Abstract
Background:
Treatment-resistant schizophrenia is prevalent and difficult to manage, as patients fail multiple antipsychotic trials before being considered as treatment-resistant. Currently clozapine is the only Food and Drug Administration-approved pharmacotherapy for treatment-resistant schizophrenia but remains under-prescribed. The purpose of this study is to investigate recent literature on clozapine in order to identify barriers to prescribing clozapine and categorize the recommended solutions.
Methods:
We conducted a systematic review following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Using free text and the medical subject headings, we searched MEDLINE/PubMed electronic bibliographic database from 2017 until 2020. Eligible studies included peer-reviewed English language articles with multiple methodologies aiming to identify clozapine barriers in treatment-resistant schizophrenia. We used search terms combining clozapine AND treatment OR treatment-resistant schizophrenia AND barriers AND prescribing OR prescription OR prescriber. We merged search results in a citation manager software, removed duplicates, and screened the remaining articles based on the study eligibility criteria.
Results:
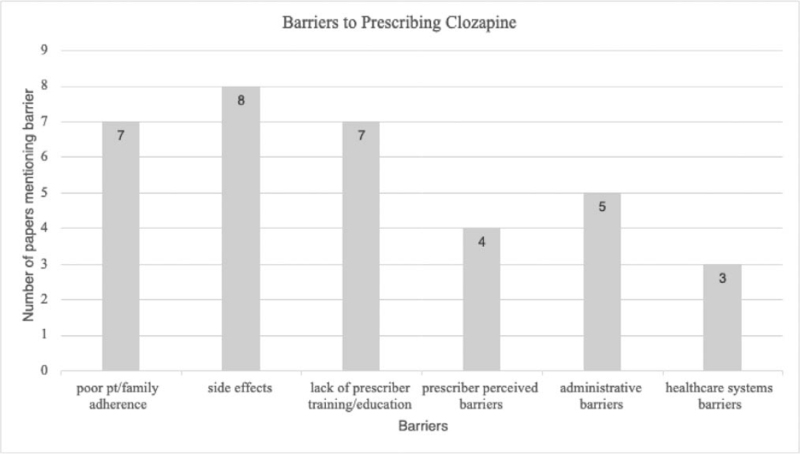
We retrieved 123 studies, however, only 10 articles exclusively met the study inclusion criteria for full text review. These studies represented 20 countries; 6 were exclusively conducted in the US. The top barriers delineated by the studies include: providers’ lack of knowledge and training (n = 7), concern about side effects (n = 8), and poor adherence (n = 7). All studies described more than 1 barrier. Other barriers included prescriber-perceived barriers (n = 4), administrative barriers (n = 5), and other healthcare systems-related barriers (n = 3). Top recommendations to overcome clozapine prescription barriers included improving prescriber clozapine education/training, utilizing interdisciplinary teams and providing integrated care via clozapine clinics, and simplifying blood test monitoring.
Conclusion:
Clozapine remains under-prescribed for patients with treatment-resistant schizophrenia due to multiple barriers related to the individual prescriber, system of care, and technology. It is recommended that by improving prescriber knowledge and training, use of integrated care, and use of technology that can enable continuous, real-time blood test monitoring, these barriers may be overcome.
Keywords: clozapine, drug-related side effects and adverse reactions, medication adherence, prescriptions, schizophrenia
1. Introduction
Schizophrenia is a treatable psychiatric disorder affecting more than 20 million people globally and is associated with significant disability.[1] It is estimated that 30% of patients with schizophrenia are considered treatment-resistant.[2] Clozapine remains the gold standard pharmacotherapy for treatment-resistant schizophrenia but is under-utilized.[3] Treatment-resistant schizophrenia is defined as insufficient treatment response after 2 adequate trials of different antipsychotics, once medication adherence is ensured.[2] Clozapine is a second generation (atypical) antipsychotic drug that targets dopamine and serotonin to improve thinking, mood, and behavior. It is the only drug approved by the US Food and Drug Administration for patients with treatment-resistant schizophrenia whose symptoms are not fully controlled with other antipsychotics. Clozapine is also recommended for recurrent suicidal ideation in patients with schizophrenia or schizoaffective disorder.[4–6] Despite its efficacy, only a relatively small percentage of eligible patients are prescribed and treated with clozapine.
While clozapine is highly effective, risks associated with treatment include its 5 black box warnings: agranulocytosis, cardiovascular events, dementia, hypotension, and seizures. Agranulocytosis can increase infection risk, requiring clozapine patients to undergo regular absolute neutrophil count (ANC) monitoring and tracking of side effects through Risk Evaluation and Mitigation Strategy (REMS).[4,5] However, it is important to note that agranulocytosis occurs in less than 1% of patients taking clozapine and is rarely seen in clinical practice.[6]
Additionally, growing evidence shows that clozapine use can be relatively safe with minimal side effects if closely monitored.[7] A recent study by Blackman et al[8] did not find a significant difference in cell count at 12 weeks after clozapine treatment compared to baseline. Furthermore, multiple patient surveys show that despite the extensive monitoring requirements of clozapine, patients who are already on clozapine are very satisfied with the treatment and want to continue.[9] Interestingly, Clozapine-Induced Gastrointestinal Hypomotility is the most common yet underdiagnosed cause of clozapine-related death due to inadequate information in current prescribing guidelines. Signs of Clozapine-Induced Gastrointestinal Hypomotility include severe constipation, ileus, and bowel obstruction.[10] However, it can be successfully treated with simple interventions in an acute inpatient setting.[11]
If side effects can be closely monitored and managed, why are prescribing rates so low? Previous studies reveal that top clozapine barriers include fears of side effects and prescriber discomfort with clozapine.[7] Nevertheless, despite advances in antipsychotic development for schizophrenia, clozapine continues to be the gold standard for treatment-resistant schizophrenia. However, it remains unclear why clozapine is underutilized. Studies in the past have investigated this gap, but this study attempts to identify and synthesize current research on barriers to prescribing/utilizing clozapine in patients with treatment-resistant schizophrenia and describe recommended solutions to overcome these barriers.
2. Methods
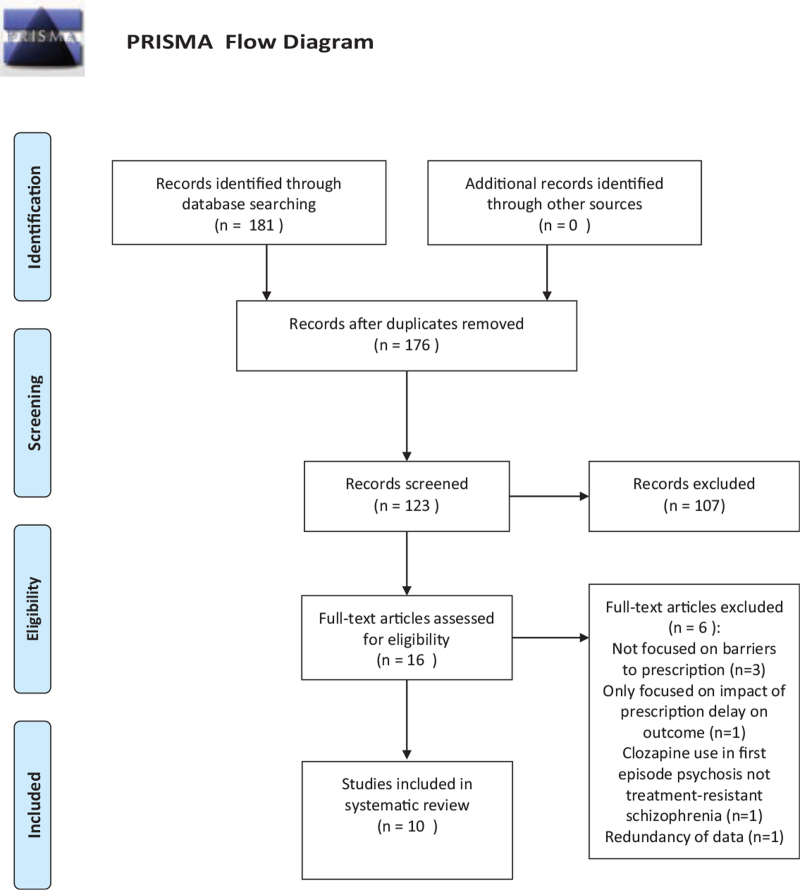
We performed a systematic review using Preferred Reporting Items for Systematic Reviews and Meta-Analyses[12] guidelines (Fig. 1). Using free text and MeSH terms, we searched MEDLINE/PubMed electronic bibliographic database from 2017 until 2020. Search terms included a combination of “clozapine” [All fields], AND “treatment” [All fields], OR “treatment-resistant schizophrenia” [All fields], AND “barriers” [All fields], AND “prescribing” [All fields], OR “prescription” [All fields], OR “prescriber” [All fields].
Figure 1.
PRISMA flow diagram.
2.1. Eligibility criteria
Our inclusion criteria consisted of multiple methodologies: quantitative observational studies, qualitative studies, systematic, and narrative reviews, as a way to make the review more comprehensive. Additionally, studies were eligible if they aimed to investigate any type of barrier to prescribing or utilizing clozapine in patients with treatment-resistant schizophrenia. We included these criteria because it incorporates the exposure and sample of interest for this study. We also included studies involving male and female adult patients, written in English, and published from 2017 to 2020, because we wanted to present new data within the past few years. We excluded studies that did not specifically mention clozapine use in treatment-resistant schizophrenia patients, the exposure of interest.
2.2. Study selection
We used Endnote reference manager software (X9; Clarivate, Philadelphia, PA) to pool research results and remove duplicates. To do this, a librarian exported all relevant articles from the MEDLINE/PubMed electronic bibliographic database to the EndNote reference management system and distributed them to the research team. Two research team members shared the results (AIB, SBH) via the Endnote library folder. During the 1st stage of screening, both reviewers, independently read the study titles and abstracts to decide whether a reference is potentially relevant to the study. Each reviewer created 2 folders: one for included studies and another for excluded studies. In the second stage, both reviewers compared included studies with each other. All disagreements were discussed and reconciled. Once a consensus was reached, the identified articles’ full text was independently screened based on the inclusion and exclusion criteria for eligibility.
2.3. Data extraction
We summarized articles included in the review by extracting clozapine prescribing barriers and organizing data by the year of study, authors, the country where the study was conducted, study design, number of participants, study sample, and risk of bias. One reviewer (AIB) extracted data from the selected articles into a Microsoft Excel spreadsheet (V2019; Microsoft, Redmond, WA), and the second reviewer (SBH) validated the extracted data. We used tables and graphs to organize and display the extracted data from each study.
2.4. Data quality and risk of bias in individual studies
The first 2 authors critically assessed and graded the studies for risk of bias using the Mixed Methods Appraisal Tool (MMAT),[13] and the Measurement Tool to Assess Systematic Reviews 2.[14] Mixed Methods Appraisal Tool is specifically developed to critically assess the methodological quality of different types of studies, including qualitative, quantitative randomized controlled trials, quantitative non-randomized, quantitative descriptive, and mixed methods.[13] The tool helps to assess the qualities of the studies in terms of study design, data collection, study selection, data analysis, presentation of findings, author's discussions and conclusions. It has 25 criteria, 5 for each study type with yes, no, unsure options. We used percentages from 0% to 100% to grade the studies. We considered studies with scores above 50% ‘yes’ as having low bias.[15]
Measurement Tool to Assess Systematic Reviews 2 has 16 items on 7 critical domains, guiding authors to interpret weaknesses of the reviewed studies in critical and non-critical domains.[16] The suggested gradings are: ‘critically low’, ‘low’, ‘moderate’, and ‘high’.[11] A ‘critically low’ grade indicates the review study has more than 1 critical flaw. A ‘low’ grade indicates that the review study has 1 critical flaw with or without non-critical flaw.[11] A ‘moderate’ grade indicates more than 1 non-critical flaw in the review study, and a ‘high’ grade means 0 or 1 non-critical flaw (Table 1). AB assessed all the studies, while SBH assessed the risk of bias on half of the studies, which were randomly selected. All differences were discussed and reconciled.
Table 1.
shows the included studies and their characteristics.
| Year authors | Country | Methodology | Types of studies/data source | Study participants | Survey sample/response rate | Barriers identified | Facilitators identified | Quality tool | Risk of bias (low, moderate, high) |
| Rubio and Kane, 2020 | USA | Narrative review | RCTs, prescriber and patient surveys, prescription registry data | Clozapine prescribers, patients with treatment-resistant schizophrenia | N/A | Prescriber lack of experience, perceived treatment burden, systems-level factors | Continued medical education, administrative partnerships, always discussing clozapine with patients despite perceived barriers/fears | AMSTAR-2 | Moderate |
| Thien et al, 2017 | UK, New Zealand, Canada, Denmark, India, Japan, Ireland, Singapore, Turkey | Narrative review | Retrospective chart reviews (n = 13) and surveys (n = 5) | Clozapine prescribers, clozapine-treated patients with treatment-resistant schizophrenia | N/A | Routine blood monitoring as a burden, physician perception of blood monitoring as a burden to patients, side effects, patient/care reluctance to comply with treatment, clinician's lack of knowledge/experience | Clinician education focused towards early eligible patient identification and care management after clozapine initiation | AMSTAR-2 | Moderate |
| Kelly and Love, 2019 | USA | Scoping review | Multiple studies | N/A | N/A | Patient and family barriers, provider barriers, resource availability, healthcare systems factors, administrative burden | Interdisciplinary teams utilizing clinical psychiatric pharmacists in managing clozapine patients, clozapine clinics, prescriber support | AMSTAR-2 | High |
| Singh et al, 2018 | USA | Survey/descriptive | N/A | 164 US psychiatry residents | 164 started but 162 completed survey, unknown actually received the survey. | Side effects, limited experience/inadequate training starting clozapine | Clozapine clinic training during residency | MMAT | Moderate |
| Leung et al, 2020 | USA, Canada | Survey/descriptive | N/A | Clozapine prescribers, including attending/consultant psychiatrists (n = 115), psychiatric nurse practitioners/physician assistants (n = 21), and psychiatric residents (n = 71), did not disclose position (n = 5) | n = 211/1152, response rate = 18.3% | Lack of clozapine-eligible patients, no REMS registration, too much trouble, too many side effects and risk of blood dyscrasias (38.5%), more than 1 reason (38%), administrative burden (68.3%), perceived as burden for patient (60.6%), poor patient adherence (67.3%), presence of medical comorbidities (52.3%), poor social support (46.2%) | Standardized clozapine clinic model, multidisciplinary approach to clozapine management, improved patient education by pharmacists, point of care testing | MMAT | Moderate low- |
| Moody and Eatmon, 2019 | USA | Survey/descriptive | N/A | 97 clozapine prescribers (physicians, advanced practice nurses, physician assistants) in VHA | Low response rate. 98 responded but 1 removed due to administrative role. | Side effects, monitoring concerns, prescribing logistics, lack of experience | Appointing administrative contact person for assistance with administrative tasks, clozapine clinics, utilize outside laboratories, prescriber education/training | MMAT | Moderate |
| Kelly et al, 2019 | USA | Survey/descriptive | N/A | 277 psychiatrists (psychiatrists, psychiatry residents, and fellows) in Maryland | 32% response rate but only 255 included in final analysis due to 16 incomplete and 6 declined | Patient adherence to blood work, patient discomfort with bloodwork, lack of education, dosing effects, registration, delayed labs | Point of care device for blood and antipsychotic level monitoring | MMAT | Moderate |
| Ismail et al, 2018 | Qatar, Bahrain, Oman, KSA, UAE, Kuwait | Semi-structured interviews/mixed methods | N/A | 13 participants: psychiatrists, psychiatric residents, psychiatric nurses, clinical/non-clinical pharmacists, mental health medical directors | Low response rate but excluded if non-English speaking or did not sign informed consent | Emergent side effect monitoring, hematological monitoring requirements, lack of patient education, poor patient adherence, diverse prescriber practices | Using interdisciplinary teams to support prescribers/assist with monitoring (clozapine clinics), patient/family education | MMAT | Moderate-high |
| Farooq et al, 2019 | UK | Systematic review | Surveys (n = 5), case note reviews (n = 4), semi-structured interview/consultations with stakeholders (n = 3), interventional/quality improvement studies (n = 3) | Psychiatrist/resident/pharmacist/advanced practice nurses/mental health leadership staff, patients on clozapine, researchers, consultants | N/A | Patient non-adherence/refusal of bloodwork, concern for poor tolerance, lack of knowledge/experience, side effect management | Point of care devices, patient, provider, and family online education, prescriber support/consumer decision-making tools (shared decision making), clozapine training in residency | AMSTAR-2 | Moderate |
| Verdoux et al, 2019 | UK, Denmark, Netherlands, France, Serbia, USA, Canada, India, Thailand, New Zealand, Brazil | Systematic review | n = 13 (all studies describing clozapine use in adult TRS patients, 1 in children, and in 1 in elderly) | Psychiatrists, psychiatry trainees, physicians, Medicaid prescribers, mental health department officials, nurses, pharmacy staff | N/A | Lack of prescriber experience, monitoring and adverse event concerns, prescribing practices/preferences | Clozapine clinic, simplified blood monitoring, prescriber education, increased clozapine access | AMSTAR-2 | Moderate |
AMSTAR-2 = Measurement Tool to Assess Systematic Reviews 2, MMAT = Mixed Methods Appraisal Tool, REMS = Risk Evaluation and Mitigation Strategy, RCT = Randomized Controlled Trials, TRS = Treatment-Resistant Schizophrenia, VHA = Veterans Health Administration.
2.5. Ethical considerations
This study exclusively utilized previously published data, and the authors did not collect patient data themselves. Thus, there was no need for obtaining patient consent or institutional board review. All authors had complete access to the data and were involved in reviewing and approving the final manuscript. The authors have no conflicts of interest to declare.
3. Results
One hundred and eighty-one records were identified in the PubMed search during the selected time period, and 176 remained after we removed the duplicates. Of these, only 123 mentioned clozapine use and, we removed 107 since they were not eligible based on the lack of key search terms used in the titles and abstracts. We reviewed the full text of the remaining 16 studies and excluded 3 (n = 3) due to lack of mentioning barriers to clozapine prescription, 1 due to use of clozapine in conditions other than treatment-resistant schizophrenia (n = 1), and 2 since the primary focus was clozapine prescription delay on outcomes (n = 2). Included studies examined barriers to clozapine use and mentioned facilitators to improve its prescription and utilization (Fig. 1).
3.1. Characteristics of included studies
Of the 10 studies included in this review, 4 were surveys, 1 was a semi-structured in-depth interview, 2 were narrative reviews, 1 was a scoping review, and 2 were systematic reviews. Six of these studies involved eliciting views of clozapine prescribing barriers from psychiatrists and other clozapine-prescribing mental health professionals. While the breakdown of the study participants by gender was not clear, participants included 589 physicians (including psychiatrists and psychiatry residents), 33 advanced practitioners (including nurses and physician assistants), and 4 pharmacists. These characteristics are summarized in Table 1.
3.2. Barriers to clozapine utilization in patients with treatment-resistant schizophrenia
Barriers to clozapine prescribing were roughly divided into prescriber-related barriers, patient-related barriers, and administrative/healthcare system-associated barriers. All included articles described more than 1 barrier, although there is some overlap in barrier type. Prescribers tend to overestimate adverse effects and anticipate poor patient adherence to clozapine and its monitoring requirements, regardless of clozapine knowledge or experience level. However, concern for adverse effects and their management appears to depend somewhat on clozapine knowledge and experience level. Moody and Eatmon[17] conducted a nationwide survey addressing Veterans Health Administration mental health providers eligible to prescribe clozapine. Results showed that the most frequent prescriber barriers were adverse event concerns; monitoring concerns; and prescribing logistics, all of which were more prevalent than prescribing concerns due to lack of experience prescribing clozapine.[17]
Farooq et al[18] conducted a systematic review of clozapine barriers and further identified prescribers’ limitation in clozapine knowledge resulting in difficulty identifying appropriate patients and reluctance to start clozapine in eligible patients. The most record barriers were found to be patient non-adherence and refusal of bloodwork and delaying treatment initiation, followed by concerns about clozapine tolerance, lack of knowledge/experience regarding side effects, and side effect management.[18] Thien and O’Donoghue[19] conducted a narrative review examining delays in initiating clozapine and barriers to prescription. They found that prescribers overestimated patients’ adherence difficulties and experienced challenges in identifying eligible patients. The limited existing literature on clozapine use in elderly patients and hematologic monitoring difficulties also contributed to reduced clozapine use.[19]
Ismail et al[20] conducted a mixed-method study by interviewing clozapine prescribers, including psychiatrists, psychiatry trainees, nurses, and pharmacists in Arabian Gulf nations. The most common barriers were reported to be emergent side effect management and hematological monitoring concerns, as well as lack of patient education and poor adherence.[20] Aware that clozapine frequent bloodwork monitoring is a major barrier to prescribing, Kelly et al[21] administered a survey to psychiatrists in Maryland to understand the impact of bloodwork and other barriers to clozapine prescription, as well as interest in a novel technology using a point of care (POC) device to measure clozapine levels. Among 28 listed barriers, the most common was poor patient adherence to bloodwork and patient discomfort with bloodwork.[21]
Singh et al[3] surveyed 165 US resident psychiatrists only from Accreditation Counsel for Graduate Medical Education-accredited programs. They found that about 41% of residents felt comfortable prescribing clozapine, while only 18% felt very comfortable despite having clozapine-eligible patients.[3] Additionally, 63% of residents had prescribed clozapine regardless of training level, suggesting inadequate clozapine exposure during residency.[3] The most frequent barriers to prescribing were blood monitoring, limited experience, and side effects.[3]
In addition to presenting the most common clozapine prescription barriers mentioned above, Leung et al,[22] Verdoux et al,[23] and Rubio and Kane[9] also highlighted administrative and healthcare systems-level barriers affecting clozapine monitoring and utilization. Verdoux et al[23] conducted a systematic review exploring prescribing practices of mental health professionals, including psychiatrists and psychiatry trainees, institutional factors affecting prescription, and interventions to improve clozapine prescription. They found that the most common barriers were lack of experience prescribing clozapine, clozapine monitoring, adverse event concerns, and variance in prescriber preferences/practices.[23] Rubio and Kane[9] completed a narrative review of clozapine use and found that the most common barriers to clozapine prescription were prescribers’ lack of experience with clozapine, their perceived treatment barriers on patients, and systems-level factors. Leung et al[22] administered a survey assessing clozapine knowledge and perception to psychiatrists, advanced practice providers, and psychiatry trainees (including residents and fellows) in the US and Canada; they noted the following prescribing barriers: lack of clozapine-eligible patients, lack of REMS registration, too much trouble, too many side effects, or other (more than 1 barrier).[22]
In addition to replicating the above findings regarding prescriber-related barriers, data from multiple studies show the impact of patients, resources, communities, and logistical and administrative healthcare systems barriers affecting clozapine prescription and utilization.[17,19,20,24,25] Thien and O’Donoghue[19] noted that additional patient-level and systems-level barriers include the complexities of initiating clozapine, difficulties convincing patients/family to start trials, lack of community support, and shortage of hospital beds to initiate clozapine. Ismail et al[20] shared other findings such as barriers, including problems dispensing clozapine, inpatient bed shortage; lack of communication across care settings; and lack of pharmacist training regarding patient medication counselling. Although prescribers did not consider the lack of a national monitoring system to be a major barrier to clozapine utilization, the authors recommended adopting and following international guidelines regarding clozapine prescription.[20]
Farooq et al[18] also commented on barriers such as difficulty in identifying eligible clozapine patients, service fragmentation, lack of community support, shortage of inpatient beds (required to initiate clozapine), and difficulty registering patients for bloodwork. Moody and Eatmon[17] mentioned that updates to the clozapine REMS no longer allow pharmacists to enroll patients in the registry, which places a greater burden on healthcare providers prescribing clozapine.
3.3. Facilitators to improve clozapine prescription/utilization and potential solutions to overcoming barriers
The most common facilitators to increase clozapine prescription and utilization included providing clozapine education and support through dedicated clozapine clinics and implementing POC testing to facilitate monitoring. Multiple articles recommended creating and utilizing standardized clozapine clinics, including during residency, to help improve prescriber knowledge and training regarding clozapine management.[3,17,18,20,22–24] Ismail et al[20] and Leung et al[22] mentioned utilizing a multidisciplinary team, including pharmacists in clozapine clinics to improve clozapine management; support prescribers; and assist with monitoring.
Leung et al[22] and Kelly and Love[25] further recommended improving and utilizing pharmacists to educate patients on clozapine side effects/management, drug interactions, smoking cessation, and monitoring. Moody and Eatmon[17] and Rubio and Kane[9] also advised designating an administrative contact and making administrative partnerships to assist with administrative tasks. Increasing prescriber education and training through continued medical education can help address adverse events, side effect monitoring, and prescribing logistics.[9,17,22,23] Furthermore, improving clinician education regarding early identification of eligible patients and care management after clozapine initiation may encourage and increase clozapine prescription.[19] In addition to improving prescriber and patient/patient family education regarding clozapine, providing shared decision-making tools can increase clozapine utilization.[18,20] Rubio and Kane[9] suggested that prescribers always discuss clozapine with patients, regardless of perceived fears. Kelly and Love[25] also recommended encouraging clozapine prescription whenever possible and increasing access to clozapine by adding clozapine to all hospital formularies and correctional facilities.[24] Leung et al[22] suggested making it easier to pick up prescriptions by reducing the required steps.
4. Discussion
Across studies, the most common barriers to clozapine prescription were side effect concerns, lack of prescriber training/experience, and poor patient/family adherence to treatment/monitoring (Fig. 2). Of note, prescribers’ perceptions of patient barriers are often exaggerated and may be associated with a lack of clozapine knowledge and clozapine-prescribing experience.[18] These findings were replicated by Kelly et al[24] in an Open Forum based on deliberations of the National Association of State Mental Health Program Directors’ workgroup to identify and address barriers to clozapine prescription. It was found that prescribers tend to overestimate the prevalence and negative impact of adverse effects of clozapine on patients,[24] including agranulocytosis and its duration.[18] Lack of prescriber clozapine education resulted in overestimation of agranulocytosis and underestimation of patient satisfaction.[21] In contrast, other studies show that most patients on clozapine may find hematological monitoring to be challenging but still prefer to continue clozapine treatment despite its side effect potential and monitoring requirements.[9,18] A patient survey revealed that 87% felt that clozapine benefits outweighed risks and preferred it despite disliking frequent blood tests, while only 1.6% discontinued clozapine due to blood monitoring.[3]
Figure 2.
Barriers to prescribing clozapine graph.
Multiple studies investigated the relationship between clozapine knowledge, experience level, and clozapine prescription with varying results. Moody and Eatmon[17] found no significant difference in prescribing based on a clozapine knowledge assessment given to prescribers, which included clozapine indications, initial dose, adverse effects, baseline ANC and ANC monitoring requirements. However, there is mixed data regarding clozapine prescription and overall clinical practice experience level. In a survey of clozapine prescribers of varying education and experience levels, Leung et al[22] found that there was no significant difference in knowledge assessment based on experience/practice level. Although 82.9% of responders believed they had adequate training and education regarding clozapine management, 68.3% still perceived initiating and maintaining clozapine therapy as an administrative burden, while more than half of respondents believed clozapine therapy to be burdensome for patients.[22] Clozapine clinic availability and perceived adequate knowledge by prescribers were noted to be additional potential barriers.[22] Other studies suggested that US psychiatry residents were not receiving adequate exposure to clozapine during residency training,[3] but even prescribers who reported adequate clozapine exposure during training still had difficulty identifying eligible patients.[18] These findings highlighted the relative overestimation of prescriber-perceived barriers compared to patient perceptions. Further, they supported the importance of the need of providing clozapine clinic training during residency and clozapine education at all experience levels as a potential solution to increasing clozapine utilization.[3,22] Additionally, they revealed an overlap of multiple barriers, resulting in additional barriers to clozapine prescription, which persisted even when prescribers reported adequate clozapine education and training.
Despite adequate clozapine knowledge and education, prescribers may hesitate or delay clozapine prescription for other reasons. Individual prescriber preferences and attitudes resulted in testing multiple antipsychotic before starting clozapine.[19] Although most providers agreed with the idea of starting clozapine, many hesitated until patients were already trailed on up to 3 different antipsychotics before starting clozapine or combining 2 antipsychotics rather than initiating clozapine in patients with treatment-resistant schizophrenia. Additionally, clozapine was prescribed less in patients aged greater than 30 years compared to those less than 30 years, suggesting discomfort with clozapine prescription in elderly individuals.[18] Furthermore, lack of clozapine clinic training during residency may affect the identification of clozapine-eligible patients by prescribers (another barrier) which will reduce clozapine prescription.
Prescribers interested in initiating clozapine may still have to overcome administrative and logistical barriers to clozapine prescribing and monitoring. Specific barriers within these categories include lack of shared decision-making tools for providers and patients, complex monitoring protocols, consultation service requirements, and ancillary service costs.[24] Kelly et al[24] and Kelly and Love[25] added that barriers include issues involving patients, family, providers, resource availability, healthcare system issues, and administrative problems. Other barriers to prescribing clozapine included dosing effects, clozapine registration, and delay in treatment initiation due to delayed lab results.[21] Further significant barriers included lack of multidisciplinary teams/care coordination, transportation, access to medical services, and prescription monitoring services.[9] Additionally, the lack of interdisciplinary teams available in clozapine clinics will increase the administrative burden on prescribers, which will make it harder to prescribe clozapine. Kelly et al[24] also acknowledged the existence of perceived barriers in addition to actual barriers to clozapine prescription and the possibility that perceived barriers can become additional barriers. Kelly and Love[25] realized the limitations in prescribers’ abilities to overcome their clozapine-prescribing barriers and highlighted the important role of psychiatric pharmacists and other advanced practice nurses and physician assistants to aid psychiatrists in reducing barriers to clozapine prescription and utilization. These findings emphasize the need for team-based care to improve clozapine prescription and monitoring. Furthermore, limited clozapine access (including limited availability on hospital and correctional facility formularies) will reduce the number of clozapine prescriptions for patients with treatment-resistant schizophrenia being cared for in these settings and contribute to healthcare disparities.
In this review, the most common solutions to overcoming barriers to clozapine prescription were the utilization of interdisciplinary teams and clozapine clinics to support prescribers, increasing prescriber education about clozapine, and simplifying hematologic monitoring. Blood monitoring can be facilitated by improving REMS and utilizing POC testing tools and outside laboratories for blood and antipsychotic level monitoring, reducing the burdens associated with obtaining labs.[23]Requiring clozapine clinic experience during residency training can help providers identify clozapine-eligible patients and thus increase clozapine prescription.[3,18,19] Leung et al's[22] study emphasized the need for continued clozapine medical education for all prescribers, regardless of clinical education level or years of clinical experience. Additionally, providers should always discuss clozapine with eligible patients during treatment planning and consult with a psychiatric pharmacist regarding dose adjustment, drug interactions, and monitoring.[9,25] Creating shared clinical decision-making tools that are readily available can further assist prescribers and patients considering clozapine therapy.[18,24] These interventions are especially important due to the national psychiatrist shortage and prescribers’ preference to initiate clozapine much later than after the recommended 2 failed antipsychotic trials.[25]
While clozapine has some potentially dangerous side effects, many of them can be easily managed.[9] Improving prescriber education regarding side effect recognition and management and recognizing the important role of the clinical pharmacist in educating patients about clozapine can improve clozapine prescription and adherence.[25] Another way to aid prescribers is by providing improved drug manufacturer package inserts to help tailor clozapine management to patients’ unique clinical profiles. Certain patients require lower clozapine doses, and adverse effects such as fever, flu-like symptoms, or pneumonia can be managed by halving the clozapine dose and temporarily holding clozapine until symptoms resolve.[26] Patients/family needs to alert the psychiatrist if these adverse effects occur to facilitate quick action and reduce risk of mortality. Patients should be instructed to inform their prescriber if clozapine treatment interruption exceeds 48 hours, as they may experience withdrawal symptoms and will require clozapine re-titration starting at the initial dose of 12.5 mg.[9]
4.1. Limitations
Limitations of this review include heavy utilization of subjective reported views about barriers to clozapine prescription from providers through surveys and interviews. Data from these studies are prone to moderate to high levels of bias due to poor or unknown response rates, poor methods of distribution, and questionable validity of survey/questionnaire tools, with survey non-responders becoming lost to follow-up. This can falsely cause under or over-reporting. The multiple study methodologies also made it challenging to compare study qualities and assess the risk of bias. Additionally, most of the data included views elicited from prescribers rather than patients eligible for or already receiving clozapine. Of note, this review included data only from previously published studies, as the investigators did not collect their own data.
5. Conclusion
Although clozapine is still the standard of care for patients with treatment-resistant schizophrenia, prescribing rates remain inadequate due to multiple barriers to the individual prescriber, system of care, and technology, despite its clinical efficacy. Many prescribers hesitate to prescribe clozapine and delay prescription, even to the point of waiting until after 3 or more antipsychotic trials are completed due to concerns about potential adverse effects.[18] If these fears are perpetuated, a growing population of clozapine-eligible patients will remain inadequately treated, worsening the global burden of treatment-resistant schizophrenia.
Overall, compared to previous studies, this review revealed that many of the same barriers to clozapine prescription persist today, thus underlining the continued unmet needs of patients with treatment-resistant schizophrenia who are not being prescribed clozapine. These findings suggest an urgent need for action, as untreated schizophrenia is associated with poor outcomes, including higher risk of relapse, increased hospitalizations, increased morbidity,[27] and higher healthcare treatment costs compared to patients treated with clozapine.[28]
COVID-19 further complicates management and prescription of clozapine in eligible patients, as low absolute ANC can increase risk of infection and mortality. Due to limited available data on clozapine use and COVID-19 infection, prescribers must be vigilant while treating and managing these patients. COVID-19 infection may also present as additional barrier to clozapine prescription. New data suggest that the acute phase of COVID-19 infection can cause mild neutropenia in established patients treated with clozapine, although neutropenia was attributed to the COVID infection, itself rather than due to the clozapine.[29] Despite the multitude of strategies included in this review, there is still a lot of work to be done as these strategies’ effectiveness is unknown and requires further research.
Acknowledgments
The authors would like to thank Joshua Shulman, MLIS from Charles R Drew University of Medicine & Science for assistance with acquiring articles used in this review.
Author contributions
Conceptualization: Anum Iqbal Baig, Shahrzad Bazargan-Hejazi.
Data curation: Anum Iqbal Baig, Jaziel Rodriguez-Lara.
Formal analysis: Anum Iqbal Baig, Shahrzad Bazargan-Hejazi.
Funding acquisition: Shahrzad Bazargan-Hejazi.
Investigation: Anum Iqbal Baig.
Methodology: Anum Iqbal Baig, Shahrzad Bazargan-Hejazi.
Project administration: Shahrzad Bazargan-Hejazi, Gul Ebrahim.
Writing – original draft: Anum Iqbal Baig, Jaziel Rodriguez-Lara.
Writing – review & editing: Anum Iqbal Baig, Shahrzad Bazargan-Hejazi, Gul Ebrahim.
Footnotes
Abbreviations: ANC = absolute neutrophil count, POC = point of care, REMS = Risk Evaluation and Mitigation Strategy.
How to cite this article: Baig AI, Bazargan-Hejazi S, Ebrahim G, Rodriguez-Lara J. Clozapine prescribing barriers in the management of treatment-resistant schizophrenia: a systematic review. Medicine. 2021;100:45(e27694).
Research supported partly by NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881.
The manuscript does not contain clinical studies or patient data.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Organization WH. Schizophrenia. Available at: https://www.who.int/news-room/fact-sheets/detail/schizophrenia. 2019. Accessed August 20, 2020. [Google Scholar]
- [2].Kane JM, Agid O, Baldwin ML, et al. Clinical guidance on the identification and management of treatment-resistant schizophrenia. J Clin Psychiatry 2019;80:18com12123. [DOI] [PubMed] [Google Scholar]
- [3].Singh B, Hughes AJ, Roerig JL. Comfort level and barriers to the appropriate use of clozapine: a preliminary survey of US psychiatric residents. Acad Psychiatry 2020;44:53–8. [DOI] [PubMed] [Google Scholar]
- [4].Administration UFaD. Information on Clozapine. Available at: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-clozapine. 2019. Accessed December 23, 2020. [Google Scholar]
- [5].Administration UFaD. FDA Drug Safety Communication: FDA modifies monitoring for neutropenia associated with schizophrenia medicine clozapine; approves new shared REMS program for all clozapine medicines. 2016. [Google Scholar]
- [6].Health NAoM. Clozapine (Clozaril and FazaClo). 2016. [Google Scholar]
- [7].Shah P, Iwata Y, Plitman E, et al. The impact of delay in clozapine initiation on treatment outcomes in patients with treatment-resistant schizophrenia: a systematic review. Psychiatry Res 2018;268:114–22. [DOI] [PubMed] [Google Scholar]
- [8].Blackman GL, Jenny EL, Zafar R, et al. Clozapine response in schizophrenia and hematological changes. J Clin Psychopharmacol 2021;21:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rubio JM, Kane JM. How and when to use clozapine. Acta Psychiatr Scand 2020;141:178–89. [DOI] [PubMed] [Google Scholar]
- [10].Every-Palmer S, Ellis PM. Clozapine-induced gastrointestinal hypomotility: a 22-year bi-national pharmacovigilance study of serious or fatal ‘slow gut’ reactions, and comparison with international drug safety advice. CNS Drugs 2017;31:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Blackman G, Kapila A, Grosskopf CM, Dratcu L. Focussing on the fundaments - assessing and treating Clozapine Induced Gastrointestinal Hypomotility. Int J Psychiatry Clin Pract 2020;24:18–9. [DOI] [PubMed] [Google Scholar]
- [12].Moher D LA, Tetzlaff J, Altman DG. The PRISMA flow diagram. 2009. [Google Scholar]
- [13].Hong QN, Pluye P, Fàbregues S, et al. Improving the content validity of the mixed methods appraisal tool: a modified e-Delphi study. J Clin Epidemiol 2019;111:49–59. [DOI] [PubMed] [Google Scholar]
- [14].Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ivanova O, Rai M, Kemigisha E. A systematic review of sexual and reproductive health knowledge, experiences and access to services among refugee, migrant and displaced girls and young women in Africa. Int J Environ Res Public Health 2018;15:1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dettori JR, Skelly AC, Brodt ED. Critically low confidence in the results produced by spine surgery systematic reviews: an AMSTAR-2 evaluation from 4 spine journals. Global Spine J 2020;10:667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Moody BL, Eatmon CV. Perceived barriers and facilitators of clozapine use: a national survey of Veterans affairs prescribers. Fed Pract 2019;36: (Suppl 6): S22–7. [PMC free article] [PubMed] [Google Scholar]
- [18].Farooq S, Choudry A, Cohen D, Naeem F, Ayub M. Barriers to using clozapine in treatment-resistant schizophrenia: systematic review. BJPsych Bull 2019;43:08–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Thien K, O’Donoghue B. Delays and barriers to the commencement of clozapine in eligible people with a psychotic disorder: a literature review. Early Interv Psychiatry 2019;13:18–23. [DOI] [PubMed] [Google Scholar]
- [20].Ismail D, Tounsi K, Zolezzi M, Eltorki Y. A qualitative exploration of clozapine prescribing and monitoring practices in the Arabian Gulf countries. Asian J Psychiatr 2019;39:93–7. [DOI] [PubMed] [Google Scholar]
- [21].Kelly DL, Ben-Yoav H, Payne GF, et al. Blood draw barriers for treatment with clozapine and development of a point-of-care monitoring device. Clin Schizophr Relat Psychoses 2018;12:23–30. [DOI] [PubMed] [Google Scholar]
- [22].Leung JG, Cusimano J, Gannon JM, et al. Addressing clozapine under-prescribing and barriers to initiation: a psychiatrist, advanced practice provider, and trainee survey. Int Clin Psychopharmacol 2019;34:247–56. [DOI] [PubMed] [Google Scholar]
- [23].Verdoux H, Quiles C, Bachmann CJ, Siskind D. Prescriber and institutional barriers and facilitators of clozapine use: a systematic review. Schizophr Res 2018;201:10–9. [DOI] [PubMed] [Google Scholar]
- [24].Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing barriers to clozapine underutilization: a national effort. Psychiatr Serv 2018;69:224–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kelly DL, Love RC. Psychiatric pharmacist's role in overcoming barriers to clozapine use and improving management. Ment Health Clin 2019;9:64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].de Leon J, Ruan CJ, Schoretsanitis G, De las Cuevas C. A rational use of clozapine based on adverse drug reactions, pharmacokinetics, and clinical pharmacopsychology. Psychother Psychosom 2020;89:200–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chan SKW, Chan HYV, Honer WG, et al. Predictors of treatment-resistant and clozapine-resistant schizophrenia: a 12-year follow-up study of first-episode schizophrenia-spectrum disorders. Schizophr Bull 2021;47:485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Verma M, Grover S, Chakrabarti S. Cost of the illness of treatment-resistant schizophrenia: a mirror image study comparing clozapine with other antipsychotics. J Clin Psychopharmacol 2021;41:36–44. [DOI] [PubMed] [Google Scholar]
- [29].Gee S, Taylor D. COVID-19 infection causes a reduction in neutrophil counts in patients taking clozapine. J Psychiatry Neurosci 2021;46:E233–7. [DOI] [PMC free article] [PubMed] [Google Scholar]