Abstract
Objectives:
To evaluate comparative outcomes of routine abdominal drainage (RAD) and non-routine abdominal drainage (NRAD) during elective hepatic resection for hepatic neoplasms.
Materials and Methods:
We systematically searched MEDLINE, EMBASE, The Cochrane Library, Web of Science. The searching phrases included “liver resection,” “hepatic resection,” “hepatectomy,” “abdominal drainage,” “surgical drainage,” “prophylactic drainage,” “intraperitoneal drainage,” “drainage tube,” “hepatectomy,” “abdominal drainage” and “drainage tube.” Two independent reviewers critically screened literature, extracted data and assessed the risk of bias. Post-operative morbidity and mortality were the outcome parameters. Combined overall effect sizes were calculated using fixed-effect or random-effect model.
Results:
We have identified 9 RCTs and 3 comparative studies reporting total of 5726 patients undergoing elective hepatectomy under RAD (n = 3084) or NRAD (NRAD group, n = 2642). RAD was associated with significantly higher overall complication rate [odds risk = 1.79, 95% CI (1.10, 2.93), P = .02] and biliary leakage rate [odds risk = 2.41, 95% CI (1.48, 3.91), P = .0004] compared with NRAD. Moreover, it significantly increased hospital stays [mean difference = 0.95, 95% CI (0.02, 1.87), P = .04] compared with NRAD. RAD showed no difference regarding intra-abdominal hemorrhage, wound complications, liver failure, subphrenic complications, pulmonary complications, infectious complications, reoperation and mortality compared with NRAD.
Conclusions:
Although routine abdominal drainage may help surgeons to observe post-operative complication, it seems to be associated with increased post-operative morbidity and longer hospital stays. Non-routine abdominal drainage may be an appropriate option in selected patients undergoing hepatic resection. Higher level of evidence is needed.
Keywords: complications, drainage, hepatectomy, meta-analysis
1. Introduction
Hepatectomy is the main treatment modality for hepatic neoplasm with acceptable postoperative morbidity and mortality.[1] Abdominal drainage was accepted as routine and mandate procedure whenever hepatectomy was performed. The main reason was to drain residual abdominal fluid and diagnose postoperative bleeding and biliary leakage as early as possible.[2,3] Recent years, the practice of preoperative precise evaluation, meticulous surgical dissection and post-operative management drastically reduced post-hepatectomy morbidity and mortality. Although, routine abdominal drainage after elective hepatectomy may help surgeons to observe postoperative bleeding and possible leakage as early as possible, some studies showed even better clinical outcome when the abdominal drainage was not routinely placed. Therefore, questions were raised concerning the necessity for routine abdominal drainage in patients undergoing elective hepatic resections.[4–6]
Despite the “sentinel” nature of abdominal drainage during elective hepatectomy, it may increase postoperative complication and hospital stay.[7,8] The necessity and clinical efficacy for routine abdominal drainage in elective hepatectomy are still inconclusive.[9] Herein, we report a systematic review and meta-analysis to compare the clinical outcomes of routine abdominal drainage (RAD) and non-routine abdominal drainage (NRAD) during elective hepatic resection.
2. Methods
This study was approved by the ethical committee of the first affiliated hospital of Xinjiang Medical university and conducted according to the recommendations of the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses, PRISMA”.[10]
2.1. Literature search
The patient-intervention-comparison-outcome scheme combining patient (hepatic resection), intervention (placing abdominal drainage), control (without abdominal drainage), and outcome (postoperative outcomes) characteristics was used. A thorough search was conducted by using electronic databases including PubMed, EMbase, Ovid Medline, The Cochrane Library, Web of Science to March 2020 for relevant trials that compare clinical outcome of routine abdominal drainage and non-routine abdominal drainage during elective hepatectomy. Searching phrases included “liver resection,” “hepatic resection,” “hepatectomy,” “abdominal drainage,” “surgical drainage,” “prophylactic drainage,” “intraperitoneal drainage,” “drainage tube,” “hepatectomy,” “abdominal drainage” and “drainage tube.” The retrieval strategy follows the Cochrane system evaluation manual.
2.2. Study selection
RCTs and comparative studies that compare the clinical outcomes of RAD and NRAD in patients undergoing elective hepatectomy were enrolled into current study. Animal studies, conference abstracts, duplicate publications and as well as non-English literatures were excluded; Besides, studies that reported emergent hepatectomy were excluded from current analysis.
2.3. Data extraction and outcomes
Data extraction was carried out independently by 2 authors. The included parameters were detailed in Table 1. The outcome indicators included ascites, intra-abdominal hemorrhage, wound complications (wound infection, drainage tube orifice leakage ascites, incision dehiscence, etc), bile leakage, liver failure, subphrenic complications (subphrenic effusion requiring puncture and drainage, subphrenic infection, etc), pulmonary complications (pulmonary infection, pleural effusion, pneumothorax, atelectasis, etc), infectious complications (wound infection, pulmonary infection, sepsis, etc), reoperation, the complication rate, postoperative hospital stay, and mortality.
Table 1.
Basic characteristics of included studies.
| RAD | NRAD | |||||||||
| References | Year | Country | Study design | No. of patients | n | Sex (M/F) | Age | n | Sex (M/F) | Age |
| Belghiti J[10] | 1993 | France | RCT | 81 | 42 | 13/29 | 47 ± 16 | 39 | 18/21 | 51 ± 12 |
| Fong Y[11] | 1996 | USA | RCT | 120 | 60 | 31/29 | 57 ± 2 | 60 | 35/25 | 57 ± 2 |
| Fuster J[12] | 2004 | Spain | RCT | 40 | 20 | 15/5 | 60 ± 6 | 20 | 15/5 | 58 ± 8 |
| Liu CL[13] | 2004 | China | RCT | 104 | 52 | 42/10 | 53.6 ± 1.5 | 52 | 44/8 | 52.8 ± 1.4 |
| Aldameh A[14] | 2005 | New Zealand | RCT | 211 | 126 | 60/66 | 60 (4–81)∗ | 85 | 30/55 | 61 (1–84)∗ |
| Lu L[15] | 2006 | China | RCT | 462 | 357 | 276/81 | 50.4 ± 0.6 | 105 | 80/25 | 50.9 ± 1.2 |
| Sun HC[16] | 2006 | China | RCT | 120 | 60 | 47/13 | 50.2 ± 13.1 | 60 | 45/15 | 49.2 ± 12.1 |
| Kim YI[17] | 2014 | Korea | RCT | 200 | 100 | 74/26 | 56.3 ± 13.4 | 100 | 74/26 | 54.9 ± 12.9 |
| Squires MH 3rd[18] | 2015 | USA | CCS | 1041 | 564 | 264/300 | 57.2 ± 13.3 | 477 | 214/263 | 57.1 ± 14.0 |
| Shwaartz C[19] | 2016 | USA | CCS | 1005 | 500 | 236/264 | 58.3 ± 14.3 | 505 | 253/252 | 55.7 ± 14.1 |
| Brauer DG[20] | 2016 | USA | RCT | 1868 | 934 | 450/484 | 59.3 ± 13.1 | 934 | 470/464 | 59.2 ± 13.2 |
| Wada S[21] | 2017 | Japan | CCS | 474 | 269 | 208/61 | 67 (26–88)∗ | 205 | 151/54 | 65 (31–90)∗ |
CCS = Case control study, NRAD = non-routine abdominal drainage, RAD = routine abdominal drainage, RCT = randomized controlled study.
Median (range).
2.4. Risk of bias assessment
Risk of bias for enrolled RCTs and comparative studies were assessed and given in Figure 1 and Table 2, respectively.
Figure 1.
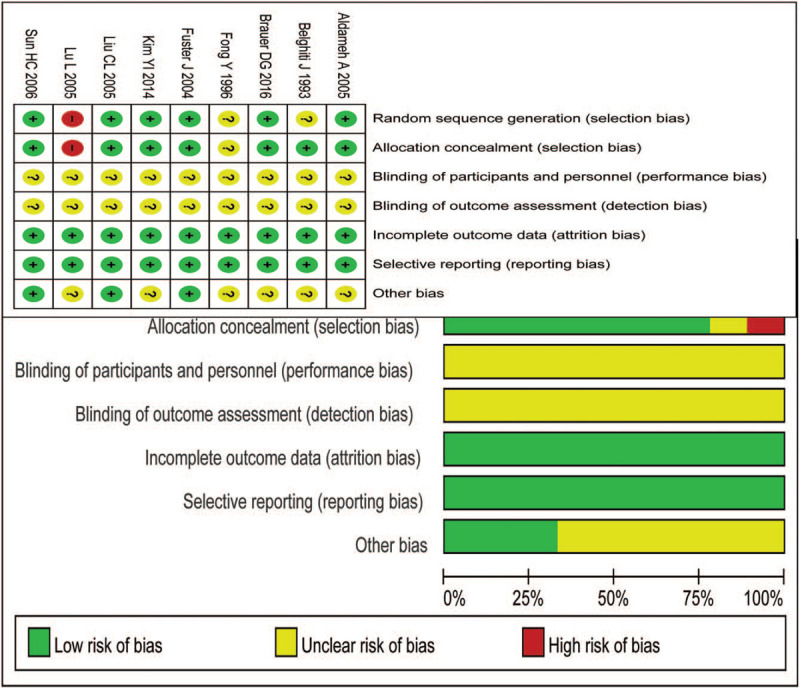
Risk-of-bias summary for the RCT studies.
Table 2.
Evaluation of risk of bias in included studies.
| Articles | ① | ② | ③ | ④ | ⑤A | ⑤B | ⑥ | ⑦ | ⑧ | NOS score |
| Squires MH 3rd | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 8 |
| Shwaartz C | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 8 |
| Wada S | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
NOS = Newcastle-Ottawa Scale.
2.5. Statistical analysis
The RevMan version 5.3 software, recommended by Cochrane collaboration, has been applied for analysis. Heterogeneity magnitude was estimated by I2 (if P < .1, I2 > 40%, it is judged as heterogeneity; if P > .1, I2≤40%, it is judged as no heterogeneity). If there was no heterogeneity among study results, the fixed-effect model was used for meta-analysis; if there was heterogeneity in the results of various studies, the random effect model was used for analysis after excluding the effect of significant clinical heterogeneity. Odds risk (OR) was used to describe count data, and mean difference was used to describe continuous variables with the same units of measurement. Summary effect measures are presented together with their corresponding 95 per cent confidence intervals (95% CI). P < .05 was considered statistically significant.
3. Results
3.1. Study selection
The systematic literature search produced 2610 matches for this study. Among them, 828 duplicate publications and 1754 irrelevant publications were excluded. Of remaining 28 publications, 12 met the inclusion criteria after careful full-text review (Fig. 2).[11–22] We have identified 5726 patients including 3084 in RAD and 2642 in NRAD group, respectively.
Figure 2.
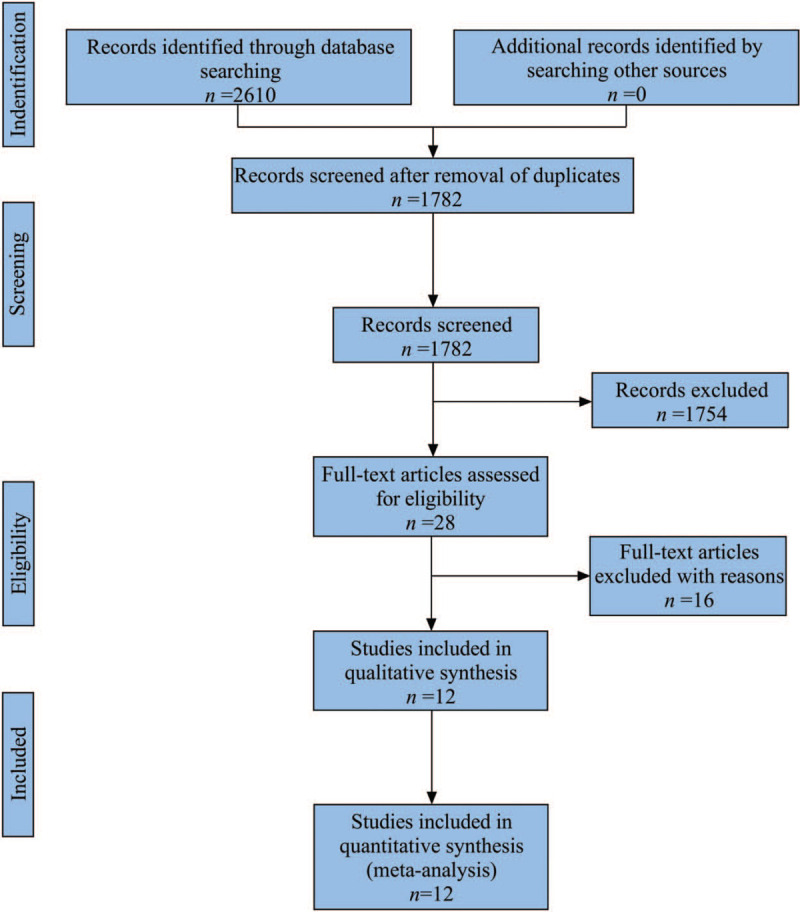
PRISMA flow chart showing selection of articles for review.
3.2. Pooled results for incidence of ascites
Ten studies reported the incidence of postoperative ascites in both groups. The results of I2 suggested no significant heterogeneity among studies (P = .56, I2 = 0%), and the fixed-effect model was selected for analysis. The results showed higher incidence of ascites in NRAD group than that in RAD group [OR = 0.72, 95% CI (0.52, 0.98), P = .04] (Fig. 3A).
Figure 3.

Pooled analysis of incidence of post-operative ascites/intra-abdominal hemorrhage/wound complications/ bile leakage in patients undergoing elected hepatectomy treated with and without routine abdominal drainage.
3.3. Pooled results for incidence of intra-abdominal hemorrhage
Six studies reported the incidence of postoperative intra-abdominal hemorrhage in both groups. No significant heterogeneity was observed among studies (P = .96, I2 = 0%), and the fixed-effect model was selected. The results showed no significant difference between RAD and NRAD groups. [OR = 1.77, 95% CI (0.61, 5.09), P = .29] (Fig. 3B).
3.4. Pooled results for incidence of wound complications
Ten studies reported the incidence of postoperative wound complications in both groups. Heterogeneity existed among studies (P < .00001, I2 = 85%), and thus random effects model was used for analysis. The results showed no significant difference between the 2 groups [OR = 2.03, 95% CI (1.01, 4.06), P = .05] (Fig. 3C).
3.5. Pooled results for incidence of postoperative bile leakage
The incidence of postoperative bile leakage was reported in all included studies both for RAD and NRAD group. Heterogeneity was significant among studies (P = .04, I2 = 49%), and therefore random effects model was selected for analysis. Higher incidence of postoperative bile leakage was shown in RAD group compare to NRAD group [OR = 2.41, 95% CI (1.48, 3.91), P = .0004] (Fig. 3D).
3.6. Pooled results for incidence of postoperative liver failure
Nine studies reported the incidence of postoperative liver failure in both groups. No significant heterogeneity among studies were shown (P = .60, I2 = 0%), and the fixed-effect model was selected for analysis. The results showed no significant difference between the 2 groups regarding postoperative liver failure [OR = 1.09, 95% CI (0.85, 1.40), P = .49] (Fig. 4A).
Figure 4.
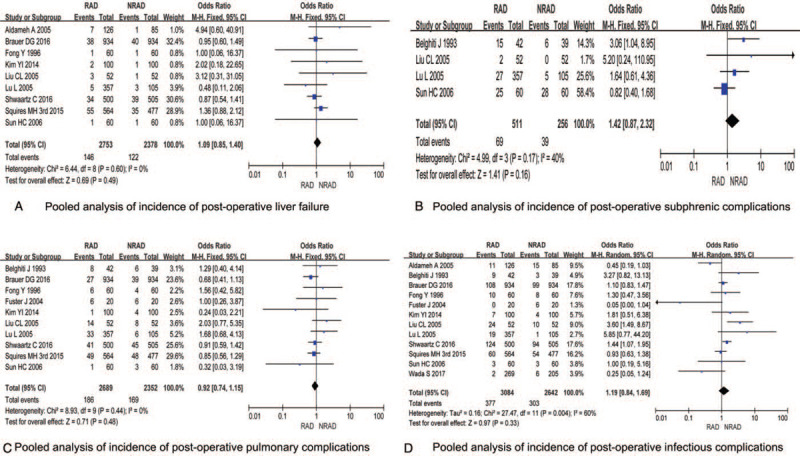
Pooled analysis of incidence of post-operative liver failure/subphrenic complications/ pulmonary complications/infectious complications in patients undergoing elected hepatectomy treated with and without routine abdominal drainage.
3.7. Pooled results for incidence of subphrenic complications
Four studies reported the incidence of postoperative subphrenic complications in both groups. No significant heterogeneity among studies (P = .17, I2 = 40%), and the fixed-effect model was selected for analysis. The results showed no significant difference between the 2 groups [OR = 1.42, 95% CI (0.87, 2.32), P = .16] (Fig. 4B).
3.8. Pooled results for incidence of postoperative pulmonary complications
Ten studies reported the incidence rate of postoperative pulmonary complications in both groups. No significant heterogeneity among studies was found (P = .44, I2 = 0%), and the fixed-effect model was selected for analysis. The results showed no significant difference between the 2 groups [OR = 0.92, 95% CI (0.74, 1.15), P = .48] (Fig. 4c).
3.9. Pooled results for incidence of postoperative infectious complications
All 12 studies reported the incidence of postoperative infectious complications in the RAD and NRAD groups. The results of heterogeneity test showed statistical heterogeneity among studies (P = .004, I2 = 60%), and random effects model was selected for analysis. The results showed no significant difference between the 2 groups [OR = 1.19, 95% CI (0.84, 1.69), P = .33] (Fig. 4D).
3.10. Pooled results for incidence of postoperative reoperation
The incidence of postoperative reoperation in RAD group and NRAD group was reported in 11 included studies. No significant heterogeneity among studies was found (P = .14, I2 = 33%), and the fixed-effect model was selected for analysis. The results showed no significant difference between the 2 groups [OR = 1.37, 95% CI (1.00, 1.87), P = .05] (Fig. 5A).
Figure 5.
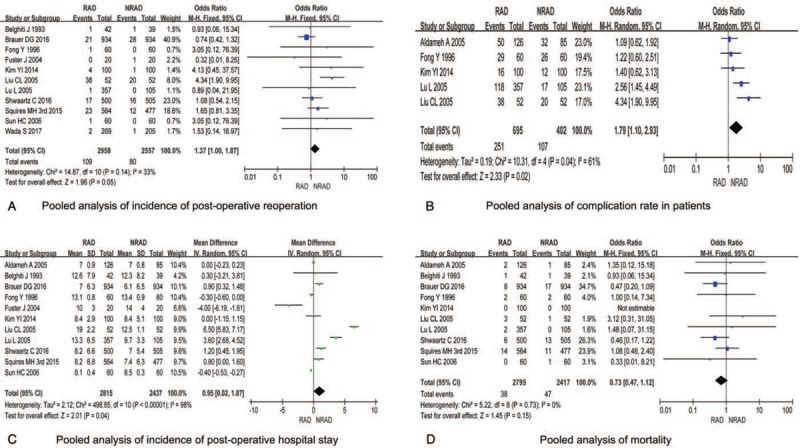
Pooled analysis of incidence of post-operative reoperation/complication rate/post-operative hospital stay/mortality in patients undergoing elected hepatectomy treated with and without routine abdominal drainage.
3.11. Pooled results for the overall complication rate
Five studies reported the overall complication rate in both groups. Heterogeneity was found among studies (P = .04, I2 = 61%), and random model was selected for analysis. Higher overall complication rate was shown in RAD compare to NRAD group [OR = 1.79, 95% CI (1.10, 2.93), P = .02] (Fig. 5B).
3.12. Pooled results for postoperative hospital stay
All 12 studies reported the comparison of postoperative hospital stay in both groups. The relevant data in a literature[22] are expressed in the form of median, which cannot be counted. Therefore, no statistical analysis is performed in the figure. The results of heterogeneity test showed statistical heterogeneity among studies (P < .00001, I2 = 98%), and random effects model was selected for analysis. The results showed that the postoperative hospital stay was shorter in NRAD compare to RAD group [mean difference = 0.95, 95% CI (0.02, 1.87), P = .04] (Fig. 5C).
3.13. Pooled results for postoperative mortality
Ten studies reported postoperative mortality in both groups. No significant heterogeneity was found among studies (P = 0.73, I2 = 0%), and the fixed-effect model was selected for analysis. The results showed no significant difference between the 2 groups [OR = 0.73, 95% CI (0.47, 1.12), P = .15] (Fig. 5D).
3.14. Publication bias analysis
The results of funnel plot analysis using the incidence of post-operative ascites, intra-abdominal hemorrhage and mortality showed that the symmetry of the funnel plot was good, and all scattered points in the plot were distributed in the funnel, indicating that publication bias had little effect on the results of the meta-analysis (Fig. 6).
Figure 6.
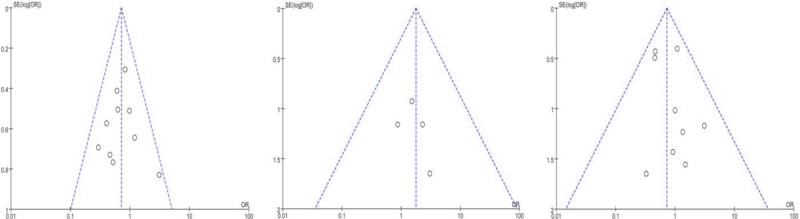
Funnel plot of meta-analysis of literature publication bias.
4. Discussion
Routine abdominal drainage was considered to play important role in collecting post-hepatectomy abdominal fluid and thus help to diagnose intra-abdominal bleeding and biliary leakage during elective hepatectomy.[2,3,23] For such reasons, abdominal drainage has been a worldwide accepted as a routine procedure after abdominal surgery.[24] More recently, the surgical advancement, introduction of enhanced recovery after surgery and “TUBELESS” surgical practice urged surgeons to perform hepatic resection without routine abdominal drainage as long as possible and yielded better clinical outcome.[25] Recent studies showed that non-routine abdominal drainage after elective hepatic resection was associated with lower post-operative morbidity.[11,14]
This meta-analysis claimed that routine abdominal drainage after elective hepatic resection was associated with increased rate of overall complication, postoperative bile leakage and post-operative hospital stay compared to patients with no abdominal drainage. Our results support the practice of “tubeless” resection during hepatic surgery with lower morbidity and higher quality of life in selected patients.
The main reason for using drainage was better observation of postoperative intra-abdominal conditions.[26] Bleeding is 1 of the drastic complications after hepatectomy. Routine abdominal drainage may seem to be helpful for potential postoperative bleeding, however, postoperative bleeding rarely occurs after elective hepatectomy. Even if the patient with postoperative massive bleeding could be judged by electrocardiogram monitoring of vital signs and laboratory tests, reoperation could be applied timely as long as it is necessary.[27] Besides, drainage placement is only observational and has no favor for hemostasis.
Biliary leakage is one of the common complications after hepatic resection. Our results showed that routine abdominal drainage increases postoperative biliary leakage and mostly happen after drainage removal. This is possibly due to the re-opening of biliary ducts after irritated by tube removal. Whenever postoperative biliary leakage happens, percutaneous puncture and drainage under ultrasound and CT guidance could easily solve the situation.[28] Moreover, routine placement of drainage tube may increase complication such as retrograde infection, pain, possible bowel injury and loss of excessive ascites. Furthermore, NRAD may reduce costs and promote early ambulation of the patients. The abdominal drainage tube restricts the patient's activities, aggravates patient's pain as well as increases the workload of medical staff.[29]
We have some limitations for current study. Surgical skills may vary for different center and this may influence on the placement of drainage and postoperative complications. Besides, both RCTs and retrospective controlled studies were included analysis and this may increase the selection bias.
5. Conclusion
Although routine abdominal drainage may provide better observation in patients after hepatectomy, however, it is associated with increased postoperative morbidity and longer hospital stays. Hepatectomy with non-abdominal drainage is practicable in high volume center and sophisticated hands with acceptable clinical outcomes.
Author contributions
TT and LT developed study concept. Z JM helped and gave methodological advice. G SS and WJ carried out the literature search and data extraction. NA, SA and ZQ conducted statistical analysis. NA, SA and ZQ wrote the first draft of the manuscript. TT, Z JM and LT provided scientific input for the study's background and rationale. SA and NA interpreted the results. All authors read, critically revised, and approved the final manuscript.
Conceptualization: Shensen Gu, Tuerhongjiang Tuxun.
Data curation: Nuerzatijiang Anweier, Shadike Apaer, Qi Zeng, Jing Wu.
Formal analysis: Nuerzatijiang Anweier, Shadike Apaer, Qi Zeng.
Funding acquisition: Tuerhongjiang Tuxun.
Investigation: Jing Wu.
Resources: Shadike Apaer, Jing Wu, Shensen Gu.
Software: Shadike Apaer, Qi Zeng, Jing Wu.
Supervision: Tao Li, Jinming Zhao, Tuerhongjiang Tuxun.
Validation: Jinming Zhao.
Writing – original draft: Nuerzatijiang Anweier, Shadike Apaer.
Writing – review & editing: Tao Li, Tuerhongjiang Tuxun.
Glossary
Abbreviations: NRAD = non-routine abdominal drainage, OR = odds risk, RAD = routine abdominal drainage.
References
- [1].Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg 2014;260:329–40. [DOI] [PubMed] [Google Scholar]
- [2].Uetsuji S, Kwon AH, Komada H, et al. Clinical evaluation of closed suction drainage following hepatectomy. Surg Today 1997;27:298–301. [DOI] [PubMed] [Google Scholar]
- [3].Bona S, Gavelli A, Huguet C. The role of abdominal drainage after major hepatic resection. Am J Surg 1994;167:593–5. [DOI] [PubMed] [Google Scholar]
- [4].Antoniou S, Koch O, Antoniou G, et al. Routine versus no drain placement after elective laparoscopic cholecystectomy: meta-analysis of randomized controlled trials. Minerva Chir 2014;69:185–94. [PubMed] [Google Scholar]
- [5].van der Wilt AA, Coolsen MM, de Hingh IH, et al. To drain or not to drain: a cumulative meta-analysis of the use of routine abdominal drains after pancreatic resection. HPB (Oxford) 2013;15:337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yamashita S, Ishizawa T, Ichida A, et al. Advantages and disadvantages of prophylactic abdominal drainage in distal pancreatectomy. World J Surg 2016;40:1226–35. [DOI] [PubMed] [Google Scholar]
- [7].Jeong O, Kim HG. Implementation of Enhanced Recovery after Surgery (ERAS) program in perioperative management of gastric cancer surgery: a nationwide survey in Korea. J Gastric Cancer 2019;19:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wong-Lun-Hing EM, van Woerden V, Lodewick TM, et al. Abandoning prophylactic abdominal drainage after hepatic surgery: 10 years of no-drain policy in an enhanced recovery after surgery environment. Dig Surg 2017;34:411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xiuwen Wu, Weiliang Tian, Nejla Zeynep Kubilay, et al. Is it necessary to place prophylactically an abdominal drain to prevent surgical site infection in abdominal operations? A systematic Meta-review. Surg Infect 2016;17:730–8. [DOI] [PubMed] [Google Scholar]
- [10].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Belghiti J, Kabbej M, Sauvanet A, et al. Drainage after elective hepatic resection. A randomized trial. Ann Surg 1993;218:748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fong Y, Brennan MF, Brown K, et al. Drainage is unnecessary after elective liver resection. Am J Surg 1996;171:158–62. [DOI] [PubMed] [Google Scholar]
- [13].Fuster J, Llovet JM, Garcia-Valdecasas JC, et al. Abdominal drainage after liver resection for hepatocellular carcinoma in cirrhotic patients: a randomized controlled study. Hepatogastroenterology 2004;51:536–40. [PubMed] [Google Scholar]
- [14].Liu CL, Fan ST, Lo CM, et al. Abdominal drainage after hepatic resection is contraindicated in patients with chronic liver diseases. Ann Surg 2004;239:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aldameh A, McCall JL, Koea JB. Is routine placement of surgical drains necessary after elective hepatectomy? Results from a single institution. J Gastrointest Surg 2005;9:667–71. [DOI] [PubMed] [Google Scholar]
- [16].Lu L, Sun HC, Qin LX, et al. Abdominal drainage was unnecessary after hepatectomy using the conventional clamp crushing technique. J Gastrointest Surg 2006;10:302–8. [DOI] [PubMed] [Google Scholar]
- [17].Sun HC, Qin LX, Lu L, et al. Randomized clinical trial of the effects of abdominal drainage after elective hepatectomy using the crushing clamp method. Br J Surg 2006;93:422–6. [DOI] [PubMed] [Google Scholar]
- [18].Kim YI, Fujita S, Hwang VJ, et al. Comparison of abdominal drainage and no-drainage after elective hepatectomy: a randomized study. Hepatogastroenterology 2014;61:707–11. [PubMed] [Google Scholar]
- [19].Squires MH, 3rd, Lad NL, Fisher SB, et al. Value of primary operative drain placement after major hepatectomy: a multi-institutional analysis of 1041 patients. J Am Coll Surg 2015;220:396–402. [DOI] [PubMed] [Google Scholar]
- [20].Shwaartz C, Fields AC, Aalberg JJ, et al. Role of drain placement in major hepatectomy: a NSQIP analysis of procedure-targeted hepatectomy cases. World J Surg 2016;41:1–9. [DOI] [PubMed] [Google Scholar]
- [21].Brauer DG, Nywening TM, Jaques DP, et al. Operative site drainage after hepatectomy: a propensity score matched analysis using the american college of surgeons NSQIP targeted hepatectomy database. J Am Coll Surg 2016;223:774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wada S, Hatano E, Yoh T, et al. Is routine abdominal drainage necessary after liver resection? Surg Today 2017;47:712–7. [DOI] [PubMed] [Google Scholar]
- [23].Sarr MG, Parikh KJ, Minken SL, et al. Closed-suction versus Penrose drainage after cholecystectomy. A prospective, randomized evaluation. Am J Surg 1987;153:394–8. [DOI] [PubMed] [Google Scholar]
- [24].McMillan MT, Malleo G, Bassi C, et al. Defining the practice of pancreatoduodenectomy around the world. HPB (Oxford) 2015;17:1145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kaibori M, Matsui K, Ishizaki M, et al. Effects of implementing an “enhanced recovery after surgery” program on patients undergoing resection of hepatocellular carcinoma. Surg Today 2017;47:42–51. [DOI] [PubMed] [Google Scholar]
- [26].Burt BM, Brown K, Jarnagin W, et al. An audit of results of a no-drainage practice policy after hepatectomy. Am J Surg 2002;184:441–5. [DOI] [PubMed] [Google Scholar]
- [27].Yang J, Zhang XH, Huang YH, et al. Diagnosis and treatment of abdominal arterial bleeding after radical gastrectomy: a retrospective analysis of 1875 consecutive resections for gastric cancer. J Gastrointest Surg 2016;20:510–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jin S, Fu Q, Wuyun G, et al. Management of post-hepatectomy complications. World J Gastroenterol 2013;19:7983–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yong Lv, Guang Bai. Abdominal drainage versus no abdominal drainage for laparoscopic cholecystectomy: A systematic review with meta-analysis and trial sequential analysis. Int J Surg 2016;36:358–68. [DOI] [PubMed] [Google Scholar]


