Abstract
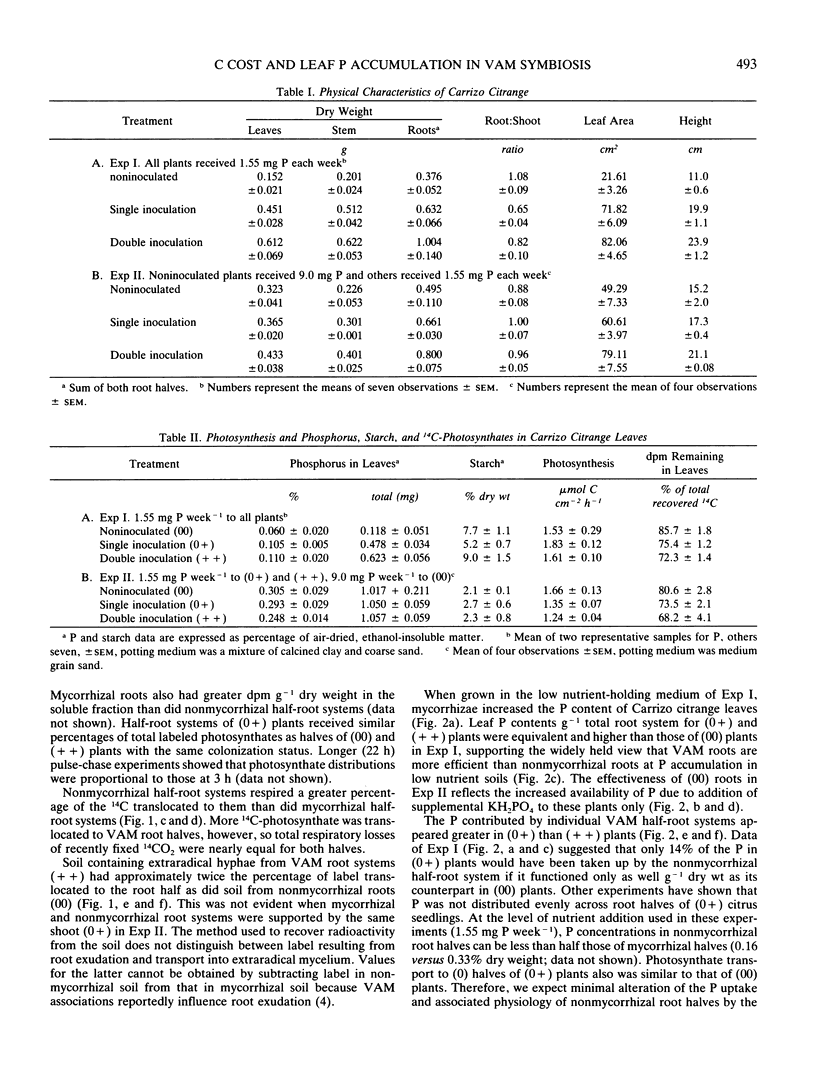
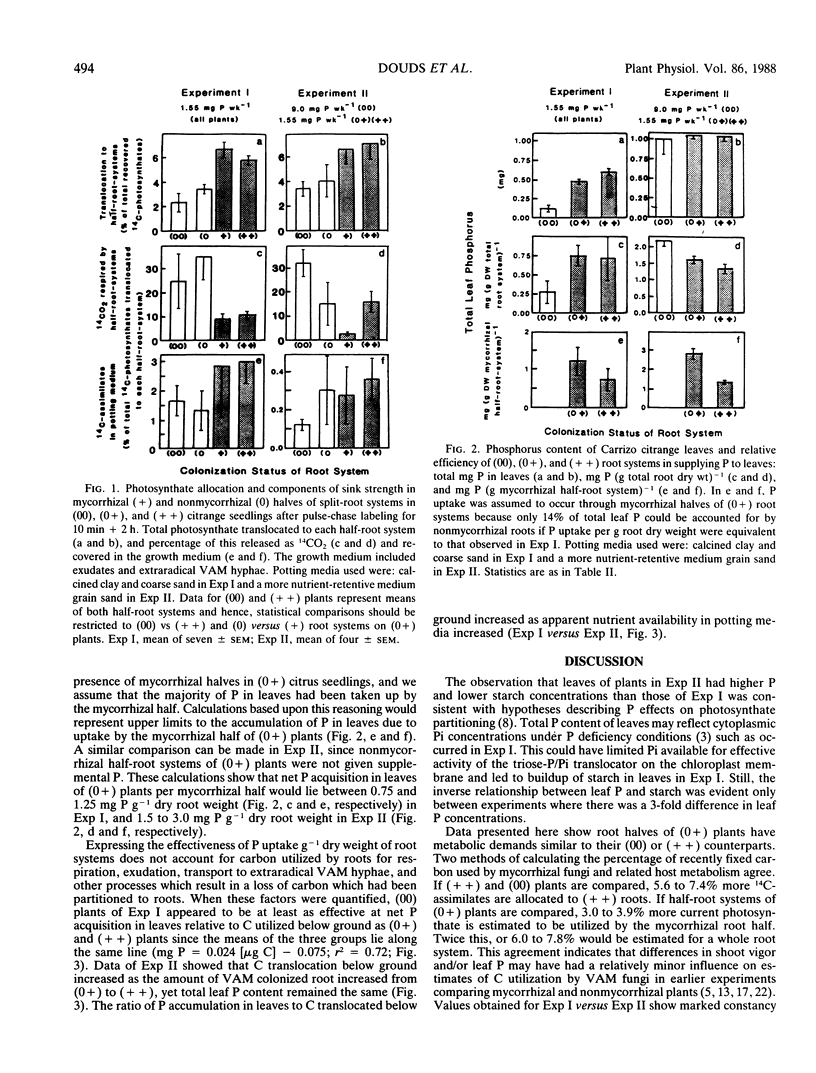
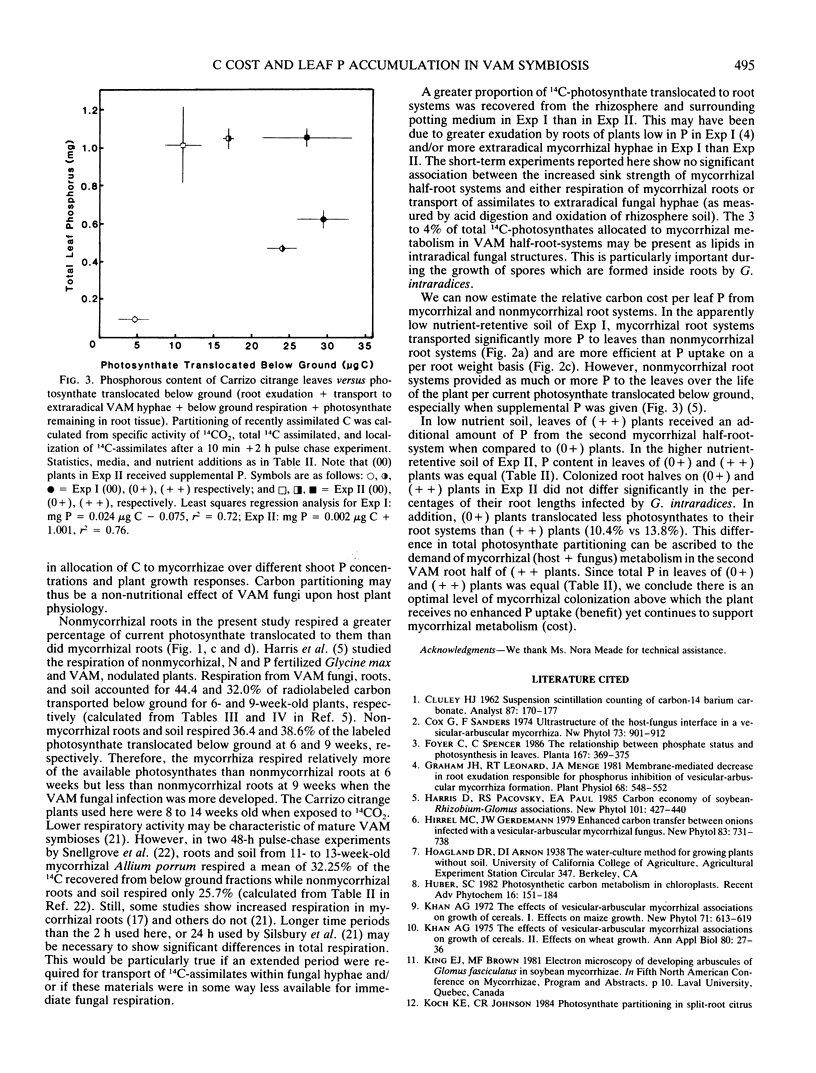
Translocation of 14C-photosynthates to mycorrhizal (+ +), half mycorrhizal (0+), and nonmycorrhizal (00) split-root systems was compared to P accumulation in leaves of the host plant. Carrizo citrange seedlings (Poncirus trifoliata [L.] Raf. × Citrus sinensis [L.] Osbeck) were inoculated with the vesicular-arbuscular mycorrhizal fungus Glomus intraradices Schenck and Smith. Plants were exposed to 14 CO2 for 10 minutes and ambient air for 2 hours. Three to 4% of recently labeled photosynthate was allocated to metabolism of the mycorrhiza in each inoculated root half independent of shoot P concentration, growth response, and whether one or both root halves were colonized. Nonmycorrhizal roots respired more of the label translocated to them than did mycorrhizal roots. Label recovered in the potting medium due to exudation or transport into extraradical hyphae was 5 to 6 times greater for (+ +) versus (00) plants. In low nutrient media, roots of (0+) and (+ +) plants transported more P to leaves per root weight than roots of (00) plants. However, when C translocated to roots utilized for respiration, exudation, etc., as well as growth is considered, (00) plant roots were at least as efficient at P uptake (benefit) per C utilized (cost) as (0+) and (+ +) plants. Root systems of (+ +) plants did not supply more P to leaves than (0+) plants in higher nutrient media, yet they still allocated twice the 14C-photosynthate to the mycorrhiza as did (0+) root systems. This indicates there is an optimal level of mycorrhizal colonization above which the plant receives no enhanced P uptake yet continues to partition photosynthates to metabolism of the mycorrhiza.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball S. G., Tree M., Morton J. J., Inglis G. C., Fraser R. Circulating dopamine: its effect on the plasma concentrations of catecholamines, renin, angiotensin, aldosterone and vasopressin in the conscious dog. Clin Sci (Lond) 1981 Oct;61(4):417–422. doi: 10.1042/cs0610417. [DOI] [PubMed] [Google Scholar]
- Graham J. H., Leonard R. T., Menge J. A. Membrane-mediated decrease in root exudation responsible for phorphorus inhibition of vesicular-arbuscular mycorrhiza formation. Plant Physiol. 1981 Sep;68(3):548–552. doi: 10.1104/pp.68.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K. E., Johnson C. R. Photosynthate partitioning in split-root citrus seedlings with mycorrhizal and nonmycorrhizal root systems. Plant Physiol. 1984 May;75(1):26–30. doi: 10.1104/pp.75.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]


