Abstract
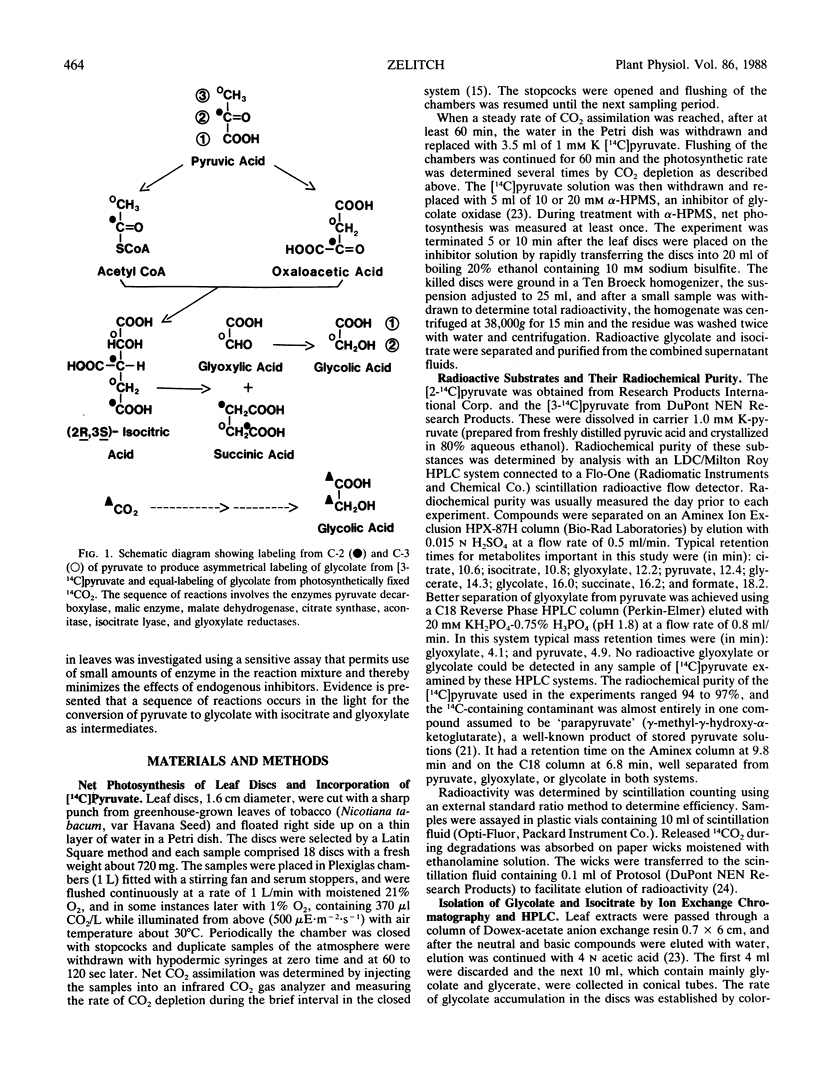
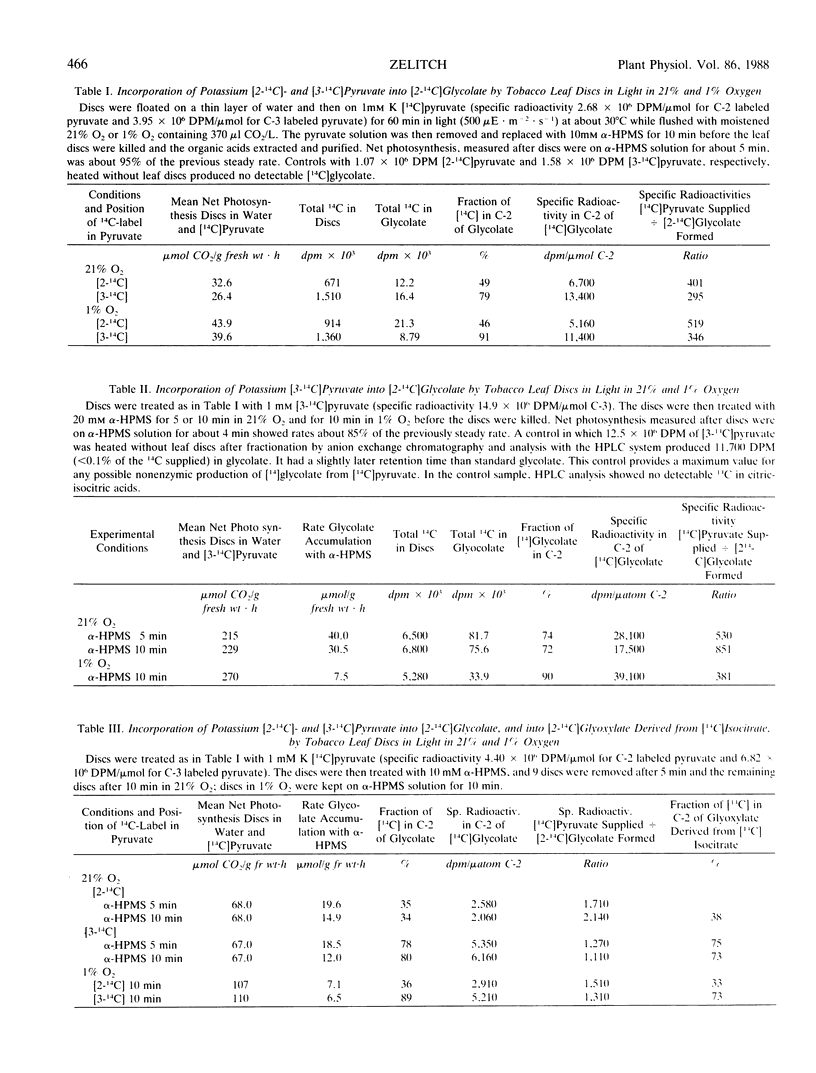
Tobacco (Nicotiana tabacum var Havana Seed) leaf discs were supplied tracer quantities of [2-14C]- and [3-14C]pyruvate for 60 minutes in steady state photosynthesis with 21% or 1% O2, and the glycolate oxidase inhibitor α-hydroxy-2-pyridinemethanesulfonic acid was then added for 5 or 10 minutes to cause glycolate to accumulate. The [3-14C]pyruvate was converted directly to glycolate as shown by a 50% greater than equallabeled 14C in C-2 of glycolate, and the fraction of 14C in C-2 increased in 1% O2 to 80% greater than equal-labeled. This suggests the pathway using pyruvate is less O2-dependent than the oxygenase reaction producing glycolate from the Calvin cycle. The formation of glycolate from pyruvate in the leaf discs was time-dependent and with [2-14C]- and [3-14C]pyruvate supplied leaf discs the C-2 of glyoxylate derived from C-2 of isocitrate was labeled asymmetrically in a manner similar to the asymmetrical labeling of C-2 of glycolate under a number of conditions. Thus glycolate was probably formed by the reduction of glyoxylate. Isocitric lyase activity of tobacco leaves was associated with leaf mitochondria, though most of the activity was in the supernatant fraction after differential centrifugation of leaf homogenates. The total enzyme activity was at least 35 micromoles per gram fresh weight per hour. The relative contribution of the pathway to the glycolate pool is unknown, but the results support the existence of a sequence of reactions leading to glycolate synthesis during photosynthesis with pyruvate, isocitrate, and glyoxylate as intermediates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry J. A., Osmond C. B., Lorimer G. H. Fixation of O(2) during Photorespiration: Kinetic and Steady-State Studies of the Photorespiratory Carbon Oxidation Cycle with Intact Leaves and Isolated Chloroplasts of C(3) Plants. Plant Physiol. 1978 Dec;62(6):954–967. doi: 10.1104/pp.62.6.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes G., Ogren W. L., Hageman R. H. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1971 Nov 5;45(3):716–722. doi: 10.1016/0006-291x(71)90475-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Godavari H. R., Badour S. S., Waygood E. R. Isocitrate lyase in green leaves. Plant Physiol. 1973 May;51(5):863–867. doi: 10.1104/pp.51.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski L. A., Randall D. D., Blevins D. G. Purification and characterization of a novel NADPH(NADH)-dependent glyoxylate reductase from spinach leaves. Comparison of immunological properties of leaf glyoxylate reductase and hydroxypyruvate reductase. Biochem J. 1986 Nov 1;239(3):653–659. doi: 10.1042/bj2390653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B. Enhanced Incorporation of Tritium into Glycolate during Photosynthesis by Tobacco Leaf Tissue in the Presence of Tritiated Water. Plant Physiol. 1982 Jan;69(1):192–197. doi: 10.1104/pp.69.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B., Zelitch I. Relationship between Net CO(2) Assimilation and Dry Weight Accumulation in Field-Grown Tobacco. Plant Physiol. 1982 Sep;70(3):677–685. doi: 10.1104/pp.70.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwitzguebel J. P., Siegenthaler P. A. Purification of peroxisomes and mitochondria from spinach leaf by percoll gradient centrifugation. Plant Physiol. 1984 Jul;75(3):670–674. doi: 10.1104/pp.75.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Yamazaki R. K., Hageman R. H., Kisaki T. A survey of plants for leaf peroxisomes. Plant Physiol. 1969 Jan;44(1):135–147. doi: 10.1104/pp.44.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VICKERY H. B., HANSON K. R. The metabolism of the organic acids of tobacco leaves. 18. Effect of culture of excised leaves in solutions of potassium Ls-isocitrate. J Biol Chem. 1961 Aug;236:2370–2375. [PubMed] [Google Scholar]
- VICKERY H. B., WILSON D. G. Preparation of potassium dihydrogen Ls(+)-isocitrate from Bryophyllum calycinum leaves. J Biol Chem. 1958 Jul;233(1):14–17. [PubMed] [Google Scholar]
- ZELITCH I., GOTTO A. M. Properties of a new glyoxylate reductase from leaves. Biochem J. 1962 Sep;84:541–546. doi: 10.1042/bj0840541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELITCH I. THE RELATION OF GLYCOLIC ACID SYNTHESIS TO THE PRIMARY PHOTOSYNTHETIC CARBOXYLATION REACTION IN LEAVES. J Biol Chem. 1965 May;240:1869–1876. [PubMed] [Google Scholar]
- Zelitch I. Alternate pathways of glycolate synthesis in tobacco and maize leaves in relation to rates of photorespiration. Plant Physiol. 1973 Feb;51(2):299–305. doi: 10.1104/pp.51.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I., Berlyn M. B. Altered glycine decarboxylation inhibition in isonicotinic Acid hydrazide-resistant mutant callus lines and in regenerated plants and seed progeny. Plant Physiol. 1982 Jan;69(1):198–204. doi: 10.1104/pp.69.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. The photooxidation of glyoxylate by envelope-free spinach chloroplasts and its relation to photorespiration. Arch Biochem Biophys. 1972 Jun;150(2):698–707. doi: 10.1016/0003-9861(72)90088-4. [DOI] [PubMed] [Google Scholar]


