Abstract
Igarashi T, Hayashi S, Ogawa K, Matsui S, Nishimatsu T. Relationship between daily rehabilitation time and functional gain in inpatient rehabilitation medicine of hospitalized older adults with subacute stroke. Jpn J Compr Rehabil Sci 2022; 13: 56-63.
Objective
Although there have been reports examining the relationship between daily rehabilitation time and functional gain, few have fully considered background factors such as severity of motor paralysis and comorbidities. This study aimed to examine the relationship between the daily rehabilitation time and improvement in functional status, longitudinally in hospitalized older adults with subacute stroke.
Method
From the results of the Functional Independence Measure (FIM), we calculated the FIM gain and FIM effectiveness, a measure that is less sensitive to the ceiling effect of FIM. Adjusted for covariates, multiple regression analysis was performed for daily rehabilitation time and FIM gain and effectiveness.
Results
This study enrolled 298 hospitalized older adults with subacute stroke (mean age, 78.1 ± 8.1 years, 112 females). The total scores of functional independence measure gain and effectiveness were 31.6 ± 22.5 points and 54.4 ± 35.2%, respectively. There was an association between FIM gain (total score) and total rehabilitation time (β = 0.29, p < 0.01) and between FIM effectiveness (total score) and total rehabilitation time (β = 0.22, p < 0.01).
Conclusions
Although prognosis after stroke is poorer in older adults than in young adults, this study shows that increased daily rehabilitation time may improve functional status.
Keywords: functional limitation, stroke, acute phase, rehabilitation, functional recovery
Introduction
Stroke is the second leading cause of death and the third leading cause of disability worldwide [1]. Furthermore, stroke-related costs due to healthcare services and medications are rising [2]. Therefore, improvement in post-stroke functional impairment and disability is one of the main goals of rehabilitation interventions [3].
Older adults have an increased incidence of comorbidities and multimorbidity [4]. It is estimated that more than half of stroke patients aged >65 years have reduced mobility, making it a major cause of serious long-term disability [2]. Acutely hospitalized stroke patients are prone to disuse syndrome due to prolonged bed rest [5], therefore it is important that interventions for physical functions are performed earlier in older stroke patients than in young patients.
Several reports have examined the relationship between rehabilitation time and functional recovery [6, 7, 8, 9, 10, 11]. In inpatients with stroke, increasing the number of days of physical therapy (PT) and occupational therapy (OT) has a positive impact on functional recovery [8, 11]. The amount of PT, OT, and speech-language-hearing therapy (SLT) interventions is associated with the recovery of mobility and cognition [6]. However, although there have been reports examining the relationship between daily rehabilitation time and functional gain, few have fully considered background factors such as severity of motor paralysis and comorbidities. Furthermore, the relationship between the amount of these interventions and functional gain in inpatients with stroke has been shown in middle-aged adults [6] but not in older adults.
We hypothesized that daily rehabilitation time is associated with functional recovery in hospitalized older adults with subacute stroke. This study aimed to determine the relationship between daily rehabilitation time and functional recovery in hospitalized older adults with subacute stroke.
Method
1. Study design
This retrospective observational cohort study was conducted at a single acute care hospital in Japan. Data were collected from consecutive stroke patients admitted to the general wards between June 2018 and October 2020. The study cohorts were identified from clinical databases, and study indicators were extracted. Furthermore, the medical records were reviewed to identify the participants. This study was conducted with the approval of the Ethics Committees at the affiliated institution and in accordance with the Declaration of Helsinki and the “Strengthening the Reporting of Observational Studies in Epidemiology” guidelines (STROBE) [12]. As an ethical consideration, information about the research was disclosed to the participants by posting information in the hospital and on the website. We explained to the participants that they could refuse participation and guaranteed them the opportunity to opt out.
2. Study population
The participants were required to meet all of the following inclusion criteria: 1) age ≥65 years; 2) hospitalization for cerebral infarction or cerebral hemorrhage; 3) length of stay (LOS) ≥7 days; and 4) received rehabilitation 7 days/week. The exclusion criteria were as follows: 1) perfect functional independence measure (FIM) score during initial evaluation; 2) LOS ≥180 days; 3) fatal cases; 4) worsening medical conditions; 5) no motor paralysis; or 6) hospitalization for subarachnoid hemorrhage. Because functional recovery differs between cases of subarachnoid hemorrhage and other stroke subtypes, patients diagnosed with subarachnoid hemorrhage were excluded [13]. The sample size for determining the linear multiple regression was calculated using G*Power, version 3.1.9.3 (Heinrich Heine University, Düsseldorf, Germany) before enrollment. The sample size was determined as 238, based on effect size f 2 of 0.15, α-error probability of 0.05, and 1−β error probability of 0.95, which was considered sufficient to confirm a correlation.
3. Interventions
All participants received daily PT and OT as rehabilitation interventions and SLT as needed. Under Japan's public medical insurance system, rehabilitation is covered by insurance. The amount of rehabilitation therapy covered by insurance for acute stroke is limited to 3 h/day. The rehabilitation time was determined by the physician and medical team, considering the condition of each participant. The PT/OT/SLT intervention requirements were assessed based on the International Classification of Functioning, Disability, and Health and individualized by the participant's primary physician based on the treatment goals. PT included muscle strengthening exercises, static and dynamic balance exercises, walking exercises, electrical stimulation therapy, and ergometer exercises. OT included upper limb function and daily living activities exercises, while SLT included higher brain function and swallowing exercises. The intervention was not controlled for in this study. The average LOS for acute stroke patients in Japan is approximately 29.5 days [14]. Due to the characteristics of the Japanese healthcare system, acute care hospitals have consistent clinical management from the acute phase to the subacute phase of stroke [8].
4. Data collection
All information was collected from a medical records database. Demographic and clinical characteristics collected during the initial evaluation included age, sex, stroke type, lesion location, stroke treatment, comorbidities, LOS, discharge destination, unilateral spatial neglect and aphasia, time to start of rehabilitation after admission, premorbid degree of disability, and severity of motor paralysis. The premorbid degree of disability was assessed using the modified Rankin Scale (mRS) [15], and the severity of motor paralysis by Brunnstrom Recovery Stage (BRS) [16].
The functional status was assessed at 1 week after hospitalization (admission FIM) and discharge (discharge FIM) using FIM [17]. FIM assesses the performance of instrumental activities of daily living and comprises 13 motor and 5 cognitive measures. Each item is scored on a scale of 1-7, with the total score in the range of 18-126; the scores are distributed between 13-91 and 5-35 for the motor and cognitive items, respectively. A lower score indicates less independence in activities of daily living, while a higher score indicates greater independence. FIM has been shown to be reliable and valid as a functional status index in subacute stroke patients [18]. The FIM assessment was performed by a physical therapist, occupational therapist, and speech-language-hearing therapist with a thorough understanding of the evaluation method.
The daily rehabilitation time of each of PT, OT, and SLT during hospitalization and the total rehabilitation time summed were collected from the medical records database. Each of PT, OT, SLT, and total rehabilitation time were divided by LOS and calculated as the average daily rehabilitation time.
5. Statistical analysis
The descriptive statistics of demographic and clinical characteristics are presented as means and standard deviations for continuous variables and rates and frequency distributions for categorical data.
Next, FIM gain and FIM effectiveness were obtained after calculating the descriptive statistics of the initial and final motor items, cognitive items, and total of FIM. FIM gain is a measure of the improvement in FIM scores from admission to discharge, and is calculated as “discharge FIM−admission FIM.” FIM effectiveness is a measure of the percentage of potential improvement in functional status from admission to discharge, and is calculated as “FIM gain / (maximum score−admission FIM)%” [19]. FIM effectiveness has been used as a measure to interpret the degree of potential improvement in functional status by reducing the impact of hospital stay and ceiling effects. Both have been used as intervention outcomes in stroke patients [20, 21]. Both FIM gain and FIM effectiveness were calculated for motor item scores, cognitive item scores, and total scores.
To investigate the relationship between the daily rehabilitation time and functional gain, Pearson's product-moment correlation coefficient (r) between daily rehabilitation time and FIM gain and FIM effectiveness was calculated. The strength of the coefficient was determined as follows: 0.00-0.25, minimum correlation (if any); 0.26-0.49, weak correlation; 0.50-0.69, moderate correlation; 0.70-0.89, strong correlation; and 0.90-1.00, very strong correlation [22].
Next, we calculated two multivariate linear regressions (forced entry method) with FIM gain and FIM effectiveness as the respective dependent variables and daily rehabilitation time as the independent variable. In both models, all clinical characteristics were entered as adjustment variables, except LOS and discharge destination. To account for multicollinearity, the correlation between the independent variables was checked beforehand, and if the correlation coefficient was ≥0.8, one of the independent variables was excluded [23]. Furthermore, the variance inflation factor (VIF) was checked, and multicollinearity was ascertained for VIF ≥10. The goodness of fit of each model was determined by the coefficient of determination (R2).
Furthermore, we performed a partial correlation analysis between daily rehabilitation time and FIM gain and FIM effectiveness, with age, pre-morbid mRS, BRS of the lower extremities, and total FIM total score at admission as control variables.
All statistical analyses were conducted using Statistical Product and Service Solutions, version 25.0 (IBM Corp., Armonk, NY) and Microsoft Excel (Microsoft Corp., Redmond, WA, USA).
Results
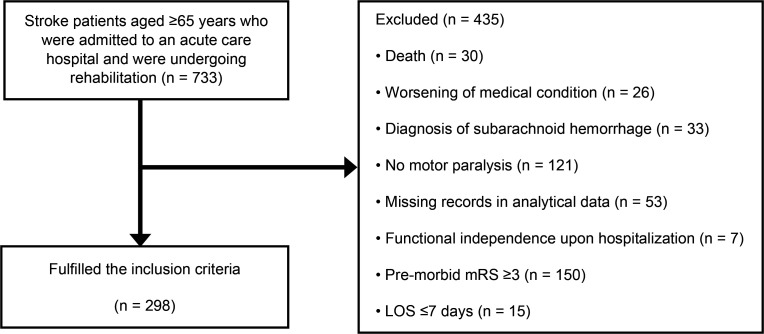
Figure 1 shows the flowchart of the study participants. Of the 733 patients hospitalized for stroke, 298 met the inclusion criteria. Table 1 shows the clinical characteristics of the participants. The total rehabilitation time was 154.8 ± 17.7 min/day. Table 2 shows the descriptive statistics of the FIM score. The total scores for FIM gain and FIM effectiveness were 31.6 ± 22.5 points and 54.4 ± 35.2%, respectively. Table 3 shows the bivariate correlations of daily rehabilitation time with FIM gain and with FIM effectiveness. Daily rehabilitation time and FIM effectiveness showed a weak positive correlation with FIM motor item scores, FIM cognitive item scores, and FIM total scores. Table 4 shows the results of multivariate linear regression with total scores of FIM gain and FIM effectiveness as the dependent variables. Daily rehabilitation time was adopted as a significant independent variable for both model FIM gain and FIM effectiveness. R2 had a model FIM gain of 0.499 and model FIM effectiveness of 0.695. In both results, the VIF of the variables was <10, and multicollinearity was absent. Table 5 shows the results of partial correlation analysis between daily rehabilitation time and FIM gain and FIM effectiveness, with age, pre-morbid mRS, BRS of the lower extremities, and total FIM total score at admission as control variables. Partial correlation coefficients between rehabilitation time and total scores of FIM gain and FIM effectiveness were 0.412 and 0.404, respectively.
Figure 1. Flowchart of the study participants.
LOS, length of stay; mRS, modified Rankin Scale.
Table1.
Clinical characteristics of participants.
| Variables | |
|---|---|
| Age (years), mean (SD) | 78.1 (8.1) |
| Sex (female), n (%) | 112 (37.6) |
| Type of stroke (cerebral hemorrhage), n (%) | 57 (19.1) |
| Stroke treatment, n (%) | |
| Conservative treatment | 273 (91.6) |
| Surgical treatment | 6 (2.0) |
| Endovascular treatment | 19 (6.4) |
| Lesion location, n (%) | |
| Basal ganglia and internal capsule | 71 (23.8) |
| Thalamus | 39 (13.1) |
| Corona radiata | 47 (15.8) |
| Brainstem | 32 (10.7) |
| Cerebellum | 9 (3.0) |
| Combined lesions | 54 (18.1) |
| Others | 46 (15.4) |
| LOS (days), mean (SD) | 29.15 (17.4) |
| Time to rehabilitation after admission (days), mean (SD) | 1.34 (1.7) |
| Discharge destination (home), (%) | 154 (51.7) |
| History of diseases, n (%) | |
| Orthopedic diseases | 84 (28.2) |
| Cardiovascular diseases | 82 (27.5) |
| Hypertension | 156 (52.3) |
| Diabetes mellitus | 60 (20.1) |
| CCI (points), mean (SD) | 1.6 (1.4) |
| Aphasia, n (%) | 54 (18.1) |
| Unilateral spatial neglect, n (%) | 66 (22.1) |
| Premorbid mRS (points), mean (SD) | 0.6 (0.8) |
| BRS (points), mean (SD) | |
| Upper limb | 4.2 (2.0) |
| Fingers | 4.1 (2.0) |
| Lower limb | 4.4 (1.8) |
| Daily rehabilitation time (min/day), mean (SD) | |
| PT | 57.2 (10.3) |
| OT | 47.5 (10.7) |
| SLT | 50.1 (13.3) |
| Total rehabilitation time | 154.8 (17.7) |
SD, standard deviation; LOS, length of stay; CCI, Charlson Comorbidity Index; mRS, modified Rankin Scale; BRS, Brunnstrom Recovery Stage; PT, physical therapy; OT, occupational therapy; SLT, speech-language-hearing therapy.
Table 2.
Descriptive statistics of FIM scores.
| Motor item scores | Cognition item scores | Total scores | |
|---|---|---|---|
| Admission (points) | 33.8 ± 21.1 | 20.9 ± 10.8 | 54.7 ± 29.5 |
| Discharge (points) | 60.7 ± 28.9 | 25.6 ± 10.1 | 86.3 ± 37.8 |
| Gain (points) | 26.9 ± 19.9 | 4.7 ± 6.2 | 31.6 ± 22.5 |
| Effectiveness (%) | 55.5 ± 36.6 | 33.2 ± 41.4 | 54.4 ± 35.2 |
The FIM gain is calculated as “discharge FIM−admission FIM,” and the FIM effectiveness as “FIM-gain / (maximum score−admission FIM).” Values are presented as means ± standard deviations.
FIM, Functional Independence Measure.
Table 3.
Bivariate correlations between daily rehabilitation time and the FIM gain and the FIM effectiveness.
| Total rehabilitation time | ||
|---|---|---|
| FIM gain | Motor item scores | 0.352** |
| Cognition item scores | 0.216** | |
| Total scores | 0.370** | |
| FIM effectiveness | Motor item scores | 0.278** |
| Cognition item scores | 0.275** | |
| Total scores | 0.286** |
Pearson's product-moment correlation coefficient (r). **p < 0.01
FIM, Functional Independence Measure.
Table 4.
Multivariate linear regression (forced entry method) with FIM gain and FIM effectiveness as the dependent variables.
| FIM gain (total scores) | FIM effectiveness (total scores) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| β | p-value | VIF | β | p-value | VIF | |
| Age | −0.26 | ** | 1.44 | −0.22 | ** | 1.44 |
| Sex | ||||||
| Male | Reference | ― | ― | Reference | ― | ― |
| Female | 0.02 | 0.63 | 1.12 | 0.03 | 0.41 | 1.12 |
| Type of stroke | ||||||
| Cerebral infarction | Reference | ― | ― | Reference | ― | ― |
| Cerebral hemorrhage | −0.02 | 0.77 | 1.76 | −0.02 | 0.71 | 1.76 |
| Stroke treatment | ||||||
| Conservative treatment | Reference | ― | ― | Reference | ― | ― |
| Surgical treatment | −0.06 | 0.23 | 1.31 | −0.03 | 0.46 | 1.31 |
| Endovascular treatment | 0.00 | 0.97 | 1.13 | 0.01 | 0.67 | 1.13 |
| Lesion location | ||||||
| Other | Reference | ― | ― | Reference | ― | ― |
| Basal ganglia and internal capsule | −0.11 | 0.08 | 2.05 | −0.10 | * | 2.05 |
| Thalamus | −0.05 | 0.38 | 1.97 | −0.04 | 0.42 | 1.97 |
| Corona radiata | −0.03 | 0.60 | 1.89 | −0.05 | 0.24 | 1.89 |
| Brainstem | 0.02 | 0.72 | 1.75 | 0.00 | 1.00 | 1.75 |
| Cerebellum | 0.01 | 0.85 | 1.27 | −0.01 | 0.76 | 1.27 |
| Combined lesions | −0.04 | 0.55 | 1.86 | −0.05 | 0.31 | 1.86 |
| Time to rehabilitation after admission | −0.10 | * | 1.35 | −0.03 | 0.42 | 1.35 |
| History of orthopedic disease | 0.06 | 0.22 | 1.11 | 0.05 | 0.17 | 1.11 |
| History of cardiovascular disease | 0.03 | 0.56 | 1.17 | 0.01 | 0.86 | 1.17 |
| History of hypertension | 0.01 | 0.77 | 1.15 | 0.00 | 0.95 | 1.15 |
| History of diabetes mellitus | 0.05 | 0.32 | 1.22 | 0.01 | 0.74 | 1.22 |
| CCI | 0.03 | 0.59 | 1.47 | 0.05 | 0.22 | 1.47 |
| Aphasia | −0.05 | 0.26 | 1.25 | −0.03 | 0.46 | 1.25 |
| Unilateral spatial neglect | −0.04 | 0.44 | 1.30 | −0.03 | 0.49 | 1.30 |
| Premorbid mRS | −0.13 | ** | 1.29 | −0.14 | ** | 1.29 |
| BRS (lower limb) | 0.63 | ** | 2.01 | 0.38 | ** | 2.01 |
| FIM total score (admission) | −0.57 | ** | 2.22 | 0.31 | ** | 2.22 |
| Total rehabilitation time | 0.29 | ** | 1.20 | 0.22 | ** | 1.20 |
Model FIM gain: R = 0.706; R2 = 0.499; adjusted R = 0.457, Model FIM effectiveness: R = 0.834; R2 = 0.695; adjusted R = 0.669, **p < 0.01; *p < 0.05
FIM, Functional Independence Measure; VIF, variance inflation factor; LOS, length of stay; CCI, Charlson Comorbidity Index; mRS, modified Rankin Scale; BRS, Brunnstrom Recovery Stage.
Table 5.
Partial correlation analysis between daily rehabilitation time and the FIM gain and the FIM effectiveness controlled for age, pre-morbid mRS, BRS-lower extremity, FIM total score at admission.
| Total rehabilitation time | ||
|---|---|---|
| FIM gain | Motor item scores | 0.387** |
| Cognition item scores | 0.176* | |
| Total scores | 0.412** | |
| FIM effectiveness | Motor item scores | 0.415** |
| Cognition item scores | 0.209** | |
| Total scores | 0.404** |
Partial correlation coefficient. **p < 0.01; *p < 0.05
mRS, modified Rankin Scale; BRS, Brunnstrom Recovery Stage; FIM, Functional Independence Measure.
Discussion
In this study, we determined the relationship between the amount of daily rehabilitation and the functional recovery in hospitalized older adults with subacute stroke. The results showed that daily rehabilitation time was associated with FIM gain independently of other variables such as comorbidities and functional disabilities.
There was a positive correlation between FIM gain (total scores) and daily rehabilitation time, with a higher correlation coefficient than those reported in previous studies [6]. Compared to the study by Wang et al. [6], this study included older and more independent individuals and the daily rehabilitation time was shorter. Similar to the results of this study, the age and FIM total score (admission) showed an independent association with FIM gain in acute stroke patients in previous studies [24, 25]. In this study, the average intervention time for PT, OT, and SLT was 57, 47, and 50 min/day, with relatively uniform time provided for each intervention type. On the other hand, a report on middle-aged stroke patients [6] showed a large difference in the intervention time depending on the type of rehabilitation. Prolongation of each rehabilitation time contributes to functional improvement even in older stroke patients. Similar to FIM gain, FIM effectiveness was independently associated with daily rehabilitation time, supporting the results of a previous study on stroke patients admitted to a convalescence rehabilitation hospital [11]. The severity of motor paralysis and premorbid function were associated with the total FIM scores [26, 27] as well as independently associated with FIM effectiveness. Lesion location in the basal ganglia and internal capsule was independently associated with FIM effectiveness. The degree of damage to the corticospinal tract is associated with the severity of motor paralysis [28], suggesting that it also affects the functional status of patients with subacute stroke.
This study has several limitations. First, we collected data on the amount of daily rehabilitation from the medical records, but the intensity of the interventions was not controlled. The content and intensity of rehabilitation interventions in stroke patients have been reported in several previous studies [29, 30, 31, 32, 33] and were expected to be highly dependent on the patient demographics and the experience and skills of the therapist. Second, in order to eliminate ceiling effects, those with a perfect FIM score during the initial assessment were excluded from the analysis. Therefore, selection bias must be taken into account when interpreting the results. However, the mean and standard deviation of the FIM total score at admission were generally similar to those reported in a previous study [8], and we believe that the target of this study was typical acute stroke severity.
This study clarified the relationship between the amount of daily rehabilitation and functional recovery in hospitalized older adults with subacute stroke. Daily rehabilitation time showed a positive correlation with FIM gain and with FIM effectiveness. The results of this study provide useful evidence for the implementation of stroke rehabilitation in hospitalized older adults with subacute stroke.
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet 2017; 390: 1211-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al.. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 2020; 141: e139-e596. [DOI] [PubMed] [Google Scholar]
- 3.Bohannon RW, Andrews AW, Smith MB.. Rehabilitation goals of patients with hemiplegia. Int J Rehabil Res 1988; 11: 181-4. [Google Scholar]
- 4.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B.. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012; 380: 37-43. [DOI] [PubMed] [Google Scholar]
- 5.Hokstad A, Indredavik B, Bernhardt J, Langhammer B, Gunnes M, Lundemo C, et al.. Upright activity within the first week after stroke is associated with better functional outcome and health-related quality of life: a Norwegian multi-site study. J Rehabil Med 2016; 48: 280-6. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Camicia M, Terdiman J, Mannava MK, Sidney S, Sandel ME.. Daily treatment time and functional gains of stroke patients during inpatient rehabilitation. PM R 2013; 5: 122-8. [DOI] [PubMed] [Google Scholar]
- 7.DiSotto-Monastero M, Chen X, Fisch S, Donaghy S, Gomez M.. Efficacy of 7 days per week inpatient admissions and rehabilitation therapy. Arch Phys Med Rehabil 2012; 93: 2165-9. [DOI] [PubMed] [Google Scholar]
- 8.Kinoshita S, Momosaki R, Kakuda W, Okamoto T, Abo M.. Association between 7 days per week rehabilitation and functional recovery of patients with acute stroke: a retrospective cohort study based on the Japan rehabilitation database. Arch Phys Med Rehabil 2017; 98: 701-6. [DOI] [PubMed] [Google Scholar]
- 9.Lohse KR, Lang CE, Boyd LA.. Is more better? Using meta- data to explore dose-response relationships in stroke rehabilitation. Stroke 2014; 45: 2053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, et al.. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One 2014; 9: e87987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimura Y, Suzuki M, Ichikawa T, Otobe Y, Koyama S, Tanaka S, et al.. Effects of different rehabilitation provision systems on functional recovery in patients with subacute stroke. PM R 2021; Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al.. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int J Surg 2014; 12: 1495-9.25046131 [Google Scholar]
- 13.Westerkam WR, Cifu DX, Keyser L.. Functional outcome after inpatient rehabilitation following aneurysmal subarachnoid hemorrhage: a prospective analysis. Top Stroke Rehabil 1997; 4: 29-37. [Google Scholar]
- 14.Kinoshita S, Kakuda W, Momosaki R, Yamada N, Sugawara H, Watanabe S, et al.. Clinical management provided by board-certificated physiatrists in early rehabilitation is a significant determinant of functional improvement in acute stroke patients: a retrospective analysis of Japan Rehabilitation Database. J Stroke Cerebrovasc Dis 2015; 24: 1019-24. [DOI] [PubMed] [Google Scholar]
- 15.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J.. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604-7. [DOI] [PubMed] [Google Scholar]
- 16.Brunnstrom S.. Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther 1966; 46: 357-75. [DOI] [PubMed] [Google Scholar]
- 17.Data management service of the Uniform Data System for Medical Rehabilitation and the Center for Functional Assessment Research. Guide for use of the Uniform Data Set for Medical Rehabilitation. version 3.1, State University of New York at Buffalo, Buffalo, 1990. [Google Scholar]
- 18.Hsueh I-P, Lin J-H, Jeng J-S, Hsieh C-L.. Comparison of the psychometric characteristics of the functional independence measure, 5 item Barthel index, and 10 item Barthel index in patients with stroke. J Neurol Neurosurg Psychiatry 2002; 73: 188-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh GCH, Chen CH, Petrella R, Thind A.. Rehabilitation impact indices and their independent predictors: a systematic review. BMJ Open 2013; 3: e003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monaco MD, Schintu S, Dotta M, Barba S, Tappero R, Gindri P.. Severity of unilateral spatial neglect is an independent predictor of functional outcome after acute inpatient rehabilitation in individuals with right hemispheric stroke. Arch Phys Med Rehabil 2011; 92: 1250-6. [DOI] [PubMed] [Google Scholar]
- 21.Spaccavento S, Cellamare F, Falcone R, Loverre A, Nardulli R.. Effect of subtypes of neglect on functional outcome in stroke patients. Ann Phys Rehabil Med 2017; 60: 376-81. [DOI] [PubMed] [Google Scholar]
- 22.Domholdt E.. Physical Therapy Research: Principles and Applications. 2nd ed. Philadelphia, PA: WB Saunders Co, pp. 347-335, 2000. [Google Scholar]
- 23.Katz MH.. Multivariable Analysis: A Practical Guide for Clinicians. Cambridge: Cambridge University Press, 1999. [Google Scholar]
- 24.Mizrahi EH, Fleissig Y, Arad M, Adunsky A.. Functional gain following rehabilitation of recurrent ischemic stroke in the elderly: experience of a post-acute care rehabilitation setting. Arch Gerontol Geriatr 2015; 60: 108-11. [DOI] [PubMed] [Google Scholar]
- 25.Ng Y, Tan K, Chen C, Senolos G, Koh G.. How do recurrent and first-ever strokes differ in rehabilitation outcomes? Am J Phys Med Rehabil 2016; 95: 709-17. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh YW, Wu CY, Lin KC, Chang YF, Chen CL, Liu JS.. Responsiveness and validity of three outcome measures of motor function after stroke rehabilitation. Stroke 2009; 40: 1386-91. [DOI] [PubMed] [Google Scholar]
- 27.Mutai H, Furukawa T, Araki K, Misawa K, Hanihara T.. Factors associated with functional recovery and home discharge in stroke patients admitted to a convalescent rehabilitation ward. Geriatr Gerontol Int 2012; 12: 215-22. [DOI] [PubMed] [Google Scholar]
- 28.Riley JD, Le V, Der-Yeghiaian L, See J, Newton JM, Ward NS, et al.. Anatomy of stroke injury predicts gains from therapy. Stroke 2011; 42: 421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jette DU, Latham NK, Smout RJ, Gassaway J, Slavin MD, Horn SD.. Physical therapy interventions for patients with stroke in inpatient rehabilitation facilities. Phys Ther 2005; 85: 238-48. [PubMed] [Google Scholar]
- 30.Shinohara T, Usuda S.. Are contents of physical therapy in nine Japanese hospitals for inpatients with stroke related to inpatients' and physical therapists' characteristics ? J Phys Ther Sci 2013; 25: 641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richards LG, Latham NK, Jette DU, Rosenberg L, Smout RJ, DeJong G.. Characterizing occupational therapy practice in stroke rehabilitation. Arch Phys Med Rehabil 2005; 86: S51-60. [DOI] [PubMed] [Google Scholar]
- 32.Latham NK, Jette DU, Slavin M, Richards LG, Procino A, Smout RJ, et al.. Physical therapy during stroke rehabilitation for people with different walking abilities. Arch Phys Med Rehabil 2005; 86: S41-50. [DOI] [PubMed] [Google Scholar]
- 33.Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, et al.. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One 2014; 9: e87987. [DOI] [PMC free article] [PubMed] [Google Scholar]