Abstract
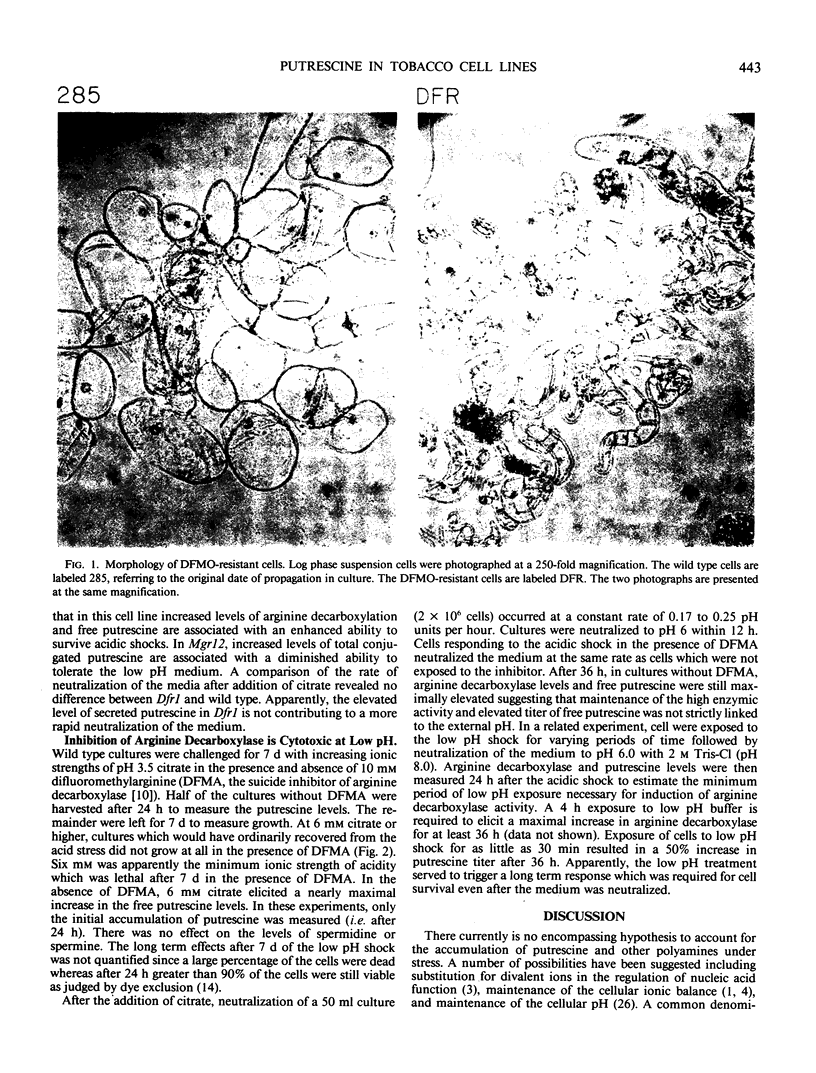
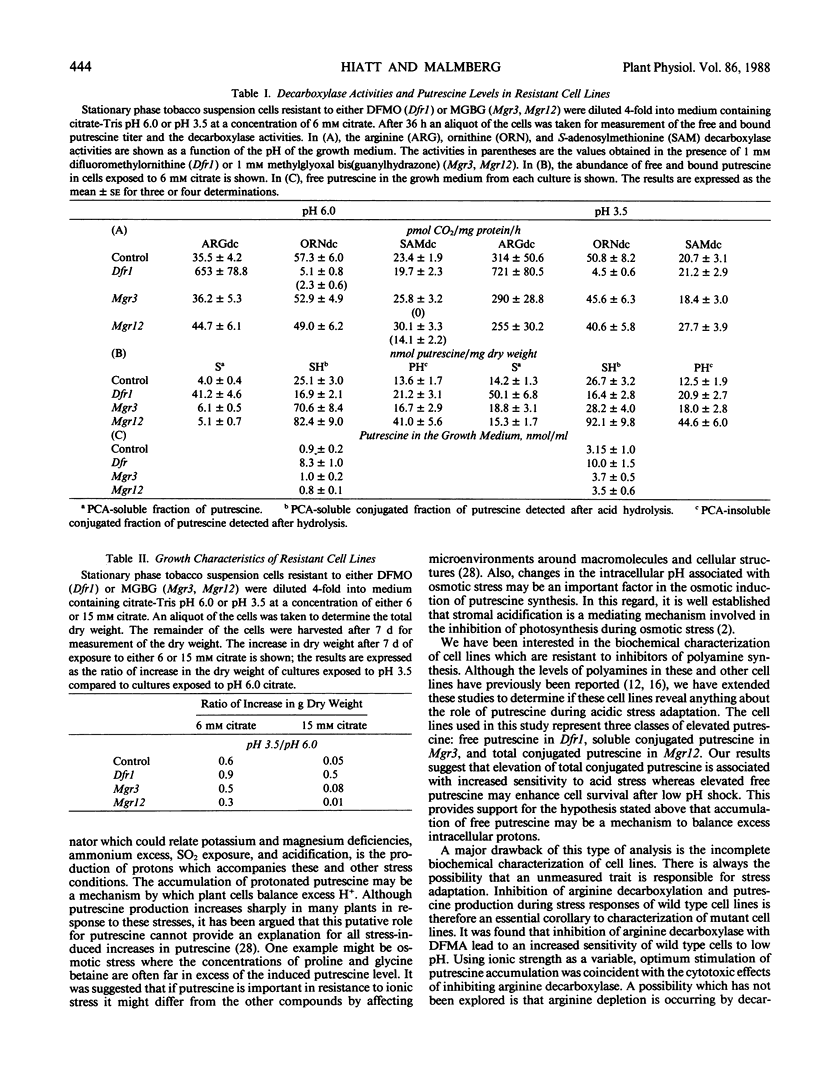
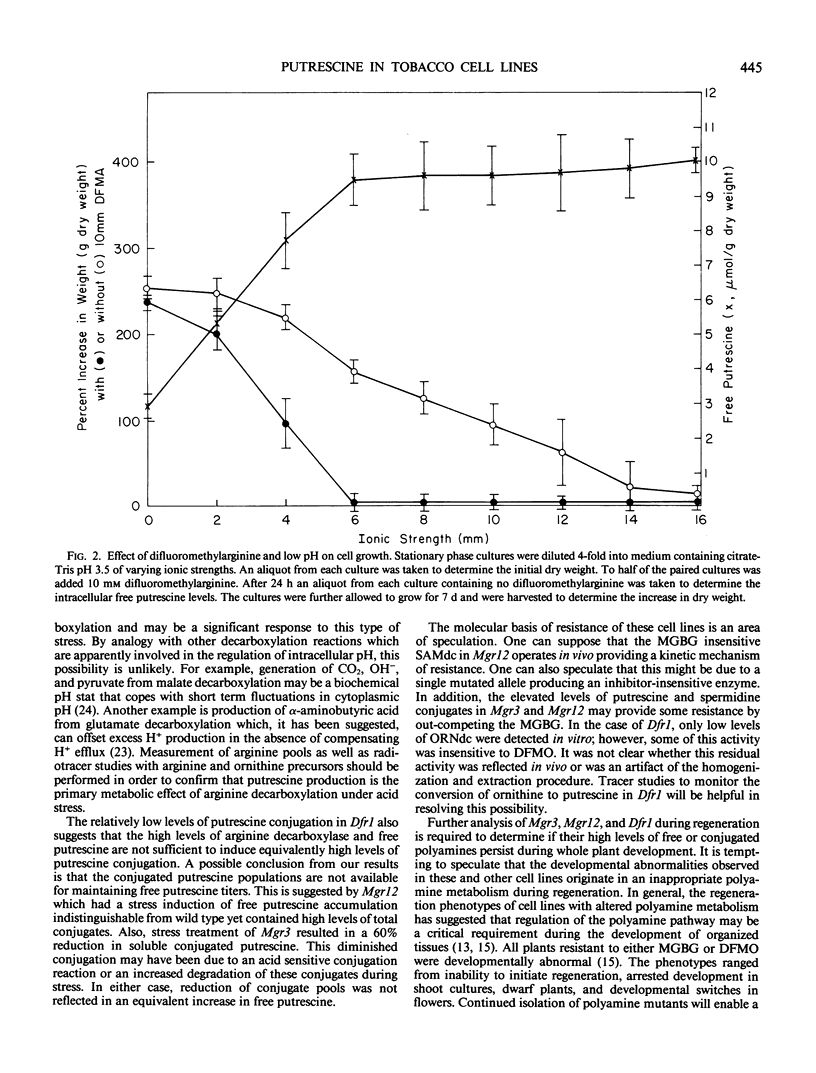
Three tobacco cell lines have been analyzed which are resistant to lethal inhibitors of either putrescine production or conversion of putrescine into polyamines. Free and conjugated putrescine pools, the enzymic activities (arginine, ornithine, and S-adenosylmethionine decarboxylases), and the growth characteristics during acidic stress were measured in suspension cultures of each cell line. One cell line, resistant to difluoromethylornithine (Dfr1) had a very low level of ornithine decarboxylase activity which was half insensitive to the inhibitor in vitro. Intracellular free putrescine in Dfr1 was elevated 10-fold which was apparently due to a 20-fold increase in the arginine decarboxylase activity. The increased free putrescine titer was not reflected in an increased level of spermidine, spermine, or putrescine conjugation. Dfr1 cultures survived acidic stress at molarities which were lethal to wild type cultures. Two other mutants, resistant to methylglyoxal bis(guanylhydrazone) (Mgr3, Mgr12), had near normal levels of the three decarboxylases and normal titers of free putrescine, spermidine, and spermine. Both mutants however had elevated levels of conjugated putrescine. Mgr12 had an increased sensitivity to acidic medium. These results suggest that increased levels of free putrescine production may enhance the ability of tobacco cells to survive acid stress. This was supported by the observation that cytotoxic effects of inhibiting arginine decarboxylase in wild type cell lines were dependent on the acidity of the medium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowitz G. A., Gibbs M. Reduced osmotic potential effects on photosynthesis : identification of stromal acidification as a mediating factor. Plant Physiol. 1983 Apr;71(4):905–911. doi: 10.1104/pp.71.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores H. E., Galston A. W. Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol. 1982 Mar;69(3):701–706. doi: 10.1104/pp.69.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores H. E., Galston A. W. Osmotic stress-induced polyamine accumulation in cereal leaves : I. Physiological parameters of the response. Plant Physiol. 1984 May;75(1):102–109. doi: 10.1104/pp.75.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores H. E., Galston A. W. Polyamines and plant stress: activation of putrescine biosynthesis by osmotic shock. Science. 1982 Sep 24;217(4566):1259–1261. doi: 10.1126/science.217.4566.1259. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Malmberg R. L. Biochemical, cellular and developmental characterization of a temperature-sensitive mutant of Nicotiana tabacum and its second site revertant. Cell. 1980 Nov;22(2 Pt 2):603–609. doi: 10.1016/0092-8674(80)90370-0. [DOI] [PubMed] [Google Scholar]
- Malmberg R. L., McIndoo J., Hiatt A. C., Lowe B. A. Genetics of polyamine synthesis in tobacco: developmental switches in the flower. Cold Spring Harb Symp Quant Biol. 1985;50:475–482. doi: 10.1101/sqb.1985.050.01.059. [DOI] [PubMed] [Google Scholar]
- Malmberg R. L. Temperature-Sensitive Variants of NICOTIANA TABACUM Isolated from Somatic Cell Culture. Genetics. 1979 May;92(1):215–221. doi: 10.1093/genetics/92.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. A. The occurrence, metabolism and functions of amines in plants. Biol Rev Camb Philos Soc. 1971 May;46(2):201–241. doi: 10.1111/j.1469-185x.1971.tb01182.x. [DOI] [PubMed] [Google Scholar]
- Young N. D., Galston A. W. Putrescine and Acid Stress : Induction of Arginine Decarboxylase Activity and Putrescine Accumulation by Low pH. Plant Physiol. 1983 Apr;71(4):767–771. doi: 10.1104/pp.71.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]



