Abstract
This study objected to evaluate the accuracy of the gamma-glutamyl transpeptidase-to-platelet ratio (GPR), aspartate aminotransferase-to-platelet ratio index (APRI), red cell distribution width (RDW), and fibrosis-4 index (FIB4) index, compared with liver biopsy (LB), in predicting the severity of inflammation in drug-induced liver injury (DILI) patients.
We evaluated patients with DILI who were followed at the First Hospital of Jilin University and underwent LB. Accuracy of each method was analyzed using ROC analysis. Classifications of liver inflammation included G0–4.
One hundred fifty six DILI patients were included with LB and complete medical records. 62.8% (98), 39.1% (61), and 16.7% (26) were classified as ≥G2, ≥G3, or G4, respectively. The AUROCs, by degree of inflammation, were: ≥G2: GPR: 0.654, RDW: 0.635, APRI: 0.728, and FIB4: 0.739; ≥G3: GPR: 0.623, RDW: 0.703, APRI: 0.777, and FIB4: 0.781; and G4: GPR: 0.556, RDW: 0.647, APRI: 0.729, and FIB4: 0.714. To predict ≥G2 inflammation, there were no differences between the AUROCs for GPR, RDW, APRI, and FIB4. To predict ≥G3 inflammation, the AUROCs for FIB4 and APRI were higher than that for GPR (0.781 vs 0.623, P < .01; 0.777 vs 0.623, P < .05). As for G4 inflammation, the AUROCs for FIB4 and APRI were also higher than GPR (0.714 vs 0.556, P < .05, 0.729 vs 0.556, P < .05).
When the level of inflammation was higher than G2 in patients with DILI, it could be predicted using APRI and FIB4 as non-invasive markers for this condition.
Keywords: biological markers, drug-induced liver injury, inflammation
1. Introduction
Drug-induced liver injury (DILI) is liver damage caused by non-prescription or prescription chemical drugs, biological preparations, natural medicines, dietary supplements and their metabolites, traditional Chinese medicines, health products, or auxiliary materials.[1–4] DILI accounts for >50% of the acute cases of liver failure in Westernized countries and has been a focus public health problem.[5] The diagnosis of DILI is mainly made by the Roussel Uclaf Causality Assessment Method (RUCAM) scale and excluding other factors.[6] After identification by a history of exposure to suspicious drugs and confirmation of liver injury, nondrug-associated liver disease etiologies should be excluded. Liver biopsy (LB) is used for the diagnosis and the differential diagnoses.[7] The presence or absence and degree of fibrosis and identification of other important conditions (e.g., inflammation, steatosis, and necrosis) can be determined using LB.[8] However, LB is an expensive and invasive procedure with associated risks and complications (e.g., pain, bleeding, and even death).[9] Due to these limitations, non-invasive DILI biomarkers of assessment have been extensively studied and improved in recent decades.
Accumulating evidence points to the gamma-glutamyl transpeptidase-to-platelet ratio (GPR), aspartate aminotransferase-to-platelet ratio index (APRI), and the fibrosis-4 index (FIB4) have good performance to predict liver fibrosis.[10–13] APRI and FIB4 are recommended by WHO to assess the stage of liver fibrosis in CHB patients.[14] In addition, APRI has been reported to predict liver necro-inflammation in patients with chronic hepatitis B.[15] The distribution of peripheral red blood cells (i.e., red cell distribution width [RDW]) indicates systemic inflammation, which positively correlated with the degree of liver fibrosis and inflammation in chronic hepatitis patients.[16,17] Liver inflammation occurs in almost all etiologies of liver disease and throughout the progression of the disease. Due to continuous chronic inflammatory stimulation and wound-healing response, liver fibrosis and cirrhosis gradually develop. Thus, serological markers that predict liver fibrosis may also be used to predict liver inflammation. This study included 156 patients with DILI who underwent LB. Calculations of GPR, RDW, APRI, and FIB4 were also performed using liver function test and peripheral blood cell count results. Our aim was to evaluate the performance of GPR, RDW, APRI, and FIB4 values for the prediction of liver inflammation and to compare their diagnostic utility. We also examined the validity of a new score that combines APRI and FIB4 values to predict liver inflammation in DILI patients.
2. Methods
2.1. Study population and ethical guidelines
We collected the data of 1180 patients who underwent routine laboratory tests and LB (January 2010 through June 2018; The First Hospital of Jilin University, Jilin, China). A total of 220 patients with DILI were considered to be enrolled in our study. DILI was diagnosed according to the American College of Gastroenterology Guidelines for the diagnosis and management of drug-induced liver injury.[3] The exclusion criteria were a history of suspected or known acetaminophen hepatotoxicity or a history of a transplant (i.e., liver or bone marrow) before the liver injury, or both. A patient was eligible if they developed superimposed DILI and currently had nonalcoholic fatty liver disease or a viral infection. Patients with other underlying chronic liver disease conditions (e.g., autoimmune liver disease) and biliary obstruction were ineligible. 53 patients accompanied with other etiologies were excluded. Eleven patients were excluded for incomplete data of routine laboratory tests, leaving 156 patients in our study. The Independent Institutional Review Board of The First Hospital of Jilin University approved the recruitment of study participants and the study protocol. Each study participant provided written informed consent prior to enrollment in the study.
2.2. Diagnosis of DILI
The diagnosis of DILI is based on a history of medication exposure, laboratory criteria, and excluding other pathogenic factors.[3]
-
1.
Patients with a history of exposure to suspected drugs and with corresponding symptoms (such as fever, pruritus, anorexia, nausea and vomiting) after taking the drugs for 1 to 4 weeks;
-
2.
Liver injury from other possible causes was ruled out. (Such as viral hepatitis; CMV, HSV, EBV infection; alcoholic hepatitis; nonalcoholic fatty hepatitis; autoimmune hepatitis; biliary obstruction; Wilson disease; hypoxic/ischaemic hepatopathy; hemochromatosis).
-
3.
Serum biological indicators meet one of the following: a) alanine aminotransferase ≥5 upper limit of normal (ULN); b) alkaline phosphatase ≥2 ULN; c) total bilirubin ≥2 ULN accompanied with alanine aminotransferase ≥3 ULN.
2.3. Liver histological examination
Local anesthesia and a 16-gauge disposable needle (Hepafix, B. Braun, Melsungen, Germany) were used during ultrasound-guided percutaneous LB. Immediately after removal, the liver samples (15-mm minimum length) were preserved in 10% buffered formalin; they were then embedded in paraffin for histopathology. A five-stage scheme was used to classify the liver inflammation present in each sample (G0: no inflammation, G1: portal inflammation with infrequent lobular necrosis, G2: mild piecemeal portal necrosis and focal or spotty lobular necrosis, G3: moderate piecemeal portal necrosis and bridging lobular necrosis, and G4:, severe piecemeal portal necrosis and multilobular necrosis).[16] Two pathologists specialized in hepatic histopathology independently interpreted the results of the samples. Both pathologists were blinded to clinical and laboratory data associated with each sample. If a consensus could not be reached for a sample, another highly experienced pathologist examined the histopathology. Final result was determined after a discussion between all evaluators.
2.4. Definition
None to mild inflammation was defined as inflammatory stage G0-G1, moderate to severe inflammation was defined as inflammatory stage G2-G4, according to a 1995 draft in China.[18]
2.5. Routine laboratory tests
Before the LB was taken, fasting blood samples were taken to be used for routine laboratory tests. An automated hematology analyzer (UniCel DxH 600 Coulter, Beckman Coulter, USA) was used for the routine blood tests (e.g., platelet count [PLT], RDW). A fully automated biochemistry analyzer (Sigma-Aldrich, USA) was used for measurement of serum biochemical parameters (e.g., AST, ALT, TBIL and gamma-glutamyl transpeptidase [GGT]).
2.6. Model calculations
GPR, APRI, and FIB4 were calculated as followed[19,20]:
-
1.
GPR = GGT (IU/L)/PLT (109/L);
-
2.
APRI = AST(IU/L)/PLT (109/L)×100;
-
3.
FIB4 = age (years) × AST(IU/L)/PLT (109/L) × (ALT (IU/L))1/2.
These parameters were measured within 1 week before the LB.
2.7. Statistical analysis
Continuous variables were described by the mean (25th and 75th percentiles), and categorical variables were displayed by counts and percentages. t-test (for continuous variables) and Chi-Squared test (for categorical variables) were used to compare statistically significant. Receiver operating characteristic curves (ROC) and the area under the ROC (AUROCs) were evaluated and compared to assess each index performance for liver inflammation and cirrhosis diagnosis.[21] MedCalc was used for the ROC curve analysis, the Z-tests, and to compute and compare the AUROC values. Take the maximum value of the sums of sensitivity and specificity or by optimizing specificity (i.e., ≥95%) as cut-offs value. Results for sensitivity, specificity, positive predictive value, negative predictive value, and the correct percentage classified were obtained to evaluate diagnostic accuracy. All significance tests were 2-tailed, the threshold for statistical significance was P < .05. SPSS 13 (SPSS Inc., Chicago, IL, USA) and the MedCalc Statistical Software version 16.1 (MedCalc Software bvba, Ostend, Belgium) to perform the statistical analyses.
3. Results
A total of 156 DILI patients were finally included in the study. Fifty years was the median age (IQR = 40–55); 40 patients were male (25.6%). The median baseline AST, ALT, and TBIL values were 101.7 IU/L (IQR = 46.88–244.65), 159 IU/L (IQR = 56.93–330.85), and 24.75 μmol/L (IQR = 11.8–104.53), respectively. The mean baseline GGT was 137.3 IU/L (IQR = 71–267.6) and platelet count was 199 × 109/L (IQR = 167.25–260). The distribution for the liver inflammation was: G0 = 5 (3.2%); G1 = 53 (34.0%); G2 = 37 (23.7%); G3 = 35 (22.4%); and G4 = 26 (16.7%). Of the 156 enrolled patients, none to mild inflammation (G0-G1) and moderate to severe inflammation (G2-G4) were 58 (37.2%) and 98 (62.8%), respectively (Table 1).
Table 1.
Demographic and clinical characteristics of the drug-induced liver injury study population.
| Variable | Total | G0-G1 (n = 58) | G2-G4 (n = 98) | P |
| Male, N (%) | 40 (25.6) | 19 (47.5) | 21 (21.6) | .126 |
| Age (years) | 50.00 (40.00,55.00) | 47.00 (35.00, 53.00) | 50.50 (41.75, 57.00) | .011 |
| AST (IU/L) | 101.70 (46.88,244.65) | 56.55 (34.10, 145.80) | 160.65 (72.83, 272.08) | <.001 |
| ALT (IU/L) | 159.00 (56.93,330.85) | 88.15 (44.75, 196.43) | 234.25 (79.18,427 .00) | <.001 |
| GGT (IU/L) | 137.3 (71.0,267.6) | 110.5 (49.85, 244.73) | 159.55 (96.15, 275.18) | .019 |
| TBIL (μmol/L) | 24.75 (11.8,104.53) | 14.75 (10.93, 41.83) | 39.30 (14.15,111.33) | .539 |
| PLT (109/L) | 199.0 (167.25, 260.00) | 232.0 (183.75, 279.25) | 189.0 (153.00,242.75) | .004 |
| GPR | 1.19 (0.63, 2.33) | 0.75 (0.36, 1.82) | 1.27 (0.79, 2.51) | .001 |
| RDW | 14.00 (12.83, 15.30) | 13.20 (12.70, 14.53) | 14.30 (13.28, 15.53) | .480 |
| APRI | 1.40 (0.52, 3.26) | 0.71 (0.37, 1.62) | 2.00 (0.85,4.24) | <.001 |
| FIB4 | 2.00 (1.09,4.06) | 1.23 (0.81, 2.16) | 2.62 (1.48, 4.81) | <.001 |
| RPR | 0.07 (0.05,0.09) | 0.06 (0.05, 0.07) | 0.08 (0.06, 0.10) | <.001 |
| Hepatic Inflammatory grade (G0/G1/G2/G3/G4) | (3.2%)/5; (34.0%)/53; (23.7%)/37; (22.4%)/35; (16.7%)/26; | |||
Continuous variables are presented as median (25th and 75th percentiles). ALT = alanine transaminase, APRI = AST-to-platelet ratio index, AST = aspartate aminotransferase, FIB4 = fibrosis-4 index, GGT = gamma-glutamyl transpeptidase, GPR = gamma-glutamyl transpeptidase-to-platelet ratio index, PLT = platelet count, RDW = red cell volume distribution width, RPR = red cell distribution width-to-platelet ratio index, TBIL = total bilirubin. P < .05 indicates a statistically significant difference between the G0–G1 and G2–G4 groups.
The results indicated that the patients with moderate to severe inflammation (G2-G4) had significantly higher age (median, 50.5 vs 47 years, P = .011), AST (median, 160.65 vs 56.55 IU/L, P < .001), ALT (median, 234.25 vs 88.15 IU/L, P < .001), GGT (median, 159.55 vs 110.5 IU/L, P = .0019), GPR (median, 1.27 vs 0.75, P = .001), APRI (median, 2 vs 0.71, P < .001), FIB4 (median, 2.62 vs 1.23, P < .001), and RPR (median, 0.08 vs 0.06, P < .001). The mean platelet counts for these patients were significantly lower, compared with patients with none to mild liver inflammation (G0-G1) (189 × 109/L vs 232 × 109/L, respectively, P = .004). There was no difference in age, TBIL level, and RDW level between G0-G1 and G2-G4.
The results for correlations of the serum-based models and stages of liver inflammation were displayed in Figure 1. As liver inflammation increased, the median APRI, RDW, and FIB4 values increased (Fig. 2A, C, and D).
Figure 1.
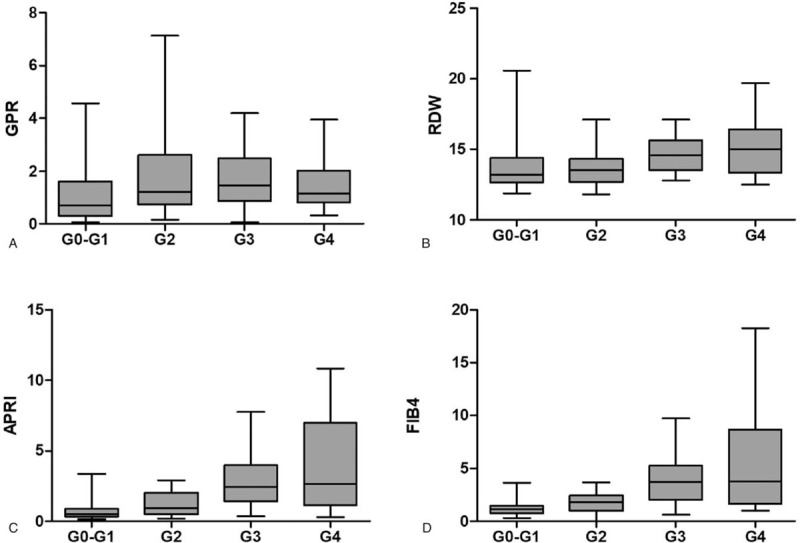
Box plots (with 95% confidence intervals) of (A) gamma-glutamyl transpeptidase-to-platelet ratio (GPR), (B) red cell volume distribution width (RDW), (C) aspartate transaminase-to-platelet ratio index (APRI) and (D) fibrosis-4 index (FIB4) index. The interquartile range is indicated by the box, and the median value is indicated by the line across the box.
Figure 2.
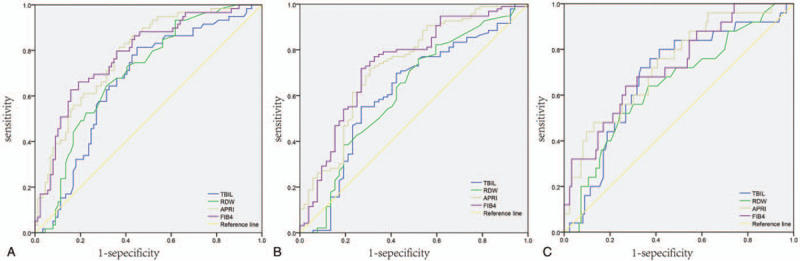
Receiver operating characteristic curves of gamma-glutamyl transpeptidase-to-platelet ratio (GPR), red cell volume distribution width (RDW), aspartate transaminase-to-platelet ratio index (APRI), fibrosis-4 index (FIB4), and total bilirubin (TBIL) for diagnosis of (A)≥G2, (B)≥G3, and (C) G4.
Examination of the AUROCs to predict ≥G2 (G2-G4) revealed no statistically significant differences in performance between GPR (AUROCs: 0.654, 95% confidence interval (CI) 0.563–0.745), RDW (AUROCs: 0.635, 95% CI: 0.541–0.729), APRI (AUROCs: 0.728, 95% CI: 0.644–0.812), FIB4 (AUROCs: 0.739, 95% CI: 0.656–0.822), and TBIL (AUROCs: 0.615, 95% CI: 0.515–0.715) (Table 2 and Fig. 2A). Examination of the AUROCs to predict ≥G3 (G3-G4) revealed that GPR performance (AUROCs: 0.623, 95% CI: 0.535–0.710) was lower than APRI (AUROCs: 0.777, 95% CI: 0.705–0.849, P < .05) and FIB4 (AUROCs: 0.781, 95% CI: 0.707–0.854, P < .01); the performance of TBIL (AUROCs: 0.663, 95% CI: 0.574–0.725) was also lower than APRI (AUROCs: 0.777, 95% CI: 0.705–0.849, P < .05) and FIB4 (AUROCs: 0.781, 95% CI: 0.707–0.854, P < .05) (Table 2 and Fig. 2B). For the prediction of G4 inflammation, GPR had lower performance (AUROCs: 0.556, 95% CI: 0.452–0.659) than that of APRI (AUROCs: 0.729, 95% CI: 0.626–0.832, P < .05) or FIB4 (AUROCS: 0.714, 95% CI: 0.608–0.820, P < .05) (Table 2 and Fig. 2C).
Table 2.
Diagnostic performance of serum-based models of liver inflammation in patients with drug-induced liver injury.
| ≥G2 | ≥G3 | =G4 | ||||
| Variables | AUROC | (95% CI) | AUROC | (95% CI) | AUROC | (95%CI) |
| GPR | 0.654 | 0.563–0.745 | 0.623 | 0.535–0.710 | 0.556 | 0.452–0.659 |
| RDW | 0.635 | 0.541–0.729 | 0.703 | 0.621–0.784 | 0.647 | 0.533–0.761 |
| APRI | 0.728 | 0.644–0.812 | 0.777 | 0.705–0.849 | 0.729 | 0.626–0.832 |
| FIB4 | 0.739 | 0.656–0.822 | 0.781 | 0.707–0.854 | 0.714 | 0.608–0.820 |
| TBIL | 0.615 | 0.515–0.715 | 0.663 | 0.574–0.725 | 0.679 | 0.567–0.791 |
| Comparison of AUROCs | ||||||
| FIB4 and APRI | >0.05 | >0.05 | >0.05 | |||
| FIB4 and GPR | >0.05 | <0.01 | <0.05 | |||
| FIB4 and RDW | >0.05 | > 0.05 | >0.05 | |||
| FIB4 and TBIL | >0.05 | <0.05 | >0.05 | |||
| GPR and APRI | >0.05 | <0.05 | <0.05 | |||
| GPR and RDW | >0.05 | >0.05 | >0.05 | |||
| GPR and TBIL | >0.05 | >0.05 | >0.05 | |||
| APRI and RDW | >0.05 | >0.05 | >0.05 | |||
| APRI and TBIL | >0.05 | <0.05 | >0.05 | |||
| RDW and TBIL | >0.05 | >0.05 | >0.05 | |||
APRI = aspartate transaminase-to-platelet ratio index, AUROCs = area under the receiver operating characteristic curves, 95% CI = 95% confidence interval, FIB4 = fibrosis-4 index, GPR = gamma-glutamyl transpeptidase-to-platelet ratio index, RDW = red cell volume distribution width, TBIL = total bilirubin. The bold values indicate a statistically significant result (P < .05).
The results for the diagnostic thresholds of the potential indicators of liver inflammation are displayed in Table 3. When the sums of sensitivity and specificity were maximized, we found that the optimal cut-offs for APRI were 0.94, 0.96, and 0.99, for diagnosis of ≥G2 (G2-G4), ≥G3 (G3-G4), and G4, respectively. The optimal cut-offs for FIB4 were 1.71, 2.72, and 3.32 for diagnosis of ≥G2 (G2-G4), ≥G3 (G3-G4), and G4, respectively. Because of the differences in the scores of each threshold in terms of diagnostic performance, and given the substantial agreement between the APRI and FIB4 scores revealed by the concordance analysis, we examined a predictive model that combined these 2 scores. The results indicated that the sensitivity was improved (from 74.5% with APRI alone and 71.4% with FIB4 alone) to 82.7% using the combined score to predict moderate to severe inflammation (≥G2). The correct percentage classified improved (from 71.8% with APRI alone and 71.1% with FIB4 alone) to 74.4% (Table 3).
Table 3.
Diagnostic thresholds of serum-based models of liver inflammation.
| Variable | Cut-offs | Se (%) | Sp (%) | PPV (%) | NPV (%) | Correct classified (%) |
| ≥G2 | ||||||
| APRI | 0.94 | 74.5 | 67.2 | 79.3 | 60.9 | 71.8 |
| FIB4 | 1.71 | 71.4 | 70.7 | 80.5 | 59.4 | 71.1 |
| Combined | 82.7 | 58.6 | 77.1 | 66.7 | 74.4 | |
| ≥G3 | ||||||
| APRI | 0.96 | 85.2 | 60.0 | 47.7 | 86.3 | 69.9 |
| FIB4 | 2.72 | 65.6 | 81.1 | 69.0 | 78.6 | 75.0 |
| Combined | 62.3 | 84.2 | 71.7 | 77.7 | 75.6 | |
| =G4 | ||||||
| APRI | 0.99 | 84.6 | 50.0 | 25.3 | 94.2 | 55.8 |
| FIB4 | 3.32 | 61.5 | 73.1 | 31.4 | 90.5 | 71.2 |
| Combined | 61.5 | 73.8 | 32.0 | 90.6 | 71.8 |
APRI = aspartate transaminase-to-platelet ratio index, FIB4 = fibrosis-4 index, NPV = negative predictive value, PPV = positive predictive value, Se = sensitivity, Sp = specificity. Take the maximum value of the sums of sensitivity and specificity to calculate Cut-offs.
4. Discussion
APRI and FIB4 are known to be 2 routine used noninvasive predictors of liver fibrosis. Only a few studies have reported their application to the prediction of liver inflammation.[15–17] The study by Wang et al showed that the AUROCs of APRI and FIB4 to predict significant liver inflammation stage (G2-G4) were 0.83 and 0.75 in chronic hepatitis patients (the study population included chronic hepatitis B, primary biliary cirrhosis and autoimmune hepatitis).[16] The results of Xu et al showed that the AUROCs of APRI and FIB4 to predict liver inflammation (G2-G4) in CHB patients is 0.802 and 0.740.[17] Our results showed that for G2-G4, AUROCs of APRI and FIB4 were 0.728 and 0.739. Our results are lower than those reported in the previous study. We speculated that there are 2 main reasons for our lower results. Firstly, both APRI and FIB4 values are calculated with AST, but our inclusion criteria of DILI patients do not take into account AST. As for the group of ≥G2 (G2-G4) in our project, AST ranged wildly from 72.83 to 272.08 IU/L. Secondly, the study population of Wang and Xu were all patients with chronic liver disease, which were associated with age. The patients with DILI are more acute liver injury, with a shorter course of the disease. In our study, there was no difference in age between G0-G1 and G2-G4 group (median: 47.00 vs 50.50 P = .011). However, FIB4 is an age-influenced biomarker. Taken together, these factors may account for the lower predictive performance of APRI and FIB4 for liver inflammation in DILI patients. Simin et al demonstrated that the AUROCs of APRI to predict liver inflammation is 0.66 in CHB,[15] significantly lower than our results. Considering that Simin applied different scoring criteria for the classification of liver inflammation than we did, the 2 results are not comparable.
We further investigated the predictive performance of APRI and FIB4 for different degrees of liver inflammation in DILI patients. We found that compared with GPR values, APRI and FIB4 values had better performance for≥G3 (G3-G4) and G4 inflammation. This conclusion is based on the results for the AUROCs for APRI vs GPR (0.777 vs 0.623, P < .05) and FIB4 vs GPR (0.781 vs 0.623, P < .01) for ≥G3 (G3-G4) inflammation, and APRI vs GPR (0.729 vs 0.556, P < .05) and FIB4 vs GPR (0.714 vs 0.556, P < .05) for G4 inflammation. However, our result indicated that APRI and FIB4 had no advantage in predicting ≥G2 (G2-G4) inflammation compared with GPR and RDW. Considering that APRI has a low AUROCs value of 0.683 for diagnosing early-stage hepatic fibrosis,[22] the liver lesions may be not severe enough to be detected by serum markers during the stage of mild inflammation.
Additionally, we obtained the Cut-offs value by maximizing the sum of sensitivity and specificity of APRI and FIB4. The Cut-offs value of APRI to predict ≥G2 (G2-G4), ≥G3 (G3-G4) and G4 inflammation were 0.94, 0.96, and 0.99, respectively. The Cut-offs value of FIB4 for each stage were, respectively, 1.71, 2.72, and 3.32. Compared to APRI and FIB4 values used alone, the combination of APRI and FIB4 improved the number correctly classified in all stages, especially in ≥G2 (G2-G4) inflammation. 71.8% and 71.1% patients were correctly classified testing APRI and FIB4, respectively. And it increased to 74.4% when combining these 2 indicators together in ≥G2 (G2-G4) inflammation. The correct percentage classified improved from 69.9% with APRI alone and 75% with FIB4 alone to 75.6% in ≥G3 (G3-G4) inflammation. And it improved from 55.8% with APRI alone and 71.2% with FIB4 alone to 71.8% in G4 inflammation. This is consistent with previously published results.[20,23] H. Ben et al found that in chronic HBV patients, combining APRI and FIB4 scores increased diagnostic performance for every stage of fibrosis. The correct percentage classified reached 67.2%, 80.3%, and 91.3% in significant fibrosis, severe fibrosis, and cirrhosis.[20] The combined application of APRI and FIB4 can be used not only to predict the stage of cirrhosis, but also to predict HCC. Paik et al demonstrated that the combination of APRI and FIB4 improved the highest sensitivity (85.7%) and specificity (88.9%) of HCC development in chronic HBV patients.[23] Thus, it is reasonable to combine these 2 indicators to predict liver inflammation.
In many liver diseases such as chronic hepatitis B, nonalcoholic fatty liver disease, primary biliary cirrhosis, and autoimmune hepatitis, RDW has been reported to be associated with the disease severity.[16,24–26] Besides, it is demonstrated that RDW increases with the progression of liver fibrosis and inflammation in chronic hepatitis patients.[16,17] However, our findings indicated that there was no difference in RDW between G0-G1 and G2-G4 (median: 13.2 vs 14.3 P = .480) in DILI patients. Patients with chronic liver disease are often accompanied with hypersplenism, in which the mononuclear phagocyte system is activated, leading to the destruction of RBCs and the release of larger immature RBCs into the peripheral blood. In addition, chronic hepatitis patients suffer from malnutrition for a chronic lack of appetite. Insufficient intake of folic acid, vitamin B12, and iron can also lead to increased RDW. But DILI most often develops rapidly and is not accompanied by these complications. Thus, RDW doesn’t change over a relatively short period. Due to the number of DILI patients enrolled in our study was small and there was no data on healthy control to match. Hence, whether RDW can be used as a potential biomarker of DILI needs further study.
Glucocorticoids are used in the treatment of DILI patients for its anti-inflammatory effects. However, corticosteroid therapy in DILI patients has been controversial. Corticosteroid therapy is not required for patients with mild or moderate DILI patients. But it is recommended for patients with severe DILI and a tendency to develop acute liver failure (ALF).[27] Accumulating evidence suggests that the higher the serum bilirubin level in DILI patients, the more severe the liver injury.[27,28] The decision to start corticosteroid treatment can be based on histological or biological (international normalized ratio >1.5 and/or bilirubin >2.5 mg/dl) indicators of severity.[29] A retrospective study of DILI (TBIL >85.5 umol/L) by Ping et al showed that serum TBIL in the corticosteroid therapy group were significantly higher than those in the non-corticosteroid group, and corticosteroid therapy was recommended when the severe DILI was accompanied by hyperbilirubinemia (TBIL >243 umol/L).[30] Data from Hou et al study of 70 DILI patients (TBIL > 10 ULN 20 of whom received corticosteroid therapy) also showed that TBIL levels were higher in the corticosteroid group than in the non-corticosteroid group, although not statistically significant.[31] Taken together, TBIL is an important parameter of corticosteroid use in DILI patients. We found that compared with TBIL, APRI and FIB4 had better performance for predicting liver inflammation, especially for ≥G3 inflammation. The differences were statistically significant; the AUROCs values were 0.777 vs 0.663 (APRI vs TBIL, P < .05), 0.781 vs 0.663 (FIB4 vs TBIL, P < .05). Therefore, APRI and FIB4 may be better indicators than TBIL for the severity of DILI and for determining glucocorticoid application.
This study is the first to report associations between non-invasive markers and liver inflammation in patients with DILI. APRI and FIB4 were better than GPR and TBIL for predicting ≥G3 (G3-G4) inflammation and had more power than GPR for predicting G4 inflammation. The combined score (APRI and FIB4) had greater power for the prediction of G2-G4 inflammation. Based on the predictive performance of APRI and FIB4 for liver inflammation, they may be new potential biomarkers for guiding corticosteroid use in DILI patients. In less developed regions, the use of non-invasive indicators is very economical and affordable. More large-scale studies are needed to determine the accuracy and validity of the use of these scores and their roles in follow-up after treatment of DILI patients.
Author contributions
Conceptualization: Xu Li.
Data curation: Dezhao Li, Le Wang.
Formal analysis: Hongqin Xu.
Funding acquisition: Xu Li.
Writing – original draft: Minjie Wan.
Writing – review & editing: Xu Li.
Glossary
Abbreviations: ALP = alkaline phosphatase, ALT = alanine aminotransferase, APRI = aspartate aminotransferase-to-platelet ratio index, AST = aspartate aminotransferase, AUROCs = area under the ROC, DILI = drug-induced liver injury, FIB4 = fibrosis-4 index, GGT = gamma-glutamyl transpeptidase, GPR = gamma-glutamyl transpeptidase-to-platelet ratio, HBV = hepatitis B virus, HCV = hepatitis C virus, LB = liver biopsy, PLT = platelet count, RDW = red cell distribution width, ROC = receiver operating characteristic curves, TBIL = total bilirubin.
References
- [1].Bjornsson ES, Bergmann OM, Bjornsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013;144:1419–25. [DOI] [PubMed] [Google Scholar]
- [2].Fontana RJ, Watkins PB, Bonkovsky HL, et al. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf 2009;32:55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 2014;109:950–66. quiz 67. [DOI] [PubMed] [Google Scholar]
- [4].Giordano C, Rivas J, Zervos X. An update on treatment of drug-induced liver injury. J Clin Transl Hepatol 2014;2:74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tujios SR, Lee WM. Acute liver failure induced by idiosyncratic reaction to drugs: challenges in diagnosis and therapy. Liver Int 2018;38:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yang H, Guo D, Xu Y, et al. Comparison of different liver test thresholds for drug-induced liver injury: updated RUCAM versus other methods. Front Pharmacol 2019;10:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hayashi PH, Fontana RJ. Clinical features, diagnosis, and natural history of drug-induced liver injury. Semin Liver Dis 2014;34:134–44. [DOI] [PubMed] [Google Scholar]
- [8].Sebastiani G, Alberti A. Non invasive fibrosis biomarkers reduce but not substitute the need for liver biopsy. World J Gastroenterol 2006;12:3682–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rustagi T, Newton E, Kar P. Percutaneous liver biopsy. Trop Gastroenterol 2010;31:199–212. [PubMed] [Google Scholar]
- [10].Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- [11].Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–26. [DOI] [PubMed] [Google Scholar]
- [12].Takyar V, Surana P, Kleiner DE, et al. Noninvasive markers for staging fibrosis in chronic delta hepatitis. Aliment Pharmacol Ther 2017;45:127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim MN, Lee JH, Chon YE, et al. Fibrosis-4, aspartate transaminase-to-platelet ratio index, and gamma-glutamyl transpeptidase-to-platelet ratio for risk assessment of hepatocellular carcinoma in chronic hepatitis B patients: comparison with liver biopsy. European J Gastroenterol Hepatol 2020;32:433–9. [DOI] [PubMed] [Google Scholar]
- [14]. Guidelines for the Prevention. Care and Treatment of Persons with Chronic Hepatitis B Infection. World Health Organization 2015. [PubMed] [Google Scholar]
- [15].Shoaei SD, Sali S, Karamipour M, et al. Non-invasive histologic markers of liver disease in patients with chronic hepatitis B. Hepat Mon 2014;14:e14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang H, Xu H, Qu L, et al. Red blood cell distribution width and globulin, noninvasive indicators of fibrosis and inflammation in chronic hepatitis patients. Eur J Gastroenterol Hepatol 2016;28:997–1002. [DOI] [PubMed] [Google Scholar]
- [17].Xu W-S, Qiu X-M, Ou Q-s, et al. Red blood cell distribution width levels correlate with liver fibrosis and inflammation: a noninvasive serum marker panel to predict the severity of fibrosis and inflammation in patients with hepatitis B. Medicine 2015;94:e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Guideline:prevention, treatment of viral hepatitis (draft). The fifth national conference on contagious disease and parasitic disease. Chin J Infect Dis 1995;13:241–7. [Google Scholar]
- [19].Maegawa FB, Shehorn L, Aziz H, et al. Association between noninvasive fibrosis markers and postoperative mortality after hepatectomy for hepatocellular carcinoma. JAMA Netw Open 2019;2:e187142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ben Ayed H, Koubaa M, Yaich S, et al. A new combined predicting model using a non-invasive score for the assessment of liver fibrosis in patients presenting with chronic hepatitis B virus infection. Med Mal Infect 2019;49:607–15. [DOI] [PubMed] [Google Scholar]
- [21].Abdollahi M, Pouri A, Ghojazadeh M, et al. Non-invasive serum fibrosis markers: a study in chronic hepatitis. Bioimpacts 2015;5:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yang XZ, Gen AW, Xian JC, et al. Diagnostic value of various noninvasive indexes in the diagnosis of chronic hepatic fibrosis. Eur Rev Med Pharmacol Sci 2018;22:479–85. [DOI] [PubMed] [Google Scholar]
- [23].Paik N, Sinn DH, Lee JH, et al. Non-invasive tests for liver disease severity and the hepatocellular carcinoma risk in chronic hepatitis B patients with low-level viremia. Liver Int 2018;38:68–75. [DOI] [PubMed] [Google Scholar]
- [24].Wang J, Huang R, Yan X, et al. Red blood cell distribution width: A promising index for evaluating the severity and long-term prognosis of hepatitis B virus-related diseases. Digestive Liver Dis 2020;52:440–6. [DOI] [PubMed] [Google Scholar]
- [25].Kim HM, Kim BS, Cho YK, et al. Elevated red cell distribution width is associated with advanced fibrosis in NAFLD. Clin Mol Hepatol 2013;19:258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hu Z, Sun Y, Wang Q, et al. Red blood cell distribution width is a potential prognostic index for liver disease. Clin Chemistry Laboratory Med 2013;51:1403–8. [DOI] [PubMed] [Google Scholar]
- [27].Hu PF, Xie WF. Corticosteroid therapy in drug-induced liver injury: pros and cons. J Dig Dis 2019;20:122–6. [DOI] [PubMed] [Google Scholar]
- [28].Verma S, Kaplowitz N. Diagnosis, management and prevention of drug-induced liver injury. Gut 2009;58:1555–64. [DOI] [PubMed] [Google Scholar]
- [29].De Martin E, Michot JM, Papouin B, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol 2018;68:1181–90. [DOI] [PubMed] [Google Scholar]
- [30].Hu PF, Wang PQ, Chen H, et al. Beneficial effect of corticosteroids for patients with severe drug-induced liver injury. J Digestive Dis 2016;17:618–27. [DOI] [PubMed] [Google Scholar]
- [31].Hou F-Q, Zeng Z, Wang G-Q. Hospital admissions for drug-induced liver injury: clinical features, therapy, and outcomes. Cell Biochemistry Biophysics 2012;64:77–83. [DOI] [PubMed] [Google Scholar]


