Abstract
Adult regeneration restores patterning of orthogonal body axes after damage in a post-embryonic context. Planarians regenerate using distinct body-wide signals primarily regulating each axis dimension: anteroposterior Wnts, dorsoventral BMP, and mediolateral Wnt5 and Slit determinants. How regeneration can coordinate perpendicular tissue axes without symmetry-breaking embryonic events is not fully understood. Here, we report that the planarian dorsoventral regulator bmp4 suppresses the posterior determinant wnt1 to provide patterning input to the anteroposterior axis. Double-FISH identified distinct anteroposterior domains within dorsal midline muscle that express either bmp4 or wnt1. Homeostatic inhibition bmp4 and smad1 expanded the wnt1 expression anteriorly, while elevation of BMP signaling through nog1;nog2 RNAi reduced the wnt1 expression domain and elevated bmp4 expression. Homeostatic BMP signal perturbation broadly affected anteroposterior identity as measured by expression of posterior Wnt pathway factors, and caused mislocalization of AP-regionalized pharynx progenitors, without strongly affecting expression domains of anterior regulators. Additionally, wnt1 inhibition elevated bmp4 expression in the tip of the tail. Therefore, dorsal BMP signals and posterior wnt1 mutually antagonize for patterning the tail. Furthermore, homeostatic bmp4 RNAi caused medial expansion of the lateral determinant wnt5 and reduced expression of the medial regulator slit. By contrast, nog1;nog2 RNAi restricted wnt5 expression. Double RNAi of bmp4 and wnt5 resulted in lateral ectopic eye phenotypes, suggesting bmp4 acts upstream of wnt5 to pattern the mediolateral axis. These results indicate bmp4 controls dorsoventral information and also, through suppression of Wnt signals, influences anteroposterior and mediolateral identity. Based on related functions across vertebrates and Cnidarians, Wnt and BMP cross-regulation could form an ancient mechanism for coordinating orthogonal axis patterning.
Author summary
Systems that coordinate long-range communication across axes are likely critical for enabling tissue restoration in regenerative animals. While individual axis pathways have been identified, there is not yet a complete understanding of how signaling allows repatterning across 3-dimensions. Here, we report an unanticipated linkage between anteroposterior, dorsoventral, and mediolateral systems in planarians through BMP signaling. We find that dorsally expressed BMP restricts posterior and lateral identity by suppressing distinct Wnt signals in adult planarians. These results reveal a potentially ancient role for separate axis systems to interact to generate coordinated growth in adult animals.
Introduction
Most animal forms are organized along orthogonal body axes. Bilaterian body plans typically involve head-to-tail (anteroposterior, AP), back-to-belly (dorsoventral, DV), and midline-to-lateral (mediolateral, ML) dimensions produced with high fidelity. Signals defining each body axis can function largely independently, and many animals use canonical Wnt signaling to regulate the AP axis and BMP signaling to regulate the DV axis [1–3]. Definitive body axes emerge through embryogenesis from initial asymmetries generated either by maternal cues such as oocyte polarity [4] or symmetry-breaking events such as sperm entry, cavitation, or gastrulation [3,5]. However, the ability of some species to undergo whole-body regeneration as adults suggests embryogenesis can be unnecessary to maintain and restore axis orthogonality. In planarians, acoels, and Cnidarians, patterning along individual tissue dimensions has been attributed to distinct spatial signaling pathways [6–13]. Because such organisms can re-establish body forms for many successive generations asexually, they could in principle inherit AP and DV asymmetries from each axis dimension separately. Alternatively, perpendicular axis systems might instead interact to enable coordinated growth.
The freshwater planarian Schmidtea mediterranea is a model for studying the principles of adult axis patterning due to its ability to regenerate nearly any surgically removed tissue and undergo perpetual homeostasis in the absence of injury. These abilities are supported by adult pluripotent stem cells termed neoblasts that differentiate into any of the approximately 150 cell types comprising the adult animal and assemble into functional and appropriately positioned and scaled tissues [14,15]. Neoblasts undergo regionalized specialization to form subsets of progenitors fated for tissues located at particular axial locations such as the eyes, pharynx, and dorsal-versus-ventral epidermal cells [16–18]. Neoblasts also hone to particular regions through their migratory ability [19–22] in order to form organs at particular locations in the body [23–25]. Therefore, spatial information is critical for controlling progenitor function in order to maintain and regenerate the planarian body plan.
Spatial organization in regeneration and homeostasis is provided by a specialized set of signaling factors termed position control genes (PCGs) that are expressed regionally from body-wall muscle [26]. Following amputations that truncate the body axis, PCG expression domains shift to provide tissue identity information, allowing for restoration of missing body regions [27,28]. The planarian AP axis is controlled by canonical β-catenin signaling involving the use of posteriorly expressed Wnts and their signaling outputs, and anteriorly expressed Wnt-inhibitors [7,9,27–30]. The Wnt ligand wnt1 and secreted Wnt inhibitor notum are expressed at the posterior and anterior poles, respectively, where they organize tail and head patterning [7,9,27–29,31]. By contrast, the DV axis is established by BMP signaling. bmp4 is expressed in a mediolateral gradient within dorsal muscle and acts to promote dorsal fates while repressing ventral fates, through feedback inhibition of a ventrally and laterally expressed admp homolog [6,10,32–35]. Fates along the ML axis are determined through the reciprocal antagonism of medial slit and the laterally expressed non-canonical Wnt ligand wnt5 [28,36]. slit inhibition results in a collapse of lateral tissues, such as eyes, onto the midline, while wnt5 inhibition causes opposite defects in which ectopic tissue, such as eyes, form laterally [28]. Inhibition of PCG factors results in mis-patterning phenotypes both in amputated animals regenerating a new blastema and also in uninjured animals using neoblasts to maintain their bodies through homeostasis [9,10,24]. Therefore, canonical Wnt, BMP, and Slit/Wnt5 signals constitutively control axis identities across three spatial dimensions. Cross-regulation among these factors has been documented previously. bmp4, wnt5, and slit all regulate the position of notum+ anterior pole progenitors to target to the DV and ML midpoint as an early step in anterior regeneration [37]. In addition, bmp4 also controls mediolateral eye placement, lateral tissue identity, regeneration of midline tissue after transverse amputation, and blastema outgrowth after longitudinal amputation [10] and regulates the epidermal injury-induced gene equinox to control head outgrowth, including the formation of the notum+ anterior pole, in transverse regeneration [38]. By contrast, beta-catenin-1(RNAi) animals regenerate ectopic heads that appear to have normal DV polarization, yet these outgrowths mainly occur at the lateral edge, corresponding to the surface DV midpoint [9]. These observations suggest a potentially complex set of interactions among the determinants of each individual body axis that still awaits full elucidation.
Results
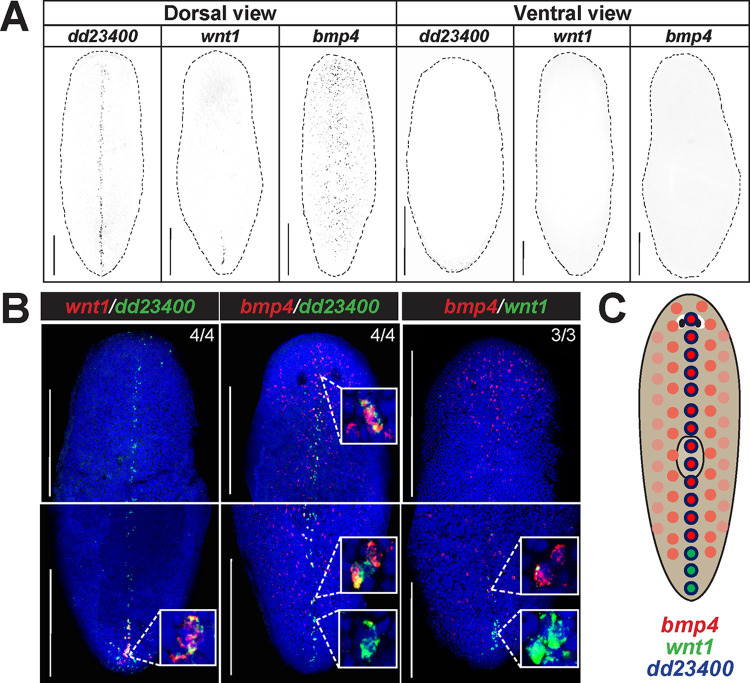
We sought to uncover possible relationships between key determinants of orthogonal body axes in planarians. Through fluorescence in situ hybridization (FISH), we examined the expression of bmp4 and observed that in addition to the prominent dorsal-versus-ventral expression pattern with highest expression on the dorsal midline [6,10,39], bmp4 expression was stronger in the anterior versus posterior of the animal and reduced at the posterior tip [10] (Fig 1A). Furthermore, we noted that expression of wnt1, a master regulator of posterior identity, is selective to the dorsal and not ventral side of animals in the posterior tail approximately where bmp4 expression is lower [9,28] (Fig 1A). wnt1 is known to be co-expressed in a posterior subset of muscle cells specific to the dorsal midline marked by expression of dd23400 [40]. Quantification of FISH intensity provided further support for these qualitative observations, and we compared replicate conditions by normalizing total intensity and position across either ML or AP axes (S1A–S1F Fig). bmp4 signal was measured to be in a graded pattern along the mediolateral axis dorsally, with highest expression at the midline, while dd23400 was expressed only in dorsal midline cells (S1A and S1D Fig). wnt1 signal had its maximum along the dorsal midline at the same location as dd23400 but only in the posterior (S1B and S1E Fig). Finally, comparing AP expression domains of wnt1 and bmp4 on the dorsal side, the wnt1-high regions also had relatively lower bmp4 expression and vice-versa (S1C and S1F Fig). To further validate these models of expression behavior, we performed double FISH to examine the features of co-expression of dorsal posterior wnt1, dorsal bmp4, and dorsal midline dd23400 (Fig 1B). dd23400 was co-expressed in the majority (88.9%) of wnt1+ cells (64/72 cells from 4 animals), similar to results reported previously [40]. Additionally, dd23400 and bmp4 were also co-expressed, as bmp4 expression was detected in 55.4% of dd23400+ cells (169/305 cells from 4 animals) on the dorsal midline. By contrast, bmp4 was only co-expressed in 12.1% of wnt1+ cells (4/33 cells from 3 animals), with double-positive cells only present at the most anterior of the wnt1 domain. By contrast, the remaining wnt1+ cells scored (29/33 from 3 animals) were more posterior and did not co-express bmp4. These results suggest a model in which dd23400+ cells are partitioned into an anterior population co-expressing bmp4 and a population in the far posterior co-expressing wnt1, with a limited overlap (Fig 1C).
Fig 1. bmp4 and wnt1 co-express with dd23400+ dorsal midline cells in a regionally distinct manner.
(A) Fluorescent in situ hybridization (FISH) detecting dd23400 on the dorsal midline, wnt1 on the dorsal posterior midline, and bmp4 in a dorsal midline-centered gradient with reduced expression in the far posterior. Dotted line indicates animal outline. Scale bars represent 150 μm with dorsal or ventral views indicated. (B) Double FISH detecting co-expression of wnt1, dd23400, and bmp4. Left: 88.9% of the cells that were wnt1+ were also dd23400+ (64/72 cells from 4 animals). Middle: 55.4% of dd23400+ cells also expressed bmp4 (169/305 cells from 4 animals). Right: Only 12.1% of cells expressing wnt1+ also co-expressed bmp4 (4/33 cells from 3 animals, top inset). The wnt1+ cells that expressed bmp4 were consistently the anterior-most cells of the wnt1 domain. By contrast, 87.9% (29/33 wnt1+ cells from 3 animals, bottom inset), all located at the posterior tip, did not co-express bmp4. (C) Schematic illustrating separation of bmp4 and wnt1 domains on the dorsal midline. Along dd23400+ dorsal midline muscle cells, wnt1 and bmp4 expression defines largely nonoverlapping AP domains within posterior. Within the wnt1+ domain of the representative image, wnt1+ cells in the anterior co-expressed bmp4 whereas cells in the posterior of the domain lacked bmp4 co-expression. Scale bars represent 150 μm.
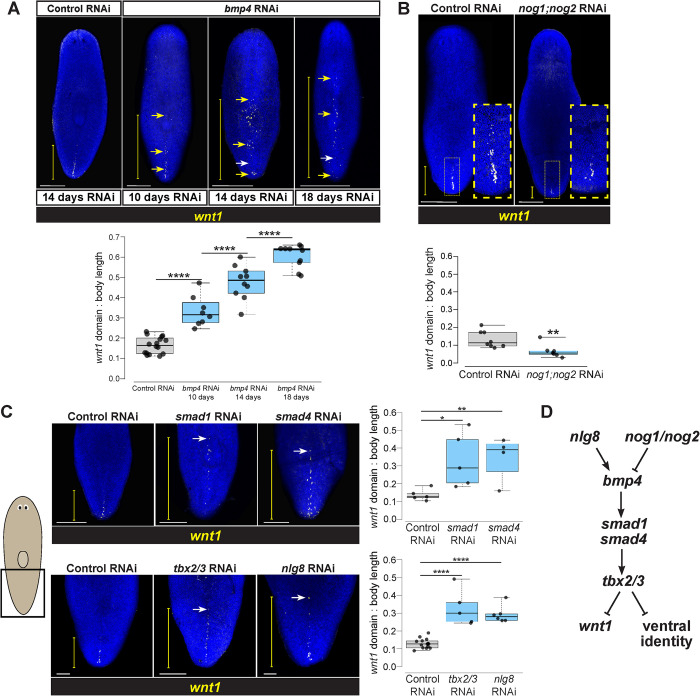
To test for possible functional relationships between bmp4 and wnt1, we first used RNA interference (RNAi) to examine the consequences of bmp4 inhibition. To circumvent the roles of bmp4 in directing expression of the injury-induced equinox gene essential for blastema outgrowth [38], we examined axis relationships using homeostatic RNAi in the absence of injury to specifically reveal possible interactions between axis patterning factors. After bmp4 RNAi, the wnt1 expression domain expanded dramatically anteriorly, but retained dorsally restricted specificity (Fig 2A). This domain increase occurred by 10 days of bmp4 RNAi and longer bmp4 RNAi treatments of 14 and 18 days resulted in wnt1+ cells progressing further toward the anterior of the animal. Numbers of wnt1+ cells increased in these conditions, indicating that the expanded range of wnt1 expression domain after bmp4 RNAi is likely due to control of expression territory and cell abundance rather than redistribution of a set number of wnt1+ cells (S2A Fig). The altered expression of wnt1 in bmp4(RNAi) animals was more sporadic and patchier compared to the normal wnt1 domain in control animals. Consistently, longer bmp4 RNAi treatments also resulted in a decrease to wnt1 expression in the posterior, suggesting both an expansion and shift of the wnt1 expression domain. In addition, wnt1 expression expanded laterally in bmp4(RNAi) animals (S2B Fig), indicative of a dual role for bmp4 in controlling both the AP and ML domain size for posterior wnt1 expression.
Fig 2. A BMP signaling pathway involved in DV identity restricts wnt1 expression to the posterior.
(A) FISH detecting wnt1 expression in animals following 14 days of homeostatic control RNAi and 10, 14, or 18 days bmp4 RNAi. Inhibition of bmp4 expanded wnt1 expression toward the anterior. Increased time of bmp4 inhibition results in further anterior expression of wnt1. The wnt1 domain expanded anteriorly and eventually had reduced expression in the posterior tip (yellow arrows show ectopic wnt1+ cells, white arrows show absence of wnt1+ cells relative to controls). (B) wnt1 expression domain size was reduced in nog1;nog2(RNAi) animals treated with dsRNA for 18 days of homeostasis (yellow boxes, magnified insets). (C) Top: FISH of wnt1 following 14 days of control, smad1, or smad4 RNAi. Bottom: FISH detecting wnt1 after 18 days of control, tbx2/3, or nlg8 RNAi. Inhibition of these BMP signaling components resulted in anterior expansion of wnt1 expression. (A-C) White scale bars represent 150 μm, and yellow brackets indicate wnt1 AP expression domain size. Box plots showing the distance from the posterior tip of the animal to the most anterior wnt1 expression relative to body length after indicated treatments. N ≥ 4 animals. Box plots show median values (middle bars) and first-to-third interquartile ranges (boxes); whiskers indicate 1.5× the interquartile ranges, and dots are data points from individual animals. *p<0.05, **p<0.01, ****p < 0.0001 by one-way ANOVA on ranks test (A,C) or by 2-tailed t-test (B). (D) Pathway model for components acting in DV determination and control of wnt1.
To gain insights into whether BMP signaling acts permissively or instructively in regulation of wnt1, we inhibited Noggin homologs nog1 and nog2 known to negatively regulate bmp4 in planarian DV determination [33]. Homeostatic inhibition of nog1 and nog2 for 18 days decreased the wnt1 expression domain (Fig 2B), which corresponded to a decline in wnt1+ cell abundance (S2C Fig). Therefore, BMP signaling likely plays an instructive role in limiting posterior wnt1 identity rather than only a permissive role.
We next examined whether this role of bmp4 in controlling a regulator of AP identity occurred via a canonical BMP pathway signaling through Smad1 and Smad4 effectors. Planarian smad1 and smad4 are known to mediate dorsoventral identity along with bmp4 [6,10]. Following a 14-day inhibition, both smad1(RNAi) and smad4(RNAi) animals had significantly anterior expansion of wnt1 expression, phenocopying the effects of bmp4 RNAi on wnt1 (Fig 2C, top). Given these results, we next investigated potential regulation of wnt1 by other factors known to act with BMP signaling to control dorsoventral identity. We examined the effects of inhibiting tbx2/3, a transcription factor shown to act downstream of bmp4 for control of DV identity in Dugesia japonica [41]. Following 18 days of tbx2/3 RNAi, wnt1 expression was likewise significantly expanded toward the anterior (Fig 2C, bottom). Similarly, we investigated nlg8, a noggin-like gene that facilitates BMP signal activation, is expressed dorsally, and whose inhibition phenocopies the ventralization phenotypes observed after bmp4 RNAi [33]. Homeostatic RNAi of nlg8 for 18 days caused expansion of the wnt1 domain (Fig 2C, bottom). While bmp4 RNAi caused a marked increase in numbers of wnt1+ cells and nog1;nog2 RNAi caused a decrease, other gene inhibitions under these conditions (smad1, smad4, tbx2/3, nlg8) only affected the domain size and did not cause a statistically significant increase to absolute cell numbers (S2D and S2E Fig). We suggest it is likely this distinction from the bmp4(RNAi) phenotype could be due to differences in expressivity, pleiotropy, or timing of RNAi effects from inhibition of other BMP pathway components in this experiment. However, we cannot rule out the possibility of the involvement of alternative pathways or other TGFbeta-related inputs controlling this phenomenon. Taken together, these experiments provide support that a bmp4 signaling pathway closely linked to dorsoventral identity determination acts to restrict the expression domain of the posterior determinant wnt1 (Fig 2D).
wnt1 also undergoes dramatic expression dynamics early in regeneration. Wound sites express wnt1 in muscle cells early after wounding, and optimal injury-induced wnt1 expression depends on bmp4 signals to induce expression of the novel secreted factor equinox, which in turn activates many injury-induced genes [1,28,38]. In addition, regenerating tail fragments undergo an extensive remodeling of pre-existing territories so that regeneration restores the overall body proportionality without restoring absolute size. In regenerating tail fragments, wnt1 expression undergoes an initial anterior expansion along the midline by 18 hours post-amputation, followed by eventual restriction and re-establishment of a new AP axis through rescaling over several days [28]. We tested whether bmp4 inhibition would affect these regeneration-dependent behaviors of wnt1 expression along the posterior midline in amputated tail fragments. In these animals, bmp4 RNAi resulted in an anterior expansion of the wnt1 domain from the homeostatic knockdown prior to amputation and so was present in animals fixed immediately after amputation (S3 Fig). By 18 hours, control tail fragments underwent an anterior expansion of wnt1 along the midline, while bmp4(RNAi) tail fragments retained an expanded wnt1 domain. However, bmp4(RNAi) animals underwent apparently normal resetting of wnt1 domains during the rescaling period by 96 hours, similar to control animals. By contrast, a prior study found that inhibition of the STRIPAK complex factor mob4 led to anteriorly expanded wnt1 but loss of regeneration-induced rescaling of wnt1 territories [40]. Therefore, although bmp4 negatively regulates wnt1 homeostatically, it is unlikely that the reduction to the wnt1 expression domain through regenerative rescaling occurs through control of bmp4 under normal conditions. Furthermore, it is likely that bmp4 and mob4 act separately to control wnt1 expression.
We next tested whether the wnt1 expansion phenotype might involve regulation of expression states in existing cells or control of cell regional identity through regulation of newly produced wnt1+ cells. To test this hypothesis, we used sublethal X-ray irradiation to deplete neoblasts and then determine whether the wnt1 expansion phenotype after bmp4 RNAi is stem-cell dependent. Animals were untreated or subjected to 1350 rads of irradiation then taken through 12 days of control or bmp4 RNAi (S4 Fig). The untreated animals robustly displayed expanded wnt1 expression after bmp4 RNAi conditions, compared to control RNAi untreated animals. However, irradiated control or irradiated bmp4(RNAi) animals lacked any detectable wnt1 expression. We interpret these results as likely indicating that neoblasts are required for both the bmp4(RNAi) wnt1 expansion phenotype and also to sustain the wnt1+ domain in control animals. We note that although a shorter-term recovery with fewer dsRNA feedings (8 days and 2 feedings) after irradiation can still support expression of wnt1 in the tail tip in normal animals [40], in order to detect the bmp4(RNAi) phenotype on wnt1, a longer irradiation recovery and dsRNA dosing period was required (12 days and 5 feedings). We cannot rule out that irradiation has some other effect to disrupt the bmp4(RNAi) wnt1 expansion phenotype, but given the prominent effect of irradiation in planarians to selectively deplete neoblasts, these results argue that bmp4 RNAi likely expands and shifts the wnt1+ domain through controlling the differentiation of cells with wnt1+ posterior identity.
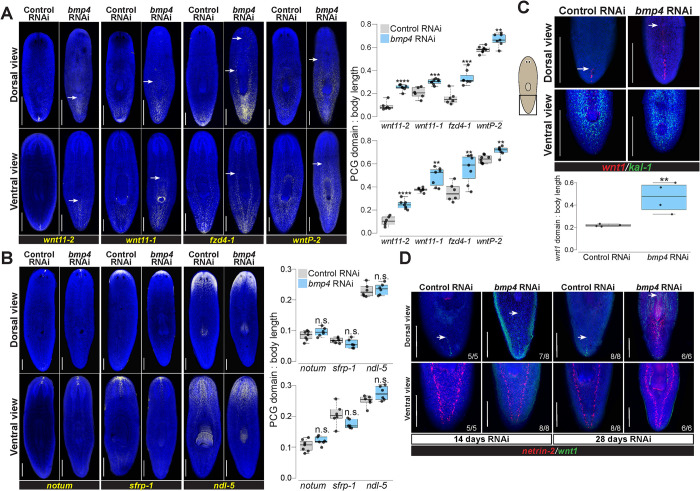
To investigate whether the role of BMP signaling was limited to wnt1 or more broadly affected posterior identity in general, we assessed expression of other posterior markers following 28 days of bmp4 RNAi. Posterior markers wnt11-2, wnt11-1, fzd4-1, and wntP-2 are expressed in successively broader posterior domains, have roles in tail and trunk patterning [7,24,30,31,42], and are expressed in a beta-catenin-dependent manner [43–45]. Most known posterior markers such as these factors are expressed both dorsally and ventrally, unlike wnt1, but in regeneration their expression depends on wnt1 [27,28]. bmp4 inhibition expanded the domains of all four posterior markers in both their dorsal and ventral domains (Fig 3A). Therefore, BMP, a dorsal signal, broadly limits posterior identity across the dorsal and ventral axes.
Fig 3. bmp4 restricts posterior identity independent of DV control.
(A) Animals were fixed after 28 days of homeostatic control or bmp4 RNAi and stained by FISH as indicated for markers of AP axis identity. bmp4 RNAi caused an anterior expansion of the posterior markers wnt11-2, wnt11-1, fzd4-1, and wntP-2 on both dorsal and ventral sides. White arrows indicate the expansion of posterior markers. (B) FISH stained animals following 28 days of control or bmp4 RNAi. bmp4 inhibition did not strongly affect AP distribution of anterior markers notum, sfrp-1, or ndl-5. (A-B), Graphs show the measurement of indicated marker domains normalized by total length of animal. N ≥ 6 animals. (C) FISH showing wnt1 and ventral marker kal-1 expression comparing 14 days of control and bmp4(RNAi). Dorsal view (upper) shows that inhibition of bmp4 expanded wnt1 expression anteriorly (arrows indicate anterior-most wnt1+ cell) at a time prior to any dorsal expression of the ventral marker kal-1 expression (4/4 animals). Boxplot comparing wnt1 expression normalized by body length between control and bmp4(RNAi) animals. N = 4 animals. (D) Dorsal (upper) and ventral view of animals fixed after 14 or 28 days of control or bmp4 RNAi and stained with wnt1 and ventral muscle and ventral nerve cord marker netrin-2. wnt1 expression expanded in bmp4(RNAi) animals by day 14 at a time prior to expression of netrin-2 dorsally while netrin-2 expression was prominently dorsal by day 28. N ≥ 5 animals. (A-C) Box plots shows median values (middle bars) and first-to-third interquartile ranges (boxes); whiskers indicate 1.5× the interquartile ranges and dots are data points from individual animals. *p<0.05, **p<0.01, ***p < 0.001, ****p<0.0001, n.s. indicates p>0.05 by 2-tailed t-test. Scale bars, 300 μm (A-B) or 150 μm (C).
By contrast, markers of far anterior identity did not appear to become restricted under these conditions of homeostatic bmp4 RNAi. We stained bmp4(RNAi) animals for notum, sfrp-1, and ndl-5 in order to assess AP identity over a range of the anterior region [7,9,28,29]. Compared to control animals, there was no significant change in these expression patterns in the AP direction on either dorsal or ventral side of the animals (Fig 3B). Quantification of notum+ cells following bmp4 RNAi revealed no change in absolute number or relative number of anterior pole cells (S5 Fig). We note, however, that decapitated and regenerating bmp4(RNAi) animals have been shown to undergo a dorsal shift to the location of their anterior pole [46], and eventually form dorsal cephalic ganglia and an extra set of dorsal eyes [10]. These transformations may impact anterior pattern expression to some degree, and we noted that the domain of ndl-5 expression, present throughout the head of normal animals, appeared mediolaterally modified after homeostatic bmp4 RNAi. However, our data indicate a strong role of bmp4 homeostatically in regulation of posterior gene expression.
Because bmp4(RNAi) animals undergo a progressive ventralization, we considered the possibility that ventral tissue identity might indirectly influence wnt1 expression in these animals. To ascertain possible relationships between ventralization and posteriorization phenotypes, we examined bmp4(RNAi) animals at an early time in their phenotypic progression after 14 days of dsRNA feeding, then simultaneously assessed both phenotypes. These animals had expanded wnt1 but not yet dorsal expression of the ventral epidermal marker kal-1 (Fig 3C). However, longer-term bmp4 RNAi ultimately results in the dorsal expression of kal-1 as the epidermis becomes ventralized during tissue turnover [16]. To provide further demonstration of the order of these events, we assessed another ventral-specific tissue by staining for netrin-2, which marks the ventral nerve cords and a muscle domain [47]. Similar experiments were performed with day 14 and day 28 time points of homeostatic bmp4 RNAi, and animals were co-stained for wnt1 and netrin-2 to evaluate posteriorization and ventralization simultaneously (Fig 3D). Day 14 bmp(RNAi) animals had expansion of wnt1 and no detectable dorsal expression of netrin-2, but by day 28 such animals had considerable dorsal netrin-2 expression. Therefore, our results suggest that the anterior wnt1 expansion after bmp4 RNAi is unlikely a secondary consequence of tissue ventralization and instead could represent a separate use of BMP signaling for planarian AP axis patterning.
Because bmp4 inhibition altered AP domains of posterior PCG factors, we sought to determine if inhibition of bmp4 also affected AP determination of any differentiated tissues or AP-restricted progenitors. We compared control and bmp4 RNAi animals to assess intestine and pharynx markers (S6A Fig). The intestine maker porcupine showed no change following bmp4 RNAi. SMU15007112, which marks a small population of cells adjacent to the posterior end of the pharynx, did not change its position with loss of bmp4. In bmp4 RNAi, the overall position of pharynx marker dd554 staining with respect to the body axis was normal relative to body size (S6A and S6B Fig), and animals did not form an ectopic pharynx, suggesting that location of the pharynx under these conditions was unaffected by bmp4 RNAi. However, we noticed that in control animals, dd554 expression forms short streams of cells located radially away from the anterior portion of the pharynx, and these were absent in bmp4(RNAi) animals (S6A Fig). Based on single-cell RNAseq, irradiation and neoblast depletion studies, dd554 has been suggested to mark migratory progenitor intermediates for at least some pharyngeal cell types [48,49]. Whether bmp4-dependent dd554 cell streams represent a migratory progenitor state is unknown, so we examined possible effects on the pharynx progenitor marker foxA, which is expressed in specified neoblasts in the central region of the body surrounding the pharynx [18,50]. Homeostatic bmp4 RNAi reduced foxA expression in the normal vicinity of the pharynx but strongly elevated its expression elsewhere in the body at more anterior and posterior locations. Similarly, inhibition of bmp4 reduced expression of the pharynx marker laminin within the pharynx but its expression became elevated in bilateral anterior locations associated with the head. Within the cohort of animals tested in this experiment, individuals with relatively more reduction to laminin expression in the pharynx also had the most elevated expression in the anterior (3 of 8 animals). The mis-regulation of dd554, foxA, and laminin may indicate complex ways that bmp4 regulates either specification or targeting of AP-restricted progenitors and/or differentiated cells. One possible explanation for these results is that in bmp4(RNAi) animals a fraction of pharynx progenitors are unable to correctly target to the pharynx and become mistargeted to aberrant locations.
We also measured effects on the AP position of eyes in homeostatic bmp4(RNAi) animals. bmp4(RNAi) animals form an extra set of medial eyes, and we noticed that long-term bmp4 RNAi resulted in a consistent displacement of the original versus ectopic eyes in these animals. Measurements of the relative position of these eyes with respect to the anterior tip revealed that the original eyes became located too anteriorly while the ectopic eyes had exactly the same relative position as control eyes (S6C Fig). Other methods to disrupt eye patterning in planarians, for example through homeostatic notum RNAi, cause ectopic eyes to form at positions located correctly with respect to body landmark tissues and PCG expression domains [25]. By contrast, pre-existing eyes in such conditions can self-sustain in spite of an incorrect location, due to the flexible targeting mechanisms used by migratory eye progenitor cells [23,25]. Therefore, we interpret our results to indicate that homeostatic bmp4 RNAi likely caused an AP tissue transformation that displaced pre-existing eyes anteriorly. Taken together, our analysis indicates bmp4 inhibits posterior Wnts and also controls varied aspects of AP cell-type organization including pharyngeal progenitors and eye location.
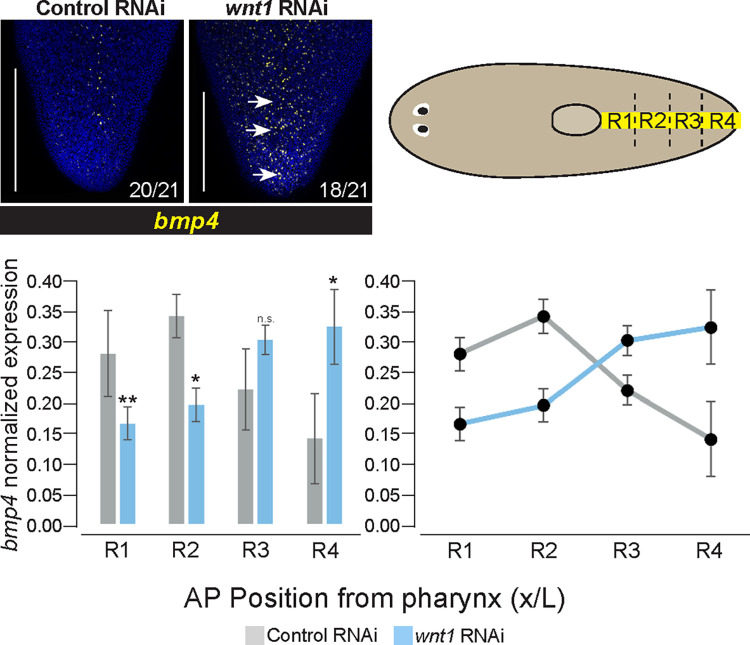
Given that bmp4 and wnt1 expression was enriched in separate anterior and posterior domains, we further examined whether these genes might undergo mutual negative regulation. To test this possibility, we inhibited wnt1 homeostatically, followed by FISH to detect expression of bmp4. Following 14 days of wnt1 RNAi, tails began to retract and become bulged, as reported previously [40]. In these animals, the bmp4 expression pattern was altered to be expressed more highly in the far posterior of the animal but retained its dorsal specificity and overall pattern elsewhere in the body (Figs 4 and S7A). Measurements of normalized FISH intensity of bmp4 expression in the posterior demonstrate that this difference is significant. Therefore, inhibition of wnt1 permitted bmp4 expression in the dorsal posterior tip of the animal in a domain normally expressing wnt1. Together with the prior results, these experiments suggest a reciprocal antagonism between wnt1 and bmp4 to define each other’s expression boundaries and consequently pattern the posterior.
Fig 4. wnt1 inhibits bmp4 expression in the posterior.
FISH staining for bmp4 expression in the tail after 14 days of control or wnt1 RNAi homeostatically. Dorsal views, arrows indicate observed ectopic bmp4 expression. Number of animals scored qualitatively as normal or elevated are indicated in the panel. Scale bars are 150 μm. N = 21 animals. Upper right cartoon depicts strategy for quantification of FISH signal. FISH intensity was measured using ImageJ along the midline using a linescan approximately 1/10 the width of the animal fragment at the pharynx and measured between the tip of the tail and posterior of pharynx, across 3 biological replicates for each sample. AP positions were normalized and intensity normalized to total intensity across this range, and individual intensities were summed across 4 equal-sized regional bins. Plots show histograms (left) and line-graphs (right) of the average intensity of each bin across biological replicates, bars show standard deviations. *p<0.05, **p<0.01, n.s. indicates p>0.05 by 2-tailed t-test applied between mean binned intensities for each region measured across biological replicates. wnt1 inhibition led to an overall increase in the relative amount of bmp4 expression in the tip of the tail (R4).
We also explored other possible regulation that could impact bmp4 expression domains. In regeneration, wnt1 controls head-versus-tail identity determination [27], but homeostatic wnt1 knockdown has only been reported to influence tail identity [40,51]. However, to exclude the possibility that the effects of homeostatic wnt1 inhibition on bmp4 expression domains might be explained by head-tail polarity reversal, we stained these animals for anterior pole marker foxD. These wnt1(RNAi) conditions resulted in bmp4 far posterior expansion and no expression of foxD in the posterior (S7B Fig). Therefore, the wnt1 and bmp4 regulation observed here is not likely due to a switch in pole identity determination. We also further explored possible influences of the anterior pole on bmp4 domains by inhibiting notum homeostatically. However, notum(RNAi) animals did not appear to have modifications to the gradient of bmp4 within the tail and did not alter the dorsal-specific expression of bmp4 under these conditions (S7C Fig). We also tested whether nog1;nog2 RNAi influenced bmp4 expression levels in the tail. We found that nog1;nog2(RNAi) animals had qualitatively higher expression of bmp4 (S7D Fig). The qualitative increase in signal intensity of bmp4 following nog1;nog2 RNAi suggests bmp4 expression could be under positive feedback control from BMP signaling.
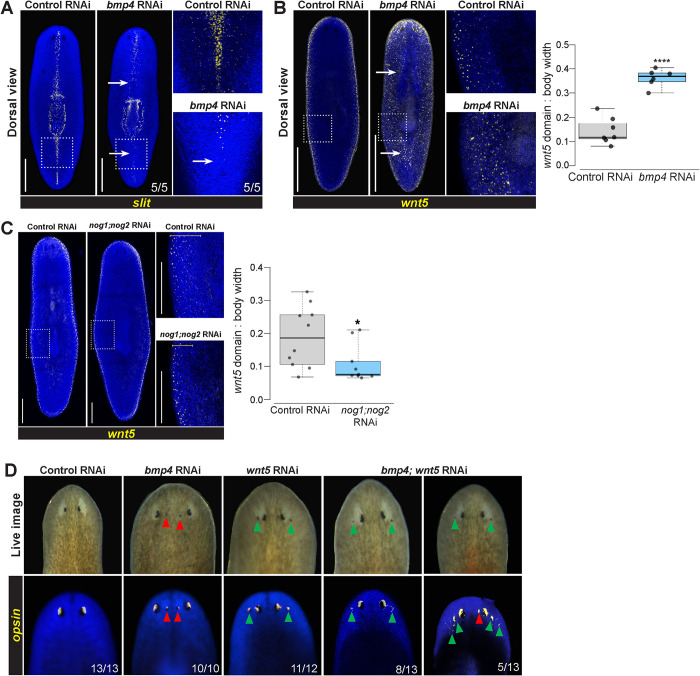
In light of the unexpected role of BMP signals in AP axis patterning, we next sought to clarify how bmp4 participates in ML axis regulation. bmp4 RNAi causes lateral tissue expansion and also failure to produce lateral tissue after lateral amputations [6,10]. In addition, bmp4 RNAi causes transverse regeneration to proceed with midline indentations [6,10], likely because of bmp4’s role in dorsoventrally positioning the notum+ anterior pole during head blastema outgrowth [46]. Furthermore, bmp4(RNAi) homeostasis animals form an extra set of eyes medially, consistent with this factor having additional roles in ML axis formation [6,10]. However, the relationship between bmp4 and other ML axis patterning factors slit and wnt5 is not fully understood. We next examined bmp4’s role in homeostatically maintaining midline marker expression. Following 28 days of bmp4 RNAi, expression of the midline determinant slit was reduced, particularly in the posterior of the animal (Fig 5A). These results suggested that slit might function downstream of bmp4 for controlling midline information. Furthermore, inhibition of bmp4 reduced and disrupted the dd23400 midline expression pattern and expanded its expression domain laterally (S8A Fig), suggesting BMP controls midline identity broadly. Taken together, these data suggest that bmp4 promotes medial identity and is important for establishing the boundaries of medial territories.
Fig 5. bmp4 regulates ML patterning upstream of lateral wnt5.
(A-C) FISH detecting slit or wnt5 following 28 days of homeostatic control or bmp4 or nog1;nog2 RNAi. Right panels show enlargements of boxed regions. Scale bars represent 300 μm. (A) bmp4 RNAi caused reduction of slit in the anterior and elimination in the posterior (arrows). (B) Inhibition of bmp4 caused medial expansion of wnt5 (arrows). (C) RNAi of nog1;nog2 reduced the size of the wnt5 lateral expression domain. (B-C) Graphs show wnt5 expression domain width (dorsal side) normalized to body width. *p<0.05, ****p<0.0001 by unpaired 2-tailed t-test; N ≥ 6 animals. Box plots show median values (middle bars) and first to third interquartile ranges (boxes); whiskers indicate 1.5× the interquartile ranges and dots are data points from individual animals. (D) Eyes assessed by live imaging (top) or opsin FISH (bottom) after 21 days of homeostatic RNAi to inhibit bmp and/or wnt5 and scored for lateral (green arrows) or medial ectopic eyes (red arrows). For single-gene RNAi, dsRNA for the targeted gene was mixed with an equal amount of control dsRNA so that the total amounts of each targeted dsRNA delivered were equal between single- and double-RNAi conditions. 100% of bmp(RNAi) animals had ectopic medial eyes and 92% wnt5(RNAi) animals had ectopic lateral eyes. By contrast, 100% of bmp4;wnt5(RNAi) animals had at least two lateral ectopic eyes, and of these 38% also formed a single medial ectopic eye (right panels) while 62% only formed ectopic lateral eyes (left panels). Co-inhibition of wnt5 reduced the penetrance of the medial ectopic eye phenotype due to bmp4 inhibition (p = 0.0027, 2-tailed Fisher’s exact test).
We next considered how bmp4 might interact with lateral regulatory factor wnt5. We first used the lateral epidermal marker laminB to confirm prior results that bmp4 inhibition generated ectopic lateral tissue (S8B Fig) [32]. We then examined a possible regulatory relationship between bmp4 and the lateral determinant wnt5. Following 28 days of bmp4 RNAi, expression of wnt5 significantly expanded to occupy distant medial territories on the dorsal side (Fig 5B), and more weakly expanded wnt5 expression on the ventral side (S8C Fig). Furthermore, we tested whether BMP pathway activation by nog1;nog2 RNAi might oppositely affect wnt5 expression. Indeed, nog1;nog2 inhibition restricted the wnt5 expression domain (Fig 5C). Together, these results suggest that BMP signaling levels might instructively determine the location of slit+ and 23400+ tissue at its highest levels medially and wnt5+ identity at the lowest levels laterally.
We next tested whether bmp4 and wnt5 undergo reciprocal negative regulation by inhibiting wnt5 for 21 days and staining for bmp4. Inhibition of wnt5 did not cause an apparent increase or decrease of the dorsal bmp4 gradient under these conditions (S9 Fig). In some wnt5(RNAi) animals, sporadic bmp4+ cells were detected ventrally, although these lacked a clear spatial enrichment and were small in number. Because the prominent dorsal mediolateral expression gradient of bmp4 was normal in these animals, these results suggested that wnt5 may not regulate mediolateral identity through control of bmp4 expression dorsally, but it may have a subtle or indirect role on dorsoventral determination. Together, these experiments indicate bmp4 strongly antagonizes wnt5 expression, promotes medial identity, and suppresses lateral identity.
To determine whether bmp4 functionally controls ML identity in part through regulation of wnt5, we conducted epistasis tests using eye placement as a readout. bmp4 RNAi causes the formation of ectopic medial eyes, whereas wnt5 RNAi produces an opposite defect of the formation of ectopic lateral eyes [6,7,10]. To test for interactions between bmp4 and wnt5, we homeostatically inhibited these genes individually or together for 21 days, examined animals visually, and stained them with an opsin riboprobe to label photoreceptor neurons (Fig 5D). Control animals had no ectopic eyes (13/13 animals), while 100% of bmp4(RNAi) animals (10/10 animals) had ectopic medial eyes, and 92% of wnt5(RNAi) animals (11/12 animals) had lateral eyes. In bmp4;wnt5(RNAi) animals, however, 100% of animals (13/13 animals, left) had at least two lateral ectopic eyes. Of these animals, 38% (5/13 animals, right) also had a single medial ectopic eye, while no animals displayed the bmp4(RNAi) phenotype of only medial ectopic eyes. Therefore, wnt5 likely does not operate exclusively upstream of bmp4, because double-RNAi animals all displayed the wnt5(RNAi) lateral ectopic eye phenotype. Additionally, the two factors likely do not operate fully independently because wnt5 co-inhibition reduced the penetrance of the bmp4(RNAi) medial ectopic eye phenotype (p = 0.0027, 2-tailed Fisher’s exact test). To ensure these results were not due to an inefficient bmp4 knockdown, we used quantitative real-time PCR to evaluate normalized expression of bmp4 and wnt5 across each condition (S10 Fig). This experiment demonstrated that bmp4 was significantly knocked down after treatment with both bmp4 dsRNA and treatment with bmp4;wnt5 dsRNAs. Similarly, wnt5 was significantly knocked down in animals treated with wnt5 dsRNA and also treated with bmp4;wnt5 dsRNA (S10 Fig). We cannot rule out an influence of differential depletion of the wnt5 and bmp4 proteins in this experiment as contributing to the outcome, though enough protein knockdown occurred in the single-RNAi conditions to generate nearly 100% penetrant patterning phenotypes. Taken together with the findings that bmp4 RNAi causes expansion of wnt5 expression, we interpret these results to indicate that bmp4 can act upstream to limit wnt5 in order to regulate ML identity.
Given the ability of bmp4 signaling to regulate wnt5 expression domains and also to promote slit expression, we tested whether bmp4’s function on midline determinant slit might depend on negative regulation of wnt5. To test this hypothesis, we performed single and double knockdown experiments between bmp4 and wnt5 and then assessed slit expression (S11 Fig). Inhibition of bmp4 again resulted in reduced slit expression, particularly in the tail. Under these homeostatic RNAi conditions, inhibition of wnt5 did not substantially alter slit expression but slit/wnt5 reciprocal antagonism has been observed previously under regeneration conditions [28]. Double inhibition of both bmp4 and wnt5 resulted in reduced slit expression, similar to bmp4 RNAi alone. Therefore, wnt5 knockdown did not influence the reduced slit expression from bmp4(RNAi), suggesting bmp4 likely does not activate slit through repression of wnt5. One possible mechanism could be that graded bmp4 activity activates midline slit expression at the highest point in the gradient at the dorsal midline and suppresses wnt5 medially, while low levels of bmp4 at the lateral edge enable expression of wnt5.
We last tested for possible ways that slit or wnt5 could participate in control of AP axis information by controlling wnt1. However, under homeostatic RNAi conditions, slit or wnt5 inhibition did not have a detectable effect on AP or ML expression of the wnt1 posterior domain (S12 Fig). These data suggest slit and wnt5 may act independently from the bmp4-dependent control mechanism regulating wnt1’s AP distribution under normal homeostatic conditions.
Discussion
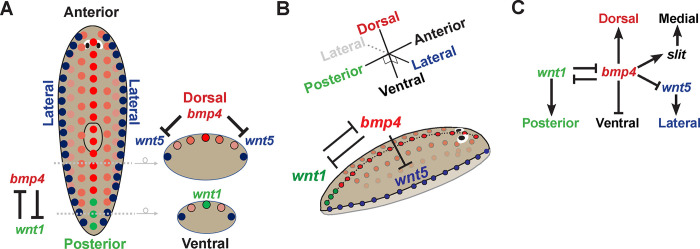
Together, these experiments identify critical roles for bmp4 in patterning multiple body axes in planarians. BMP regulation not only establishes DV polarity but additionally influences both posterior identity through regulation of wnt1 and also ML polarity through the suppression of wnt5 and activation of slit. These results contribute to prior work analyzing roles for bmp4 and slit/wnt5 on the regenerating anterior pole [37] to argue that major body patterning systems interact homeostatically in order to coordinate growth. We suggest that a regulatory logic in which the interaction of information across axes may be important for robustness of patterning across long timescales in adulthood (Fig 6).
Fig 6. Model for homeostatic regulation of DV, AP, and ML axes by bmp4.
(A-B) 2D and 3D models illustrating mutual antagonism between bmp and wnt1 in the far posterior, bmp4 inhibition of wnt5 for control of lateral identity, and expression of key components across the AP and DV axes. Not shown, bmp4 activates slit expression on the midline, nog1;nog2 negatively regulate bmp4 to promote wnt1 posteriorly, and bmp4 regulates dorsal-versus-ventral tissue identity.(C) Regulatory model highlighting key interactions connecting axis information in homeostatic animals. bmp4 controls dorsal versus ventral identity, undergoes mutual inhibition with wnt1 for control of posterior identity, and activates slit and represses wnt5 in regulation mediolateral information.
The cross-regulation we observe between BMP, Wnt1 and Wnt5 signals in overall animal domains raises the question of how signal integration is achieved. Whether inputs from distinct axes can be resolved by individual or collections of the muscle cells involved in planarian positional information is an important and unresolved question for future analysis.
Our results also clarify the relationships among the BMP, Wnt5, and Slit signals that participate in ML patterning in planarians. DV polarity and dorsal bmp4 expression were overall apparently normal in wnt5(RNAi) animals, although we found sparse bmp4-expressing cells to appear ventrally in such animals (S9 Fig) [28]. Although these RNAi conditions successfully knocked down wnt5 and the inhibition caused ectopic lateral eye formation, it is possible that more complete inhibition of Wnt5 signaling could further impact the DV axis. However, with the present analysis, we find a much stronger effect of bmp4 inhibition on wnt5 expression than vice versa (Fig 5B). Furthermore, the double RNAi experiments testing bmp4;wnt5(RNAi) presented here suggest wnt5 acts downstream of bmp4 in eye placement as tested in these conditions (Fig 5D). These results argue strongly against a hypothetical model in which wnt5 acts exclusively upstream of bmp4. Because of the above caveats, we cannot rule out the existence of wnt5/bmp4 mutual antagonism, similar to that detected between wnt1 and bmp4. However, our present results are more consistent with a mechanism for DV and ML formation in which bmp4 has a primary role to delineate a wnt5 expression domain which then exerts several effects, for example on ML eye placement, in a primarily downstream fashion. It has been previously demonstrated that BMP inhibits Wnt5 during ectoderm patterning in sea urchins [52], and BMP activity downregulates wnt5a to modulate convergence and extension in zebrafish development [53]. Therefore, roles of BMP upstream of Wnt5 factors may be conserved. slit also likely acts downstream of bmp4, because bmp4 inhibition reduced slit expression. These results collectively argue that bmp4 acts at a high point in a hierarchy for ML patterning.
Our results also identify mutual antagonism between posterior wnt1 and dorsal bmp4 expression domains in the posterior. bmp4 also regulated the expression domain sizes of several posterior Wnt and Wnt-related factors. We found that in some cases, bmp4 RNAi caused expression of posterior determinants to extend much further anterior than the tail (Figs 2 and 3). However, bmp4(RNAi) animals had normal tail size and pharynx placement (S6B Fig). On the other hand, our analysis of pharynx cell precursor markers foxA and dd554 indicates bmp4 indeed exerts effects on the distributions of AP-regionalized progenitors (S6A Fig). The relatively slow turnover of the pharynx may prevent identification of the consequences to organ position and/or maintenance in these experiments. In addition, bmp4(RNAi) animals ultimately underwent displacement of the pre-existing eyes, suggestive of complex shifts in AP identity (S6C Fig). However, the complex nature of these phenotypes suggests that a full understanding of how bmp4 couples with Wnt signals to regulate AP tissue determination will likely require additional markers and assays of progenitor behavior and potentially ways to selectively impact bmp4’s AP regulatory functions.
Our results identifying roles of BMP on the AP axis are congruous with evidence of spatiotemporal coordination between the AP and DV axes in vertebrate development. In early mammalian development, a proximal BMP signal from trophectoderm activates Wnt3 asymmetrically in epiblast cells in order to establish the future AP axis [54]. In early Xenopus development, BMP4 is required for the expression of posterior Wnt8 [55]. The integration of BMP and Wnt signaling pathways occurs by Wnt8 preventing the conserved ability of GSK3 kinase to inhibit Smad1 activity through its phosphorylation and targeted proteasomal degradation, such that Wnt signals can positively enhance BMP signaling duration. This regulation contributes to a model in which AP positional information is specified by the duration of BMP signaling [56]. Zebrafish development also involves coordination of AP and DV axis formation, with maternal Wnt signaling generating the organizer to repress bmp, and zygotic Wnt transcriptionally promoting the maintenance of BMP expression [57]. Furthermore, BMP defines DV patterning in a temporal AP gradient from head to tail [58]. By contrast, planarian Wnt/BMP integration involves signaling antagonism and operates at a transcriptional level and so likely occurs through a distinct mechanism. Uses for Wnt and BMP signals to pattern perpendicular body axes predate the bilaterians, with Cnidarians using Wnt signaling along the oral-aboral primary body axis and BMP to control the perpendicular directive axis [3,12,59,60]. In Nematostella, BMP and canonical Wnt signaling mutually antagonize to pattern endomesoderm [61], reminiscent of our identification of mutual antagonism between planarian wnt1 and bmp4. Our results suggest that interactions between BMP/Wnt signaling across axes may be a fundamental and ancient property enabling the integration of body axis information in three dimensions.
Materials and methods
Ethics statement
Procedures with Schmidtea mediterranea planarians (invertebrates) were conducted according to safety and ethics procedures in line with the Northwestern Office for Research Safety, with authorization from the Institutional Biosafety Committee (IBC), approved 12/7/21. As invertebrate animal subjects, planarians are not subject to IACUC or IRB review.
Experimental model
Asexual Schmidtea mediterranea animals (CIW4 strain) were kept in 1x Montjuic salts (1.6 mmol/l NaCl, 1.0 mmol/l CaCl2, 1.0 mmol/l MgSO4, 0.1 mmol/l MgCl2, 0.1 mmol/l KCl and 1.2 mmol/l NaHCO3 prepared in Milli-Q water) at 18–20°C. Animals were fed pureed calf liver from aliquots stored at -80°C once a week and washed twice a week. Animals were starved at least 7 days before the start of experiments.
Fluorescence in situ hybridization (FISH)
The FISH protocol used is based off previously published work [40]. Animals were treated with 7.5% NAC (w/v) in 1X PBS, fixed in 4% formaldehyde (w/v), and stored in methanol. They were rehydrated with methanol: 1x PBSTx, bleached in 6% hydrogen peroxide (v/v) in 1x PBS, permeabilized with proteinase K (20 mg/ml), then prehybridized at 56°C. Hybridization occurred with digoxigenin or fluorescein labeled riboprobes at a 1:1000 concentration (v/v), which were synthesized using T7 RNA binding sites for antisense transcription. Animals were washed in a SSC concentration series of 1:1 Pre-hybridization buffer:2xSSC, 2xSSC, and 0.2x SSC at 56°C. Anti-digoxigenin-POD (Sigma/Roche) or anti-fluorescein-POD antibodies (Sigma/Roche) were in a solution of 1x TNTx/ 10% (v/v) horse serum/ 10% (v/v) Western Blocking Reagent (Roche) at a concentration of 1:2000 (v/v). Tyramide in 1x TNTx was utilized to develop and amplify the antibody signal. For double FISH, the enzymatic activity of tyramide reactions was inhibited by sodium azide (100mM). Nuclei were stained using 1:1000 Hoescht (v/v, Invitrogen) in 1x TNTx.
RNA interference (RNAi)
RNAi treatments were performed by feeding with dsRNA (16% v/v), red food dye (4% v/v), and pureed liver (80% v/v). Worms of each condition were cultured in separate petri dishes in 1x Montjuic salts. dsRNA was synthesized as previously described [29]. For controls, the dsRNA synthesized was either Photinus pyralis luciferase (Ppluc) or C. elegans unc-22, as these genes are not present in the Schmidtea mediterranea genome. Animals were fed 1 ul RNAi food mix per worm every 2–3 days for the indicated length of the experiment. For double RNAi, control dsRNA was mixed in with the single experimental dsRNA to ensure the same amount of overall dsRNA in feedings between double and single experimental conditions. This resulted in a 1:1 dsRNA mix of Ppluc:Ppluc for control RNAi, Ppluc:bmp4 for bmp4 RNAi, Ppluc:wnt5 for wnt5 RNAi, and bmp4:wnt5 as the double RNAi knockdown (Figs 5D, S10 and S11). For RNAi treatment without injury, animals were fixed 5 days after the last feeding. For a regeneration time course following injury, animals were cut 2 days after the last feeding and fixed at the indicated time.
Image acquisition
Live animals were imaged with a Leica M210F dissecting microscope with a Leica DFC295 camera (Fig 5D). Stained animals were imaged with a Leica Stellaris confocal microscope using a 10x or 20x objective and Leica LAS-X software (Figs 1–5, S3, S4, S6–S9, S11 and S12). FISH images are maximum projections from a z-stack and representative of each condition. Unless otherwise specified, z-stacks were chosen to segment to approximately half way through the dorso-ventral axis from animals viewed either dorsally or ventrally as indicated. Adjustments to brightness and contrast were made using Adobe Photoshop or ImageJ.
Irradiation
Animals were irradiated using a Radsource RS-2000 X-ray to deliver 1350 rads to worms in petri dishes of 1x Montjuic salts (S4 Fig). Following irradiation, the first RNAi feeding was administered.
qRT-PCR expression analysis
RNA was purified from whole animals in trizol using a Turrex tissue homogenizer. Four biological replicates were generated for each RNAi condition. cDNA was synthesized using the Multiscribe reverse transcriptase kit (Applied Biosystems) following DNase treatment (Turbo DNA-free, Ambion). Control samples were generated by exclusion of reverse transcription reagents. cDNA was treated with 40% RNAse H (v/v, New England Biosciences) then diluted 1:5. The QuantStudio3 QPCR system and SYBR green (Roche KAPA biosystems) were used for analysis (S10 Fig). Detection of bmp4 and wnt5 mRNA was normalized to reference gene ubiquilin and relative expression values calculated using the delta-delta Ct method. Detection of mRNA abundance was validated by comparison between Ct values in treatments that either included or excluded the reverse transcription enzyme, and mRNA signal was evaluated for detection at least 8-fold above background as averaged across all samples. Outliers were flagged for removal by Grubb’s outlier test with alpha < 0.05 with respect to all Cts tested for each probe set. Sequences of the qRT-PCR primers used are included in S1 Table.
Cloning
Primers for dsRNA, riboprobes, and qRT-PCR are listed in S1 Table. Riboprobes for foxD, dd554, and porcupine were described previously [48,62,63]. Briefly, RNA was isolated from adult animals and fragments undergoing regeneration in a mixed-stage timeseries using Trizol and a Turrex tissue homogenizer, followed by ethanol precipitation and DNAse treatment (DNA-free, Ambion). Reverse transcription was performed using Superscript III and oligo-dT priming. Primers were designed using Primer3 (https://primer3.ut.ee/) and planarian sequences analyzed from Planmine (https://planmine.mpinat.mpg.de/planmine/begin.do) [64]. Planarian cDNAs were cloned by PCR using the primers indicated in S1 Table and either cloned into pGem-T-easy or directly used for PCR-mediated addition of T7 sequences to 3’ ends for riboprobe synthesis or to 5’ and 3’ ends for dsRNA synthesis.
Quantification and statistical analysis
Body length and PCG measurements were taken using Leica LAS X or FIJI on z-stack maximum projections of FISH images. For body length, animals were measured from their most anterior to most posterior tips. For PCG length, animals were measured from the tip of the anterior (Figs 3B, S5 and S6C), tip of the posterior (Figs 2, 3A, 3C, S2A, S2C–S2E, S3 and S12), or lateral edge (Figs 5B, 5C, S2B and S8A) to end of the fluorescence stain. For measuring head, pharynx, and tail lengths, animals were measured from anterior tip to the start of the dd554 stain, the length of the dd554 stain, the end of the dd554 stain to the posterior tip, respectively (S6B Fig). Cell counting was manually performed using maximum projections in LAS X (Figs 1, S2, S5 and S8A). Line-scan intensity measurements of AP distributions of bmp4 and wnt1 were performed in FIJI along the midline using line thickness of 30 pixels for measurements, which corresponded to ~1/10 of the width of the animal measured at the pharynx region (Figs 4 and S1A). Measurements of wnt1, bmp4, and dd23400 ML expression were taken using line thickness of 120 pixels, which corresponded to ~1/10 the length of the animal (S1B Fig). Cartoons indicate AP position of measured regions. Measurements were normalized by length along each region, and by maximum intensity across the scan, then summed normalized intensities were computed across equal-sized bins and plotted in R Studio (S1 Fig) or Microsoft Excel (Fig 4). Box plots were generated in BoxPlotR. Statistical analysis was conducted using Microsoft Excel or R. A student’s t-test was used for comparison between the means of two populations (Excel). One-way ANOVA on ranks (Kruskal-Wallis test) and Dunnett’s test were used for comparison between the means of multiple populations (R Studio). Data used to generate figures is included in S1 Data.
Supporting information
Analysis of normalized FISH line-scan intensity from dorsal-view images represented in Fig 1A measuring (A) mediolateral expression of bmp4 and dd23400 from an anterior tail domain, (B) mediolateral expression of dd23400 and wnt1 measured from a posterior tail domain, and (C) anteroposterior expression of wnt1 and bmp4 measured within the tail. 3 biological replicate images were processed in ImageJ to measure line-scan intensity with a width of 120 (A-B) or 30 pixels (C), encompassing approximately 1/10th the size of the orthogonal axis. Cartoons indicate the regions and approximate relative scale of measurements taken for each analysis (R1, R2, R3). Data were position-normalized and intensity-normalized, then summed within 15 equal-sized bins dividing each region. Averages and standard deviations across biological replicates for each bin are presented in each plot (A-C). Statistical tests were performed to assess overall expression trends using t-tests to compare sample intensities across the bins indicated by the dotted lines. t-test for ML-bmp4 distributions compares data from bins 1–3 versus bins 7–9 (N = 9 measurements for each region), t-tests for ML plots of dd23400 and wnt1 compares data from bins 4–6 versus bins 7–9 (N = 9 measurements for each region), and t-tests for AP bmp4 and wnt1 compares data from bins 1–4 versus bins 5–8 (N = 12 measurements for each region). (D-F) Plots of individual biological replicates after the normalization procedure described above. The key trends observed in this analysis are that bmp4 expression is present in a graded fashion on the mediolateral axis dorsally, dd23400 is expressed sharply at the midline, wnt1 is expressed at the midline in the posterior, and bmp4 expression along the posterior midline reduces in the far posterior at approximately the same location as wnt1 is expressed.
(PDF)
(A-E) Quantification of absolute and relative wnt1+ cell numbers after inhibition of indicated genes related to Fig 2. Graphs showing absolute numbers of wnt1+ cells and numbers of wnt1+ cells normalized to body length for (A) 14 days of control or bmp4 RNAi, (C) 18 days of control or nog1;nog2 RNAi, (D) 14 days of control, smad1, or smad4 RNAi, or (E) 18 days of control, tbx2/3, or nlg8 RNAi. (B) Graph illustrating the maximum width between wnt1+ cells relative to animal body width following 14 days of control or bmp4 RNAi. (A-E) N ≥ 4 animals. Plots shows median values (middle bars) and first-to-third interquartile ranges (boxes); whiskers indicate 1.5× the interquartile ranges and dots are data points from individual animals. *p<0.05, **p<0.01, ****p<0.0001, n.s. indicates p>0.05 by 2-tailed t-test (A-C) or by one-way ANOVA on ranks (D-E).
(PDF)
(A) FISH to detect wnt1 expression in regenerating tail fragments at 0, 18, and 96 hours after amputations conducted after 14 days of either control or bmp4 RNAi. Arrows indicate anterior-most wnt1+ cell detected along the dorsal midline for each timepoint and condition. Top panels show control animals undergoing early expansion of the dorsal midline wnt1 domain by 18 hours of regeneration followed by rescaling to reduce the domain to the tip of the animal by 96 hours. Bottom panel shows wnt1 expression dynamics in bmp4 RNAi, in which midline wnt1 expression was anterior expanded at the time of injury (0 hours), remained expanded at 18 hours of regeneration, and then restricted posteriorly by 96 hours, similar to control RNAi conditions. Therefore, BMP pathway modulation is unlikely to be responsible for the normal restriction of wnt1 by 96 hours in regenerating tail fragments. Scale bars represent 150 μm. (B) Graph showing the quantification of the length of the wnt1 domain relative to length of tail fragment. ****p<0.0001 by 2-tailed t-test and n.s. indicates p>0.05; N ≥ 3 animals. Box plots shows median values (middle bars) and first to third interquartile ranges (boxes); whiskers indicate 1.5× the interquartile ranges and dots are data points from individual animals.
(PDF)
FISH detecting wnt1 in control and bmp4(RNAi) animals given 5 dsRNA dosings over 12 days following either no irradiation or given a sublethal dose of 1350 rads of X-ray irradiation. Unirradiated bmp4(RNAi) animals underwent expansion of wnt1 expression compared to unirradiated control RNAi conditions. By contrast, wnt1 expression was not present in irradiated control or bmp4(RNAi) animals. N ≥ 5. Scale bars represent 300 μm.
(PDF)
Plots of absolute and size-normalized numbers of anterior pole notum+ cells following homeostatic inhibition of bmp4 RNAi versus control RNAi animals, related to Fig 3B. Cells were manually scored from dorsal-view images obtained at 20x. Knockdown of bmp4 did not significantly change the absolute or bodysize-relative number of notum+ cells. n.s. indicates p>0.05 by 2-tailed t-test. N ≥ 6 animals. Plots shows median values (middle bars) and first-to-third interquartile ranges (boxes); whiskers indicate 1.5× the interquartile ranges and dots are data points from individual animals.
(PDF)
(A) Following 28 days RNAi of control or bmp4 RNAi, animals were stained for gut marker porcupine and pharynx-associated markers SMU15007112, dd554, foxA, and laminin. Inhibition of bmp4 did not affect porcupine or SMU15007112 expression. bmp4 RNAi caused a subtle change to dd554 expression which kept overall pharynx staining intact but eliminated expression of streams of dd554+ cells located outside of the pharynx (zoom-ins, 6/7 animals had no dd554 expressing cells adjacent to the pharynx, compared with 7/7 control animals displaying this expression). However, inhibition of bmp4 consistently resulted in ectopic non-pharynx expression of foxA and ectopic anterior expression of laminin. 5/8 bmp4(RNAi) animals had relatively less reduction to laminin pharynx staining and less ectopic anterior laminin and 3/8 animals had coincident loss of laminin expression and stronger gain of ectopic anterior expression. N ≥ 7. Scale bars represent 300 μm. (B) Measurements of head, pharynx, and tail regions determined by dd554 expression relative to body region for control and bmp4 RNAi. bmp4 inhibition did not significantly alter AP pharynx scale or position or size of tail and anterior (head) domain as measured with respect to pharynx position. (C) Left: Hoechst-stained animals following 28 days of control or bmp4 RNAi to detect original and ectopic eyes. Right: Quantification of the length from the anterior tip of the eyes (as measured for each pair of eyes along the midline) and normalized to total body size. Top panels show zoom-in of anterior animal regions for control and bmp4(RNAi) animals of eyes. Images are max-projections with z-planes chosen to show only either the original or ectopic bmp4(RNAi) animal eyes as indicated. Compared to the position of control eyes, original eyes in bmp4(RNAi) animals were displaced anteriorly toward the tip of the head while ectopic eyes are positioned at the same relative location as normal eyes in control animals. Scale bars represent 300 um. (B-C) ****p<0.0001, n.s. indicates p>0.05 by 2-tailed t-test (B) or one way ANOVA test on ranks (C). N = 7 animals. Plots shows median values (middle bars) and first-to-third interquartile ranges (boxes); whiskers indicate 1.5× the interquartile ranges and dots are data points from individual animals.
(PDF)
(A) FISH staining for bmp4 expression in whole animals after 14 days of control or wnt1 RNAi related to Fig 4. Inhibition of wnt1 resulted in elevated expression of bmp4 on the posterior midline of the animal (arrows) and did not change the lack of ventral bmp4 expression (right). Scale bars are 150 μm. N = 21 animals. (B) Double FISH of control or wnt1 RNAi for bmp4 and anterior marker foxD. N = 8. Inhibition of wnt1 caused elevated expression of bmp4 in the posterior but did not alter the lack of posterior foxD, suggesting wnt1’s role on bmp4 expression is not likely due to control of head-versus-tail identity determination. (C) Animals stained for bmp4 following control or notum RNAi homeostatically for 14 days. Inhibition of notum did not alter bmp4 expression on either the dorsal or ventral side of the animals. Scale bars are 300 μm. N ≥ 6. (D) Dorsal posterior view of bmp4 FISH conducted on control or nog1;nog2(RNAi) animals inhibited homeostatically for 18 days. nog1;nog2 inhibition qualitatively appeared to cause an increase in overall bmp4 expression levels. N = 8. Scale bars are 150 μm.
(PDF)
(A-C) FISH for dd23400, LaminB, and wnt5 following 28 days of control or bmp4 RNAi. Scale bars represent 300 μm. (A) Inhibition of bmp4 reduces dd23400 expression (arrows), particularly in the posterior. Bottom Left: Quantification of number of dd23400+ cells normalized to animal body length. Bottom Right: Quantification of maximum width between dd23400+ cells relative to animal body width. *p<0.05, **p<0.01 by unpaired 2-tailed t-test; N ≥ 6 animals. Box plots show median values (middle bars) and first to third interquartile ranges (boxes); whiskers indicate 1.5× the interquartile ranges and dots are data points from individual animals. (B) bmp4 RNAi causes ectopic medial expression of lateral marker laminB expression on the posterior midline (arrows). (C) Knockdown of bmp4 appears to elevate wnt5 expression less dramatically on the ventral side versus dorsal side. Right panels show enlargements of boxed regions.
(PDF)
FISH staining for bmp4 after 21 days of control or wnt5 RNAi under homeostatic conditions. Inhibition of wnt5 does not cause detectable increases or decreases in bmp4 expression or distribution on the dorsal side of animals. However, some ectopic ventral expression appears following wnt5 RNAi. Scale bars are 150 μm. N = 5 animals.
(PDF)
Left: qPCR to detect expression of bmp4 transcript (normalized to ubiquilin control) to detect knockdown of bmp4 in bmp4 RNAi and bmp4;wnt5 RNAi. Animals were treated with dsRNA for 20 days (9 dsRNA feedings), followed by isolation of RNA, followed by RT-qPCR. bmp4 expression was knocked down after delivery of either bmp4 dsRNA or the combination of bmp4 and wnt5 dsRNA. Right: qPCR to detect expression of wnt5 after RNAi of wnt5 individually or in combination with bmp4 under the same conditions. Both single and double RNAi conditions caused significant wnt5 knockdown. *p<0.05, **p<0.01 by one-tailed t-test to determine if mRNA reduced after RNAi. Plots show median values (middle bars) and first-to-third interquartile ranges (boxes); whiskers indicate 1.5× the interquartile ranges and dots are data points from individual animals.
(PDF)
FISH staining for slit following 20 days of control, bmp4, wnt5, and bmp4;wnt5 RNAi. Loss of bmp4 reduces slit expression (arrows). Loss of wnt5 did not appear to alter slit expression. Knockdown of both bmp4 and wnt5 resulted in the reduced slit expression, similar to bmp4 RNAi (arrows). N ≥ 6 animals. Scale bars represent 300 μm.
(PDF)
Left: Following 18 days of control, bmp4, slit, or wnt5 RNAi, animals were stained for wnt1 expression. Inhibition of bmp4 expanded wnt1 anteriorly and reduced posterior expression. Inhibition of slit or wnt5 did not significantly affect wnt1 expression. N ≥ 6 animals. Scale bars represent 300 μm. Right: Plot showing length of wnt1 domain from the tip of the tail relative to animal body length. n.s. indicates p>0.05, ****p<0.0001 by one-way ANOVA on ranks. N ≥ 8 animals. Plots show median values (middle bars) and first-to-third interquartile ranges (boxes); whiskers indicate 1.5× the interquartile ranges and dots are data points from individual animals.
(PDF)
(PDF)
(XLSX)
Acknowledgments
We thank members of the Petersen lab for critical comments and Dr. Erik Schad for reagents and concepts.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by National Institutes of Health grant NIGMS R01GM129339 (to C.P.P.), National Institutes of Health grant NIGMS R01GM130835 (to C.P.P.), National Institutes of Health grant NIGMS R35GM149280 (to C.P.P.), and Simons/SFARI (597491-RWC) pilot project grant (to C.P.P.). E.G.C was supported in part by the Northwestern University Graduate School Cluster in Biotechnology, Systems, and Synthetic Biology, which is affiliated with the Biotechnology Training Program, and Northwestern University biotechnology training grant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell. 2009;139(6):1056–68. doi: 10.1016/j.cell.2009.11.035 [DOI] [PubMed] [Google Scholar]
- 2.De Robertis EM, Sasai Y. A common plan for dorsoventral patterning in Bilateria. Nature. 1996;380(6569):37–40. doi: 10.1038/380037a0 [DOI] [PubMed] [Google Scholar]
- 3.Niehrs C. On growth and form: a Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes. Development. 2010;137(6):845–57. doi: 10.1242/dev.039651 [DOI] [PubMed] [Google Scholar]
- 4.Roth S, Neuman-Silberberg FS, Barcelo G, Schupbach T. cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell. 1995;81(6):967–78. doi: 10.1016/0092-8674(95)90016-0 [DOI] [PubMed] [Google Scholar]
- 5.Rossant J, Tam PP. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development. 2009;136(5):701–13. doi: 10.1242/dev.017178 [DOI] [PubMed] [Google Scholar]
- 6.Molina MD, Salo E, Cebria F. The BMP pathway is essential for re-specification and maintenance of the dorsoventral axis in regenerating and intact planarians. Developmental biology. 2007;311(1):79–94. doi: 10.1016/j.ydbio.2007.08.019 [DOI] [PubMed] [Google Scholar]
- 7.Gurley KA, Rink JC, Sanchez Alvarado A. Beta-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science. 2008;319(5861):323–7. doi: 10.1126/science.1150029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iglesias M, Gomez-Skarmeta JL, Saló E, Adell T. Silencing of Smed-betacatenin1 generates radial-like hypercephalized planarians. Development. 2008;135(7):1215–21. doi: 10.1242/dev.020289 [DOI] [PubMed] [Google Scholar]
- 9.Petersen CP, Reddien PW. Smed-betacatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science. 2008;319(5861):327–30. doi: 10.1126/science.1149943 [DOI] [PubMed] [Google Scholar]
- 10.Reddien PW, Bermange AL, Kicza AM, Sanchez Alvarado A. BMP signaling regulates the dorsal planarian midline and is needed for asymmetric regeneration. Development. 2007;134(22):4043–51. doi: 10.1242/dev.007138 [DOI] [PubMed] [Google Scholar]
- 11.Srivastava M, Mazza-Curll KL, van Wolfswinkel JC, Reddien PW. Whole-body acoel regeneration is controlled by Wnt and Bmp-Admp signaling. Curr Biol. 2014;24(10):1107–13. doi: 10.1016/j.cub.2014.03.042 [DOI] [PubMed] [Google Scholar]
- 12.Holstein TW. The role of cnidarian developmental biology in unraveling axis formation and Wnt signaling. Developmental biology. 2022;487:74–98. doi: 10.1016/j.ydbio.2022.04.005 [DOI] [PubMed] [Google Scholar]
- 13.Reddy PC, Gungi A, Ubhe S, Pradhan SJ, Kolte A, Galande S. Molecular signature of an ancient organizer regulated by Wnt/β-catenin signalling during primary body axis patterning in Hydra. Communications Biology. 2019;2(1):434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fincher CT, Wurtzel O, de Hoog T, Kravarik KM, Reddien PW. Cell type transcriptome atlas for the planarian Schmidtea mediterranea. Science. 2018;360(6391):eaaq1736. doi: 10.1126/science.aaq1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plass M, Solana J, Wolf FA, Ayoub S, Misios A, Glazar P, et al. Cell type atlas and lineage tree of a whole complex animal by single-cell transcriptomics. Science. 2018;360(6391). doi: 10.1126/science.aaq1723 [DOI] [PubMed] [Google Scholar]
- 16.Wurtzel O, Oderberg IM, Reddien PW. Planarian Epidermal Stem Cells Respond to Positional Cues to Promote Cell-Type Diversity. Dev Cell. 2017;40(5):491–504.e5. doi: 10.1016/j.devcel.2017.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lapan SW, Reddien PW. Transcriptome analysis of the planarian eye identifies ovo as a specific regulator of eye regeneration. Cell Rep. 2012;2(2):294–307. doi: 10.1016/j.celrep.2012.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adler CE, Seidel CW, McKinney SA, Sánchez Alvarado A. Selective amputation of the pharynx identifies a FoxA-dependent regeneration program in planaria. eLife. 2014;3:e02238. doi: 10.7554/eLife.02238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonar NA, Petersen CP. Integrin suppresses neurogenesis and regulates brain tissue assembly in planarian regeneration. Development. 2017;144(5):784–94. doi: 10.1242/dev.139964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seebeck F, März M, Meyer A-W, Reuter H, Vogg MC, Stehling M, et al. Integrins are required for tissue organization and restriction of neurogenesis in regenerating planarians. Development. 2017;144(5):795–807. doi: 10.1242/dev.139774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abnave P, Aboukhatwa E, Kosaka N, Thompson J, Hill MA, Aboobaker AA. Epithelial-mesenchymal transition transcription factors control pluripotent adult stem cell migration in vivo in planarians. Development. 2017;144(19):3440–53. doi: 10.1242/dev.154971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guedelhoefer OCt, Sánchez Alvarado A. Amputation induces stem cell mobilization to sites of injury during planarian regeneration. Development. 2012;139(19):3510–20. doi: 10.1242/dev.082099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atabay KD, LoCascio SA, de Hoog T, Reddien PW. Self-organization and progenitor targeting generate stable patterns in planarian regeneration. Science. 2018;360(6387):404–9. doi: 10.1126/science.aap8179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lander R, Petersen CP. Wnt, Ptk7, and FGFRL expression gradients control trunk positional identity in planarian regeneration. eLife. 2016;5:e12850. doi: 10.7554/eLife.12850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill EM, Petersen CP. Positional information specifies the site of organ regeneration and not tissue maintenance in planarians. eLife. 2018;7:e33680. doi: 10.7554/eLife.33680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witchley JN, Mayer M, Wagner DE, Owen JH, Reddien PW. Muscle cells provide instructions for planarian regeneration. Cell Rep. 2013;4(4):633–41. doi: 10.1016/j.celrep.2013.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen CP, Reddien PW. A wound-induced Wnt expression program controls planarian regeneration polarity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(40):17061–6. doi: 10.1073/pnas.0906823106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurley KA, Elliott SA, Simakov O, Schmidt HA, Holstein TW, Sanchez Alvarado A. Expression of secreted Wnt pathway components reveals unexpected complexity of the planarian amputation response. Developmental biology. 2010;347(1):24–39. doi: 10.1016/j.ydbio.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen CP, Reddien PW. Polarized notum activation at wounds inhibits Wnt function to promote planarian head regeneration. Science. 2011;332(6031):852–5. doi: 10.1126/science.1202143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sureda-Gómez M, Pascual-Carreras E, Adell T. Posterior Wnts Have Distinct Roles in Specification and Patterning of the Planarian Posterior Region. Int J Mol Sci. 2015;16(11):26543–54. doi: 10.3390/ijms161125970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adell T, Salò E, Boutros M, Bartscherer K. Smed-Evi/Wntless is required for beta-catenin-dependent and -independent processes during planarian regeneration. Development. 2009;136(6):905–10. doi: 10.1242/dev.033761 [DOI] [PubMed] [Google Scholar]
- 32.Gavino MA, Reddien PW. A Bmp/Admp regulatory circuit controls maintenance and regeneration of dorsal-ventral polarity in planarians. Current biology: CB. 2011;21(4):294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molina MD, Neto A, Maeso I, Gomez-Skarmeta JL, Salo E, Cebria F. Noggin and noggin-like genes control dorsoventral axis regeneration in planarians. Current biology: CB. 2011;21(4):300–5. [DOI] [PubMed] [Google Scholar]
- 34.Orii H, Watanabe K. Bone morphogenetic protein is required for dorso-ventral patterning in the planarian Dugesia japonica. Development, growth & differentiation. 2007;49(4):345–9. doi: 10.1111/j.1440-169X.2007.00931.x [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Sastre A, Molina MD, Salo E. Inhibitory Smads and bone morphogenetic protein (BMP) modulate anterior photoreceptor cell number during planarian eye regeneration. The International journal of developmental biology. 2012;56(1–3):155–63. doi: 10.1387/ijdb.123494ag [DOI] [PubMed] [Google Scholar]
- 36.Cebrià F, Guo T, Jopek J, Newmark PA. Regeneration and maintenance of the planarian midline is regulated by a slit orthologue. Dev Biol. 2007;307(2):394–406. doi: 10.1016/j.ydbio.2007.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oderberg IM, Li DJ, Scimone ML, Gavino MA, Reddien PW. Landmarks in Existing Tissue at Wounds Are Utilized to Generate Pattern in Regenerating Tissue. Current biology: CB. 2017;27(5):733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scimone ML, Cloutier JK, Maybrun CL, Reddien PW. The planarian wound epidermis gene equinox is required for blastema formation in regeneration. Nature communications. 2022;13(1):2726. doi: 10.1038/s41467-022-30412-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orii HK K; Agata K; Watanabe K. Molecular Cloning of the Bone Morphogenetic Protein (BMP) Gene from the Planarian Dugesia japonica. Zoological science. 1998;15(6):6. [Google Scholar]
- 40.Schad EG, Petersen CP. STRIPAK Limits Stem Cell Differentiation of a WNT Signaling Center to Control Planarian Axis Scaling. Curr Biol. 2020;30(2):254–63.e2. doi: 10.1016/j.cub.2019.11.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian Q, Sun Y, Gao T, Li J, Hao Z, Fang H, et al. TBX2/3 is required for regeneration of dorsal-ventral and medial-lateral polarity in planarians. J Cell Biochem. 2021;122(7):731–8. doi: 10.1002/jcb.29905 [DOI] [PubMed] [Google Scholar]
- 42.Scimone ML, Cote LE, Rogers T, Reddien PW. Two FGFRL-Wnt circuits organize the planarian anteroposterior axis. eLife. 2016;5. doi: 10.7554/eLife.12845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stückemann T, Cleland JP, Werner S, Thi-Kim Vu H, Bayersdorf R, Liu SY, et al. Antagonistic Self-Organizing Patterning Systems Control Maintenance and Regeneration of the Anteroposterior Axis in Planarians. Dev Cell. 2017;40(3):248–63.e4. doi: 10.1016/j.devcel.2016.12.024 [DOI] [PubMed] [Google Scholar]
- 44.Tewari AG, Owen JH, Petersen CP, Wagner DE, Reddien PW. A small set of conserved genes, including sp5 and Hox, are activated by Wnt signaling in the posterior of planarians and acoels. PLoS Genet. 2019;15(10):e1008401. doi: 10.1371/journal.pgen.1008401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reuter H, Marz M, Vogg MC, Eccles D, Grifol-Boldu L, Wehner D, et al. Beta-catenin-dependent control of positional information along the AP body axis in planarians involves a teashirt family member. Cell Rep. 2015;10(2):253–65. [DOI] [PubMed] [Google Scholar]
- 46.Oderberg IM, Li DJ, Scimone ML, Gaviño MA, Reddien PW. Landmarks in Existing Tissue at Wounds Are Utilized to Generate Pattern in Regenerating Tissue. Curr Biol. 2017;27(5):733–42. doi: 10.1016/j.cub.2017.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cebria F, Newmark PA. Planarian homologs of netrin and netrin receptor are required for proper regeneration of the central nervous system and the maintenance of nervous system architecture. Development. 2005;132(16):3691–703. doi: 10.1242/dev.01941 [DOI] [PubMed] [Google Scholar]
- 48.Zhu SJ, Hallows SE, Currie KW, Xu C, Pearson BJ. A mex3 homolog is required for differentiation during planarian stem cell lineage development. eLife. 2015;4. doi: 10.7554/eLife.07025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fincher CT, Wurtzel O, de Hoog T, Kravarik KM, Reddien PW. Cell type transcriptome atlas for the planarian Schmidtea mediterranea. Science. 2018;360(6391). doi: 10.1126/science.aaq1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scimone ML, Kravarik KM, Lapan SW, Reddien PW. Neoblast specialization in regeneration of the planarian Schmidtea mediterranea. Stem cell reports. 2014;3(2):339–52. doi: 10.1016/j.stemcr.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sureda-Gomez M, Pascual-Carreras E, Adell T. Posterior Wnts Have Distinct Roles in Specification and Patterning of the Planarian Posterior Region. International journal of molecular sciences. 2015;16(11):26543–54. doi: 10.3390/ijms161125970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McIntyre DC, Seay NW, Croce JC, McClay DR. Short-range Wnt5 signaling initiates specification of sea urchin posterior ectoderm. Development. 2013;140(24):4881–9. doi: 10.1242/dev.095844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Myers DC, Sepich DS, Solnica-Krezel L. Bmp activity gradient regulates convergent extension during zebrafish gastrulation. Dev Biol. 2002;243(1):81–98. doi: 10.1006/dbio.2001.0523 [DOI] [PubMed] [Google Scholar]
- 54.Kurek D, Neagu A, Tastemel M, Tüysüz N, Lehmann J, van de Werken HJG, et al. Endogenous WNT signals mediate BMP-induced and spontaneous differentiation of epiblast stem cells and human embryonic stem cells. Stem Cell Reports. 2015;4(1):114–28. doi: 10.1016/j.stemcr.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoppler S, Moon RT. BMP-2/-4 and Wnt-8 cooperatively pattern the Xenopus mesoderm. Mech Dev. 1998;71(1–2):119–29. doi: 10.1016/s0925-4773(98)00004-5 [DOI] [PubMed] [Google Scholar]
- 56.Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, et al. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131(5):980–93. doi: 10.1016/j.cell.2007.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tuazon FB, Mullins MC. Temporally coordinated signals progressively pattern the anteroposterior and dorsoventral body axes. Semin Cell Dev Biol. 2015;42:118–33. doi: 10.1016/j.semcdb.2015.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tucker JA, Mintzer KA, Mullins MC. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell. 2008;14(1):108–19. doi: 10.1016/j.devcel.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rentzsch F, Anton R, Saina M, Hammerschmidt M, Holstein TW, Technau U. Asymmetric expression of the BMP antagonists chordin and gremlin in the sea anemone Nematostella vectensis: implications for the evolution of axial patterning. Developmental biology. 2006;296(2):375–87. doi: 10.1016/j.ydbio.2006.06.003 [DOI] [PubMed] [Google Scholar]
- 60.Saina M, Genikhovich G, Renfer E, Technau U. BMPs and Chordin regulate patterning of the directive axis in a sea anemone. Proceedings of the National Academy of Sciences. 2009;106(44):18592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wijesena N, Simmons DK, Martindale MQ. Antagonistic BMP-cWNT signaling in the cnidarian Nematostella vectensis reveals insight into the evolution of mesoderm. Proc Natl Acad Sci U S A. 2017;114(28):E5608–e15. doi: 10.1073/pnas.1701607114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scimone ML, Lapan SW, Reddien PW. A forkhead transcription factor is wound-induced at the planarian midline and required for anterior pole regeneration. PLoS genetics. 2014;10(1):e1003999. doi: 10.1371/journal.pgen.1003999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rink JC, Gurley KA, Elliott SA, Sanchez Alvarado A. Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science. 2009;326(5958):1406–10. doi: 10.1126/science.1178712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rozanski A, Moon H, Brandl H, Martin-Duran JM, Grohme MA, Huttner K, et al. PlanMine 3.0-improvements to a mineable resource of flatworm biology and biodiversity. Nucleic acids research. 2019;47(D1):D812–D20. doi: 10.1093/nar/gky1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Analysis of normalized FISH line-scan intensity from dorsal-view images represented in Fig 1A measuring (A) mediolateral expression of bmp4 and dd23400 from an anterior tail domain, (B) mediolateral expression of dd23400 and wnt1 measured from a posterior tail domain, and (C) anteroposterior expression of wnt1 and bmp4 measured within the tail. 3 biological replicate images were processed in ImageJ to measure line-scan intensity with a width of 120 (A-B) or 30 pixels (C), encompassing approximately 1/10th the size of the orthogonal axis. Cartoons indicate the regions and approximate relative scale of measurements taken for each analysis (R1, R2, R3). Data were position-normalized and intensity-normalized, then summed within 15 equal-sized bins dividing each region. Averages and standard deviations across biological replicates for each bin are presented in each plot (A-C). Statistical tests were performed to assess overall expression trends using t-tests to compare sample intensities across the bins indicated by the dotted lines. t-test for ML-bmp4 distributions compares data from bins 1–3 versus bins 7–9 (N = 9 measurements for each region), t-tests for ML plots of dd23400 and wnt1 compares data from bins 4–6 versus bins 7–9 (N = 9 measurements for each region), and t-tests for AP bmp4 and wnt1 compares data from bins 1–4 versus bins 5–8 (N = 12 measurements for each region). (D-F) Plots of individual biological replicates after the normalization procedure described above. The key trends observed in this analysis are that bmp4 expression is present in a graded fashion on the mediolateral axis dorsally, dd23400 is expressed sharply at the midline, wnt1 is expressed at the midline in the posterior, and bmp4 expression along the posterior midline reduces in the far posterior at approximately the same location as wnt1 is expressed.
(PDF)
(A-E) Quantification of absolute and relative wnt1+ cell numbers after inhibition of indicated genes related to Fig 2. Graphs showing absolute numbers of wnt1+ cells and numbers of wnt1+ cells normalized to body length for (A) 14 days of control or bmp4 RNAi, (C) 18 days of control or nog1;nog2 RNAi, (D) 14 days of control, smad1, or smad4 RNAi, or (E) 18 days of control, tbx2/3, or nlg8 RNAi. (B) Graph illustrating the maximum width between wnt1+ cells relative to animal body width following 14 days of control or bmp4 RNAi. (A-E) N ≥ 4 animals. Plots shows median values (middle bars) and first-to-third interquartile ranges (boxes); whiskers indicate 1.5× the interquartile ranges and dots are data points from individual animals. *p<0.05, **p<0.01, ****p<0.0001, n.s. indicates p>0.05 by 2-tailed t-test (A-C) or by one-way ANOVA on ranks (D-E).
(PDF)
(A) FISH to detect wnt1 expression in regenerating tail fragments at 0, 18, and 96 hours after amputations conducted after 14 days of either control or bmp4 RNAi. Arrows indicate anterior-most wnt1+ cell detected along the dorsal midline for each timepoint and condition. Top panels show control animals undergoing early expansion of the dorsal midline wnt1 domain by 18 hours of regeneration followed by rescaling to reduce the domain to the tip of the animal by 96 hours. Bottom panel shows wnt1 expression dynamics in bmp4 RNAi, in which midline wnt1 expression was anterior expanded at the time of injury (0 hours), remained expanded at 18 hours of regeneration, and then restricted posteriorly by 96 hours, similar to control RNAi conditions. Therefore, BMP pathway modulation is unlikely to be responsible for the normal restriction of wnt1 by 96 hours in regenerating tail fragments. Scale bars represent 150 μm. (B) Graph showing the quantification of the length of the wnt1 domain relative to length of tail fragment. ****p<0.0001 by 2-tailed t-test and n.s. indicates p>0.05; N ≥ 3 animals. Box plots shows median values (middle bars) and first to third interquartile ranges (boxes); whiskers indicate 1.5× the interquartile ranges and dots are data points from individual animals.
(PDF)
FISH detecting wnt1 in control and bmp4(RNAi) animals given 5 dsRNA dosings over 12 days following either no irradiation or given a sublethal dose of 1350 rads of X-ray irradiation. Unirradiated bmp4(RNAi) animals underwent expansion of wnt1 expression compared to unirradiated control RNAi conditions. By contrast, wnt1 expression was not present in irradiated control or bmp4(RNAi) animals. N ≥ 5. Scale bars represent 300 μm.
(PDF)
Plots of absolute and size-normalized numbers of anterior pole notum+ cells following homeostatic inhibition of bmp4 RNAi versus control RNAi animals, related to Fig 3B. Cells were manually scored from dorsal-view images obtained at 20x. Knockdown of bmp4 did not significantly change the absolute or bodysize-relative number of notum+ cells. n.s. indicates p>0.05 by 2-tailed t-test. N ≥ 6 animals. Plots shows median values (middle bars) and first-to-third interquartile ranges (boxes); whiskers indicate 1.5× the interquartile ranges and dots are data points from individual animals.
(PDF)
(A) Following 28 days RNAi of control or bmp4 RNAi, animals were stained for gut marker porcupine and pharynx-associated markers SMU15007112, dd554, foxA, and laminin. Inhibition of bmp4 did not affect porcupine or SMU15007112 expression. bmp4 RNAi caused a subtle change to dd554 expression which kept overall pharynx staining intact but eliminated expression of streams of dd554+ cells located outside of the pharynx (zoom-ins, 6/7 animals had no dd554 expressing cells adjacent to the pharynx, compared with 7/7 control animals displaying this expression). However, inhibition of bmp4 consistently resulted in ectopic non-pharynx expression of foxA and ectopic anterior expression of laminin. 5/8 bmp4(RNAi) animals had relatively less reduction to laminin pharynx staining and less ectopic anterior laminin and 3/8 animals had coincident loss of laminin expression and stronger gain of ectopic anterior expression. N ≥ 7. Scale bars represent 300 μm. (B) Measurements of head, pharynx, and tail regions determined by dd554 expression relative to body region for control and bmp4 RNAi. bmp4 inhibition did not significantly alter AP pharynx scale or position or size of tail and anterior (head) domain as measured with respect to pharynx position. (C) Left: Hoechst-stained animals following 28 days of control or bmp4 RNAi to detect original and ectopic eyes. Right: Quantification of the length from the anterior tip of the eyes (as measured for each pair of eyes along the midline) and normalized to total body size. Top panels show zoom-in of anterior animal regions for control and bmp4(RNAi) animals of eyes. Images are max-projections with z-planes chosen to show only either the original or ectopic bmp4(RNAi) animal eyes as indicated. Compared to the position of control eyes, original eyes in bmp4(RNAi) animals were displaced anteriorly toward the tip of the head while ectopic eyes are positioned at the same relative location as normal eyes in control animals. Scale bars represent 300 um. (B-C) ****p<0.0001, n.s. indicates p>0.05 by 2-tailed t-test (B) or one way ANOVA test on ranks (C). N = 7 animals. Plots shows median values (middle bars) and first-to-third interquartile ranges (boxes); whiskers indicate 1.5× the interquartile ranges and dots are data points from individual animals.
(PDF)
(A) FISH staining for bmp4 expression in whole animals after 14 days of control or wnt1 RNAi related to Fig 4. Inhibition of wnt1 resulted in elevated expression of bmp4 on the posterior midline of the animal (arrows) and did not change the lack of ventral bmp4 expression (right). Scale bars are 150 μm. N = 21 animals. (B) Double FISH of control or wnt1 RNAi for bmp4 and anterior marker foxD. N = 8. Inhibition of wnt1 caused elevated expression of bmp4 in the posterior but did not alter the lack of posterior foxD, suggesting wnt1’s role on bmp4 expression is not likely due to control of head-versus-tail identity determination. (C) Animals stained for bmp4 following control or notum RNAi homeostatically for 14 days. Inhibition of notum did not alter bmp4 expression on either the dorsal or ventral side of the animals. Scale bars are 300 μm. N ≥ 6. (D) Dorsal posterior view of bmp4 FISH conducted on control or nog1;nog2(RNAi) animals inhibited homeostatically for 18 days. nog1;nog2 inhibition qualitatively appeared to cause an increase in overall bmp4 expression levels. N = 8. Scale bars are 150 μm.
(PDF)
(A-C) FISH for dd23400, LaminB, and wnt5 following 28 days of control or bmp4 RNAi. Scale bars represent 300 μm. (A) Inhibition of bmp4 reduces dd23400 expression (arrows), particularly in the posterior. Bottom Left: Quantification of number of dd23400+ cells normalized to animal body length. Bottom Right: Quantification of maximum width between dd23400+ cells relative to animal body width. *p<0.05, **p<0.01 by unpaired 2-tailed t-test; N ≥ 6 animals. Box plots show median values (middle bars) and first to third interquartile ranges (boxes); whiskers indicate 1.5× the interquartile ranges and dots are data points from individual animals. (B) bmp4 RNAi causes ectopic medial expression of lateral marker laminB expression on the posterior midline (arrows). (C) Knockdown of bmp4 appears to elevate wnt5 expression less dramatically on the ventral side versus dorsal side. Right panels show enlargements of boxed regions.
(PDF)
FISH staining for bmp4 after 21 days of control or wnt5 RNAi under homeostatic conditions. Inhibition of wnt5 does not cause detectable increases or decreases in bmp4 expression or distribution on the dorsal side of animals. However, some ectopic ventral expression appears following wnt5 RNAi. Scale bars are 150 μm. N = 5 animals.
(PDF)
Left: qPCR to detect expression of bmp4 transcript (normalized to ubiquilin control) to detect knockdown of bmp4 in bmp4 RNAi and bmp4;wnt5 RNAi. Animals were treated with dsRNA for 20 days (9 dsRNA feedings), followed by isolation of RNA, followed by RT-qPCR. bmp4 expression was knocked down after delivery of either bmp4 dsRNA or the combination of bmp4 and wnt5 dsRNA. Right: qPCR to detect expression of wnt5 after RNAi of wnt5 individually or in combination with bmp4 under the same conditions. Both single and double RNAi conditions caused significant wnt5 knockdown. *p<0.05, **p<0.01 by one-tailed t-test to determine if mRNA reduced after RNAi. Plots show median values (middle bars) and first-to-third interquartile ranges (boxes); whiskers indicate 1.5× the interquartile ranges and dots are data points from individual animals.
(PDF)
FISH staining for slit following 20 days of control, bmp4, wnt5, and bmp4;wnt5 RNAi. Loss of bmp4 reduces slit expression (arrows). Loss of wnt5 did not appear to alter slit expression. Knockdown of both bmp4 and wnt5 resulted in the reduced slit expression, similar to bmp4 RNAi (arrows). N ≥ 6 animals. Scale bars represent 300 μm.
(PDF)
Left: Following 18 days of control, bmp4, slit, or wnt5 RNAi, animals were stained for wnt1 expression. Inhibition of bmp4 expanded wnt1 anteriorly and reduced posterior expression. Inhibition of slit or wnt5 did not significantly affect wnt1 expression. N ≥ 6 animals. Scale bars represent 300 μm. Right: Plot showing length of wnt1 domain from the tip of the tail relative to animal body length. n.s. indicates p>0.05, ****p<0.0001 by one-way ANOVA on ranks. N ≥ 8 animals. Plots show median values (middle bars) and first-to-third interquartile ranges (boxes); whiskers indicate 1.5× the interquartile ranges and dots are data points from individual animals.
(PDF)
(PDF)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.