Abstract
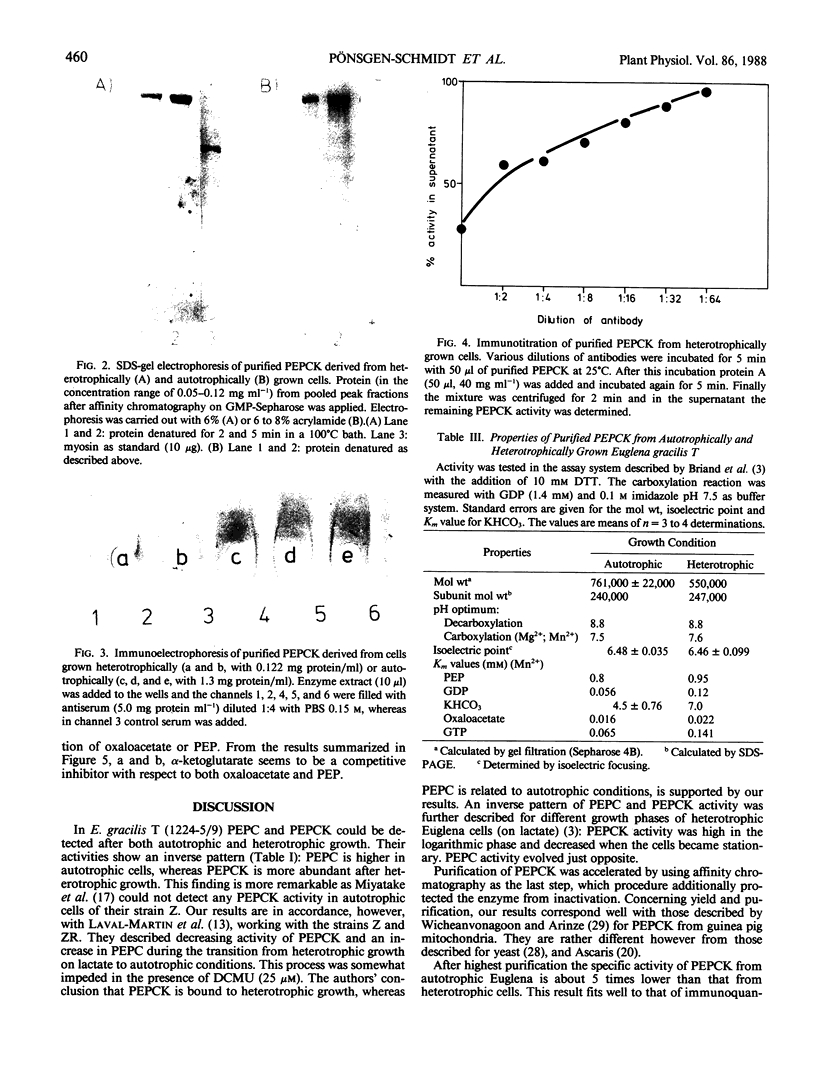
Euglena gracilis (1224-5/9) contains phosphoenolpyruvate carboxykinase when grown autotrophic with CO2 in the light. Its yield is higher when an additional carbon source like glucose has been added. The enzyme is lacking in cells provided with CO2 alone and kept in the dark, whereas highest yields result if both glucose and CO2 are provided together in the dark. The enzyme was purified by ammonium sulfate precipitation, gel filtration on Sephacryl S-300 and affinity chromatography on GMP-Sepharose. The latter step was most effective to protect the enzyme from inactivation. Its homogeneity was tested electrophoretically and immunologically. Enzymes from autotrophic and heterotrophically grown cells have identical pH optima and similar isoelectric points. The molecular weight was different: 761,000 for the enzyme from autotrophic and 550,000 for that from heterotrophic cells as determined by gel filtration. The subunit molecular weight of both enzymes is nearly the same. The kinetic data of the enzymes are slightly different. Glycolytic and tricarboxylic acid cycle intermediates are of limited influence on enzyme activity and inhibitory in unphysiological high concentrations. From Ouchterlony double immunodiffusion and enzyme-linked immunosorbent assay, it is evident that the enzyme is localized in the cytosol. With the latter quantification test the phosphoenolpyruvate carboxykinase protein content was found 10 times higher in heterotrophically grown cells than when cultivated under autotrophic conditions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis C. H., Schliselfeld L. H., Wolf D. P., Leavitt C. A., Krebs E. G. Interrelationships among glycogen phosphorylase isozymes. J Biol Chem. 1967 Oct 25;242(20):4824–4833. [PubMed] [Google Scholar]
- Hebda C. A., Nowak T. The purification, characterization, and activation of phosphoenolpyruvate carboxykinase from chicken liver mitochondria. J Biol Chem. 1982 May 25;257(10):5503–5514. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamed R., Levin Y., Wilchek M. Covalent coupling of nucleotides to agarose for affinity chromatography. Biochim Biophys Acta. 1973 Apr 28;304(2):231–235. doi: 10.1016/0304-4165(73)90239-0. [DOI] [PubMed] [Google Scholar]
- Laval M., Hernandez-Verdun D., Bouteille M. Remnant nucleolar structures and residual RNA synthesis in chick erythrocytes. Exp Cell Res. 1981 Mar;132(1):157–167. doi: 10.1016/0014-4827(81)90092-6. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Ohmann E., Plhák F. Reinigung und Eigenschaften von Phosphoenolpyruvat-Carboxylase aus Euglena gracilis. Eur J Biochem. 1969 Aug;10(1):43–55. [PubMed] [Google Scholar]
- ROSENBERG A. A COMPARISON OF LIPID PATTERNS IN PHOTOSYNTHESIZING AND NONPHOTOSYNTHESIZING CELLS OF EUGLENA GRACILIS. Biochemistry. 1963 Sep-Oct;2:1148–1154. doi: 10.1021/bi00905a042. [DOI] [PubMed] [Google Scholar]
- Rohrer S. P., Saz H. J., Nowak T. Purification and characterization of phosphoenolpyruvate carboxykinase from the parasitic helminth Ascaris suum. J Biol Chem. 1986 Oct 5;261(28):13049–13055. [PubMed] [Google Scholar]
- Satoh Y. Effects of Mn2+ on the exchange reaction of phosphoenolpyruvate carboxykinase in the presence of high concentrations of Mg2+. Biochim Biophys Acta. 1986 Aug 15;872(3):177–182. doi: 10.1016/0167-4838(86)90269-4. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tortora P., Hanozet G. M., Guerritore A. Purification of phosphoenolpyruvate carboxykinase from Saccharomyces cerevisiae and its use for bicarbonate assay. Anal Biochem. 1985 Jan;144(1):179–185. doi: 10.1016/0003-2697(85)90101-0. [DOI] [PubMed] [Google Scholar]
- Wicheanvonagoon S., Arinze I. J. Phosphoenolpyruvate carboxykinase from guinea-pig liver mitochondria. Immunological evidence for increase in enzyme amount during neonatal development. Biochem J. 1984 Jul 1;221(1):105–111. doi: 10.1042/bj2210105. [DOI] [PMC free article] [PubMed] [Google Scholar]