NA | NA | 127/129 | NA | 0.8 |
| DELIVER Trial, 2022 | RCT | 3131/3132 | Dapagliflozin | LVEF ≥ 40% | 71.8/71.5 | 56.4/55.8 | 44.7/44.8 | NA | 2.3 |
DM = diabetes mellitus.
Figure 1.
PRISMA flowchart of the search strategy for systematic review and meta-analysis.
3.2. Study and patient characteristics
Six studies with a total of 15,989 total patients were included in the final analysis.[11,13,19,20,27,28] The mean age of patients with SGLT2 inhibitors and the placebo group was 69.1 years and 69.37 years. 43% of patients in the SGLT2i group were female, while 44 % were in the placebo group. The most common comorbidities among available data was diabetes mellitus (56% vs 54%). The median follow-up duration was 2.24 years. Study characteristics, patients’ demographics, and comorbidities are presented in Table 1.
3.3. Results of meta-analysis
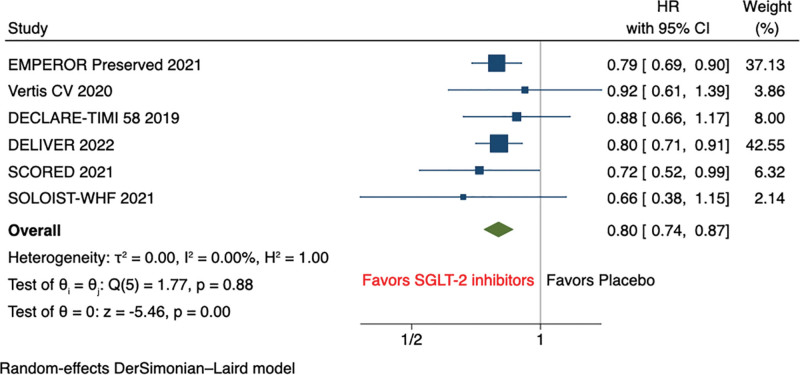
The pooled analysis of primary outcome shows that SGLT2 inhibitors significantly reduced the composite of first hospitalization for HF or cardiovascular mortality (HR, 0.80 [95% CI: 0.74–0.87], P < .001, I2 = 0%) (Fig. 2).
Figure 2.
Primary composite outcome (composite of first HFH or cardiovascular death). HFH = heart failure hospitalization.
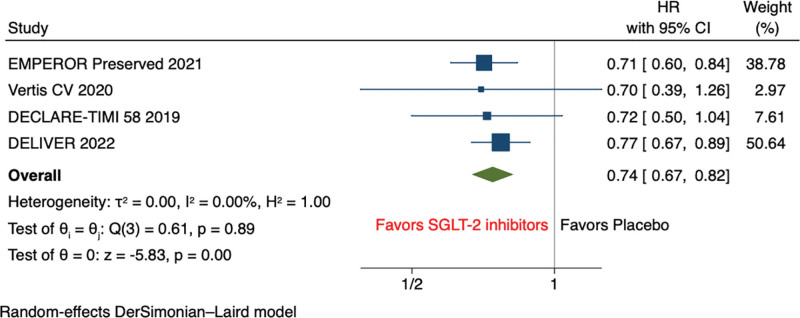
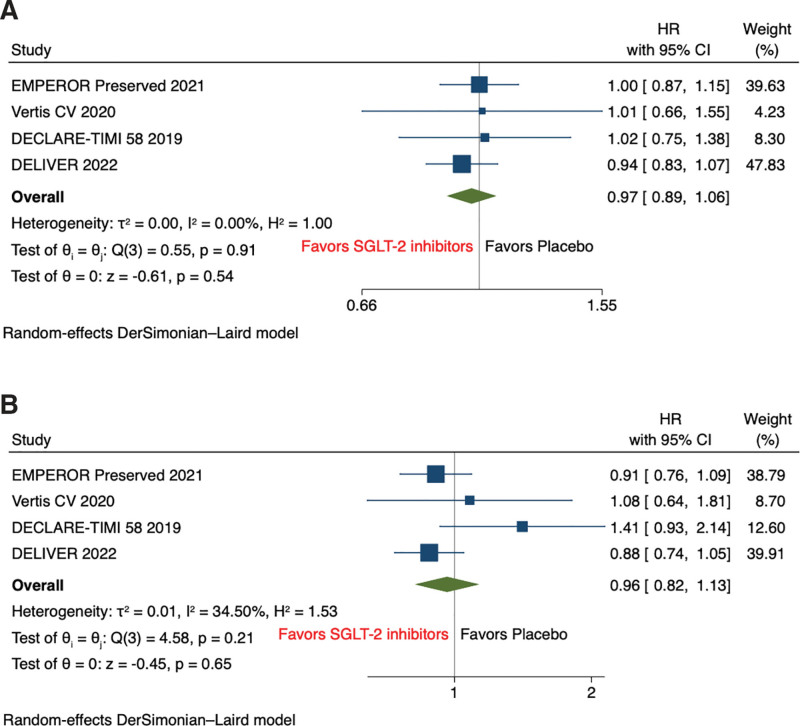
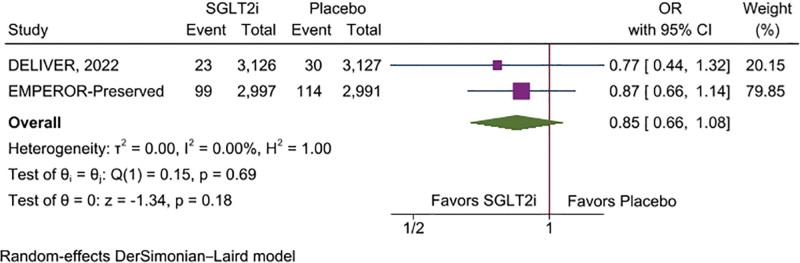
Pooled analysis of secondary outcomes shows that SGLT2 inhibitors significantly reduce the hazard of HF hospitalization (HR, 0.74 [95% CI: 0.67–0.82], P < .001, I2 = 0%) compared with placebo (Fig. 3). However, all-cause mortality (HR, 0.97 [95% CI: 0.89–1.06], P = .54, I2 = 0%), and cardiovascular mortality (HR, 0.96 [95% CI: 0.82–1.13], P = .66, I2 = 35.09%) were comparable between both groups on SGLT2 inhibitor and placebo (Fig. 4A and B). The risk of incidence of sudden cardiac death was non statistically significant reduced among SGLT2 inhibitors compared with placebo (OR, 0.85 [95% CI: 0.66–1.08], P = .18, I2 = 0%) (Fig. 5).
Figure 3.
Forest plot of secondary outcome: HFH. HFH = heart failure hospitalization.
Figure 4.
Forest plot of secondary outcome: (A) all-cause mortality and (B) cardiovascular mortality.
Figure 5.
Forest plot of secondary outcome: sudden cardiac death.
Publication bias was assessed for the primary and secondary outcomes meeting criteria as described in the methods. There appeared to be no evidence of publication bias, as evident by no funnel plot asymmetry (Figure S1–S4, Supplemental Digital Content, http://links.lww.com/MD/J757, http://links.lww.com/MD/J761, http://links.lww.com/MD/J763, http://links.lww.com/MD/J765). The included trials were found to have a low risk of bias, 1 was found to have some concerns in outcome selection and another one had high risk of bias due to bias arising in outcome selection on Cochrane’s risk of bias tool (Figure S5, Supplemental Digital Content, http://links.lww.com/MD/J767).
4. Discussion
Our study highlights that SGLT2 inhibitors among patients with HFpEF significantly reduce the primary composite end-point and HF hospitalization (HFH). However, cardiovascular and all-cause mortality were comparable between both groups (Fig. 6). In this study, we evaluated 6 RCTs (DELIVER trial, EMPEROR-Preserved, DECLARE-TIMI, VERTIS CV trial, SCORED clinical trial, and SOLOIST-WHF Clinical trials) having a total of 15,989 patients.[11,13,19,20,27,28] The DELIVER trial and EMPEROR-Preserved showed lower first HFH and HFH in SGLT2 inhibitors groups compared to placebo, concordant with our results.[11,13] The secondary outcome of our study, that is, all-cause mortality and cardiovascular mortality, were evaluated in various trials with SGLT2 inhibitors and were found comparable between the SGLT2 inhibitors and placebo groups, findings supporting our study.[19,20,27,29]
Figure 6.
Central Illustration demonstrating the effects of SGLT2 inhibitors compared with placebo groups in HFpEF. HFpEF = heart failure with reduced ejection fraction, SGLT2 = Sodium-glucose co-transporter 2.
Previously, a meta-analysis was conducted by Butler et al[30] on the effect of SGLT2 inhibitors in HFrEF and HFpEF. In patients with HFpEF, a composite of HFH or cardiovascular death and first HFH were significantly lower in the SGLT2-treated patients compared to the control. In contrast, cardiovascular death and all-cause mortality were comparable between the 2 groups, findings concordant with our study.[30] While in patients with HFrEF, the composite of first HFH or cardiovascular death, total (first or recurrent) HFH or cardiovascular death, and first HFH were significantly lower in the SGLT2 group compared to placebo.[30] Similarly, another study by Vaduganathan et al showed reduced composite cardiovascular death or first HFH in SGLT2 treated group in HFpEF and overall HF patients (HFpEF and HFrEF), findings concordant with our study. In contrast, they found significantly reduced overall mortality in SGLT2 treated group in HF patients (both HFpEF and HFrEF), results discordant with our findings.[12] Similarly, another study by Tsampsian et al[31] showed a significant reduction in composite cardiovascular death or first HFH in HFpEF patients in the SGLT2 group in comparison to place, further supporting our findings.
The SGLT2 inhibitors were developed to control hyperglycemia in T2DM by inhibiting glucose reabsorption in the proximal tubule and have been found cardioprotective through various protective mechanisms.[32] These beneficial effects of SGLT2 can be summarized as increasing diuresis/natriuresis; lowering blood pressure; preventing inflammation; improving cardiac energy metabolism; preventing cardiac remodeling and ischemic injury; increasing erythropoietin level, and improving vascular function.[32] A study by Graffin et al[33] showed that empagliflozin effectively increased natriuresis and reduced plasma volume in a small population of 20 patients with T2DM and stable HF. Another positive effect of SGLT2 inhibitors on cardiac metabolism is a shift of fatty acids to ketone bodies as the substrate for myocardial energy generation.[34] A study by Aubert et al[35] showed that a failing heart uses ketone bodies as a fuel for energy, and increased hepatic neogenesis of ketone bodies is an established effect of SGLT2 inhibitors.[36,37] Another mechanism could be SGLT2 inhibitors-associated hemoconcentration, which causes increased oxygen delivery enhancing cardiac efficiency.[38] In conclusion, the above various mechanisms could answer why SGLT2 inhibitors have cardioprotective effects in all patients, especially HF patients.
SGLT2 inhibitors were reported for causing acute kidney injury (AKI) during the early days of their introduction in clinical practice as hypoglycemic drugs. This side effect was largely driven by specific features of their mechanism of action, including the fact that its initiation is accompanied by an initial reduction of estimated glomerular filtration rate.[39] As a result, the FDA cautioned healthcare professionals regarding its use, especially in the context of the other factors that increase the chances of AKI, such as CKD, HF, and diuretic use.[39] However, none of the RCTs has proven SGLT2 inhibitors as a high-risk factor for AKI to date. EMPEROR-Reduced and DAPA-HF trials showed a similar rate of AKI in both SGLT2 inhibitors and placebo groups in HF with impaired renal function patients.[14,15] A meta-analysis conducted by Neuen et al[40] showed a significantly reduced risk of AKI, end-stage renal disease, and the risk of dialysis, transplantations, or death due to kidney diseases in the SGLT2 inhibitors group compared to placebo. Improving kidney functions results in a decreased incidence of cardiovascular events and can improve established HF.[41]
The main strength of our study is that it was conducted on a large sample size of 15989 participants and showed a significant reduction in the composite of first HFH or cardiovascular death and total HFH in the SGLT2 inhibitors group in comparison to placebo. There are certain limitations as well which need consideration. Only 6 studies qualified our inclusion criteria, and sensitivity & subgroup analyses could not be performed due to limited data. In the future, more RCTs are warranted to have more robust evidence in a different subset of patients with diabetes and CKD and its effect on mortality.
5. Conclusion
Our study finding shows that SGLT2 inhibitors significantly reduced the risk of first HF hospitalization or cardiovascular death and HF hospitalization.
Acknowledgments
We want to thank Maha Hameed for helping during the initial part of the abstract writing.
Author contributions
Conceptualization: Akash Jaiswal, Vikash Jaiswal.
Data curation: Akash Jaiswal, Vikash Jaiswal, Anagha M. Nair, Zarghoona Wajid.
Formal analysis: Vikash Jaiswal, Song Peng Ang, Angela Ishak, Zarghoona Wajid.
Funding acquisition: Vikash Jaiswal.
Investigation: Akash Jaiswal, Vikash Jaiswal, Vibhor Agrawal, Tushar Kumar.
Methodology: Akash Jaiswal, Vikash Jaiswal, Abhigan Babu Shrestha.
Project administration: Akash Jaiswal, Vikash Jaiswal, Monodeep Biswas.
Resources: Helen Huang, Abhigan Babu Shrestha.
Software: Helen Huang, Abhigan Babu Shrestha.
Supervision: Akash Jaiswal, Vikash Jaiswal, Monodeep Biswas.
Validation: Vikash Jaiswal, Muhammad Hanif, Vamsi Garimella, Angela Ishak, Helen Huang, Victor Hugo Aguilera Alvarez, Abhigan Babu Shrestha, Monodeep Biswas.
Visualization: Vikash Jaiswal, Muhammad Hanif, Angela Ishak, Helen Huang, Monodeep Biswas.
Writing – original draft: Akash Jaiswal, Vikash Jaiswal, Song Peng Ang, Muhammad Hanif, Ananya Vadhera, Tushar Kumar, VamsikalyanReddy Borra, Vamsi Garimella, Angela Ishak, Victor Hugo Aguilera Alvarez.
Writing – review & editing: Akash Jaiswal, Vikash Jaiswal, Song Peng Ang, Muhammad Hanif, Ananya Vadhera, Vibhor Agrawal, Tushar Kumar, Anagha M. Nair, VamsikalyanReddy Borra, Vamsi Garimella, Angela Ishak, David Song, Abdelrahman M. Attia, Helen Huang, Victor Hugo Aguilera Alvarez, Abhigan Babu Shrestha, Monodeep Biswas.
Supplementary Material
Abbreviations:
- AKI
- acute kidney injury
- CI
- confidence interval
- CKD
- chronic kidney disease
- HF
- heart failure
- HFH
- heart failure hospitalisation
- HFrEF
- heart failure with reduced ejection fraction
- HFpEF
- heart failure with reduced ejection fraction
- HR
- hazard ratio
- SGLT2
- sodium-glucose co-transporter 2
- T2DM
- type 2 diabetes mellitus
AJ and VJ contributed equally this work.
The authors have no funding and conflicts of interest to disclose.
Prospero registration: CRD42023388472.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
The abstract of this analysis has been presented at the ESC ASIA conference (https://doi.org/10.1093/eurheartj/ehac779.041).
How to cite this article: Jaiswal A, Jaiswal V, Ang SP, Hanif M, Vadhera A, Agrawal V, Kumar T, Nair AM, Borra V, Garimella V, Ishak A, Wajid Z, Song D, Attia AM, Huang H, Aguilera Alvarez VH, Shrestha AB, Biswas M. SGLT2 inhibitors among patients with heart failure with preserved ejection fraction: A meta-analysis of randomised controlled trials. Medicine 2023;102:39(e34693).
Contributor Information
Akash Jaiswal, Email: vikash29jaxy@gmail.com.
Vikash Jaiswal, Email: vikash29jaxy@gmail.com.
Song Peng Ang, Email: hestonang23@gmail.com.
Muhammad Hanif, Email: hanifafridi273@gmail.com.
Ananya Vadhera, Email: Ananyavadhera@gmail.com.
Vibhor Agrawal, Email: 2000.vibhor@gmail.com.
Tushar Kumar, Email: tushar.radir@gmail.com.
Anagha M. Nair, Email: anaghanairc201@gmail.com.
VamsikalyanReddy Borra, Email: drpam30@gmail.com.
Vamsi Garimella, Email: vcg24@med.miami.edu.
Angela Ishak, Email: Angela.ishak.10@gmail.com.
Zarghoona Wajid, Email: hj2051@wayne.edu.
David Song, Email: xdavidsong@gmail.com.
Abdelrahman M. Attia, Email: abdelrahman-m-attia@students.kasralainy.edu.eg.
Victor Hugo Aguilera Alvarez, Email: dr.vicaguilera@gmail.com.
Monodeep Biswas, Email: mbiswasmd@gmail.com.
References
- [1].Kanagala P, Arnold JR, Singh A, et al. Characterizing heart failure with preserved and reduced ejection fraction: an imaging and plasma biomarker approach. PLoS One. 2020;15:e0232280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mohammed SF, Hussain S, Mirzoyev SA, et al. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure – abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–9. [DOI] [PubMed] [Google Scholar]
- [4].Kraigher-Krainer E, Shah AM, Gupta DK, et al.; PARAMOUNT Investigators. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–20. [DOI] [PubMed] [Google Scholar]
- [6].Butler J, Anker SD, Packer M. Redefining heart failure with a reduced ejection fraction. JAMA. 2019;322:1761–2. [DOI] [PubMed] [Google Scholar]
- [7].Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396:121–8. [DOI] [PubMed] [Google Scholar]
- [8].Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2017;376:896–7. [DOI] [PubMed] [Google Scholar]
- [9].Pitt B, Pfeffer MA, Assmann SF, et al.; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92. [DOI] [PubMed] [Google Scholar]
- [10].Solomon SD, Claggett B, Lewis EF, et al.; TOPCAT Investigators. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016;37:455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–61. [DOI] [PubMed] [Google Scholar]
- [12].Vaduganathan M, Docherty KF, Claggett BL, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022;400:757–67. [DOI] [PubMed] [Google Scholar]
- [13].Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–98. [DOI] [PubMed] [Google Scholar]
- [14].McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- [15].Packer M, Anker SD, Butler J, et al.; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–24. [DOI] [PubMed] [Google Scholar]
- [16].Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al.; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–46. [DOI] [PubMed] [Google Scholar]
- [17].McDonagh TA, Metra M, Adamo M, et al.; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726. [DOI] [PubMed] [Google Scholar]
- [18].Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2022;145:e876–94. [DOI] [PubMed] [Google Scholar]
- [19].Bhatt DL, Szarek M, Steg PG, et al.; SOLOIST-WHF Trial Investigators. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–28. [DOI] [PubMed] [Google Scholar]
- [20].Bhatt DL, Szarek M, Pitt B, et al.; SCORED Investigators. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384:129–39. [DOI] [PubMed] [Google Scholar]
- [21].Jaiswal V, Ang SP, Yaqoob S, et al. Cardioprotective effects of influenza vaccination among patients with established cardiovascular disease or at high cardiovascular risk: a systematic review and meta-analysis. Eur J Prev Cardiol. 2022;29:1881–92. [DOI] [PubMed] [Google Scholar]
- [22].Jaiswal V, Khan N, Jaiswal A, et al. Early surgery vs conservative management among asymptomatic aortic stenosis: a systematic review and meta-analysis. Int J Cardiol Heart Vasc. 2022;43:101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jaiswal V, Nain P, Mukherjee D, et al. Symptomatology, prognosis, and clinical findings of Monkeypox infected patients during COVID-19 era: a systematic-review. Immun Inflamm Dis. 2022;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Higgins JPT, Altman DG, Gotzsche PC, et al.; Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Borenstein M, Hedges LV, Higgins JPT, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. [DOI] [PubMed] [Google Scholar]
- [26].Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wiviott SD, Raz I, Bonaca MP, et al.; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57. [DOI] [PubMed] [Google Scholar]
- [28].Cannon CP, Pratley R, Dagogo-Jack S, et al.; VERTIS CV Investigators. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–35. [DOI] [PubMed] [Google Scholar]
- [29].Cosentino F, Cannon CP, Cherney DZI, et al.; VERTIS CV Investigators. Efficacy of ertugliflozin on heart failure–related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation. 2020;142:2205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Butler J, Usman MS, Khan MS, et al. Efficacy and safety of SGLT2 inhibitors in heart failure: systematic review and meta-analysis. ESC Heart Fail. 2020;7:3298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tsampasian V, Elghazaly H, Chattopadhyay R, et al. Sodium glucose co-transporter 2 inhibitors in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Prev Cardiol. 2022;29:e227–9. [DOI] [PubMed] [Google Scholar]
- [32].Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of Sodium Glucose Co-Transporter 2 (SGLT2) inhibitors. JACC Basic Transl Sci. 2020;5:632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Griffin M, Rao VS, Ivey-Miranda J, et al. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation. 2020;142:1028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rådholm K, Figtree G, Perkovic V, et al. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program. Circulation. 2018;138:458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Aubert G, Martin OJ, Horton JL, et al. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ferrannini E, Baldi S, Frascerra S, et al. Shift to fatty substrate utilization in response to sodium–glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190–5. [DOI] [PubMed] [Google Scholar]
- [37].Kappel BA, Lehrke M, Schütt K, et al. Effect of empagliflozin on the metabolic signature of patients with type 2 diabetes mellitus and cardiovascular disease. Circulation. 2017;136:969–72. [DOI] [PubMed] [Google Scholar]
- [38].Staels B. Cardiovascular protection by sodium glucose cotransporter 2 inhibitors: potential mechanisms. Am J Med. 2017;130:S30–9. [DOI] [PubMed] [Google Scholar]
- [39].Lioudaki E, Joslin JR, Trachanatzi E, et al. The role of sodium-glucose co-transporter (SGLT)-2 inhibitors in heart failure management and implications for the kidneys. Rev Cardiovasc Med. 2022;23:082. [DOI] [PubMed] [Google Scholar]
- [40].Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7:845–54. [DOI] [PubMed] [Google Scholar]
- [41].Jankowski J, Floege J, Fliser D, et al. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143:1157–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.