Abstract
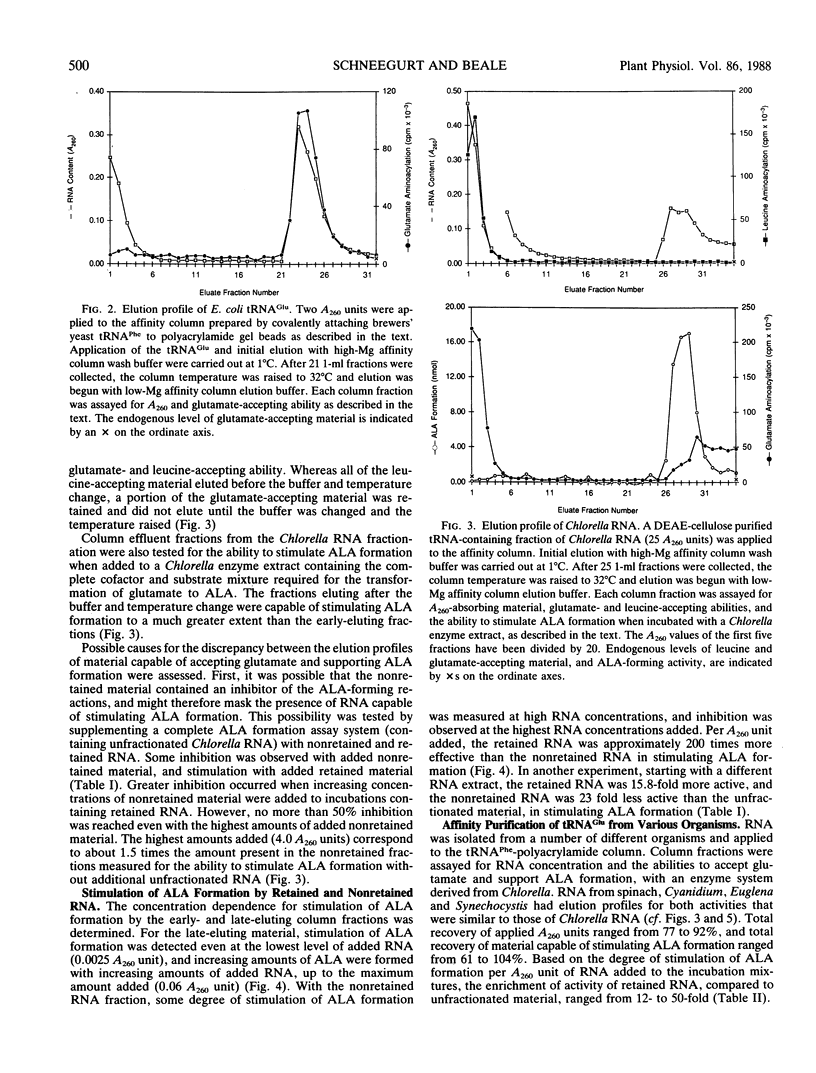
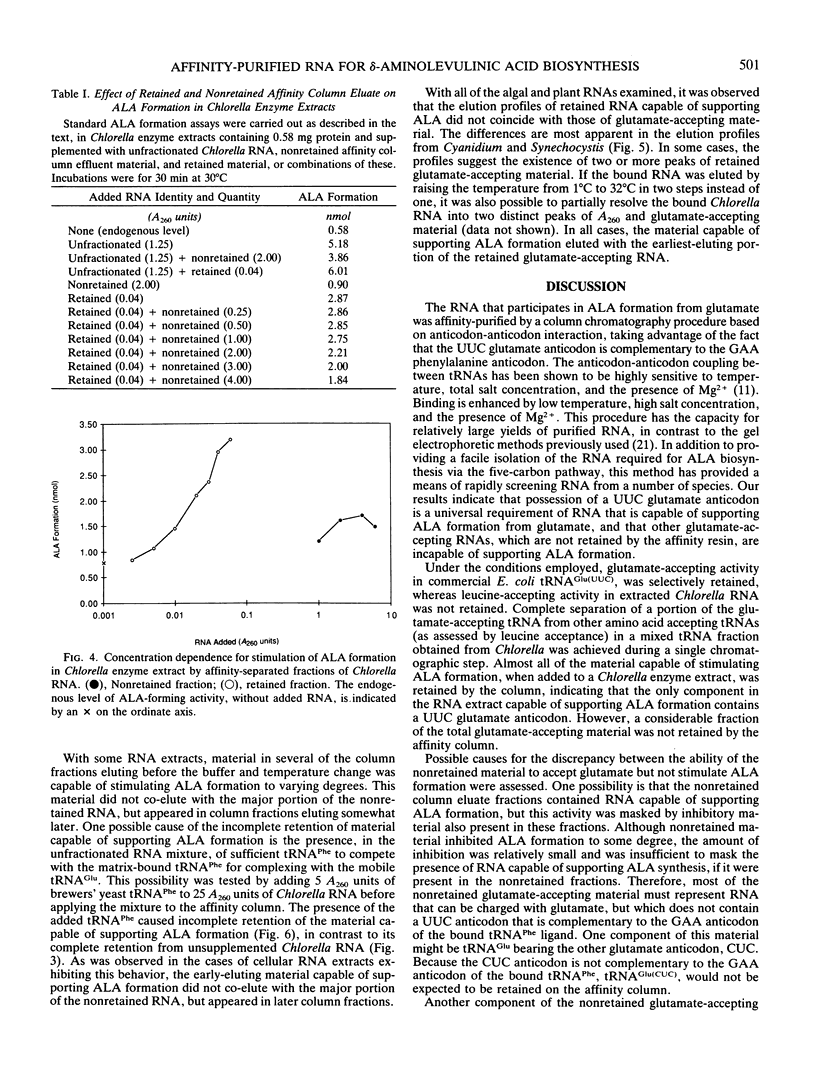
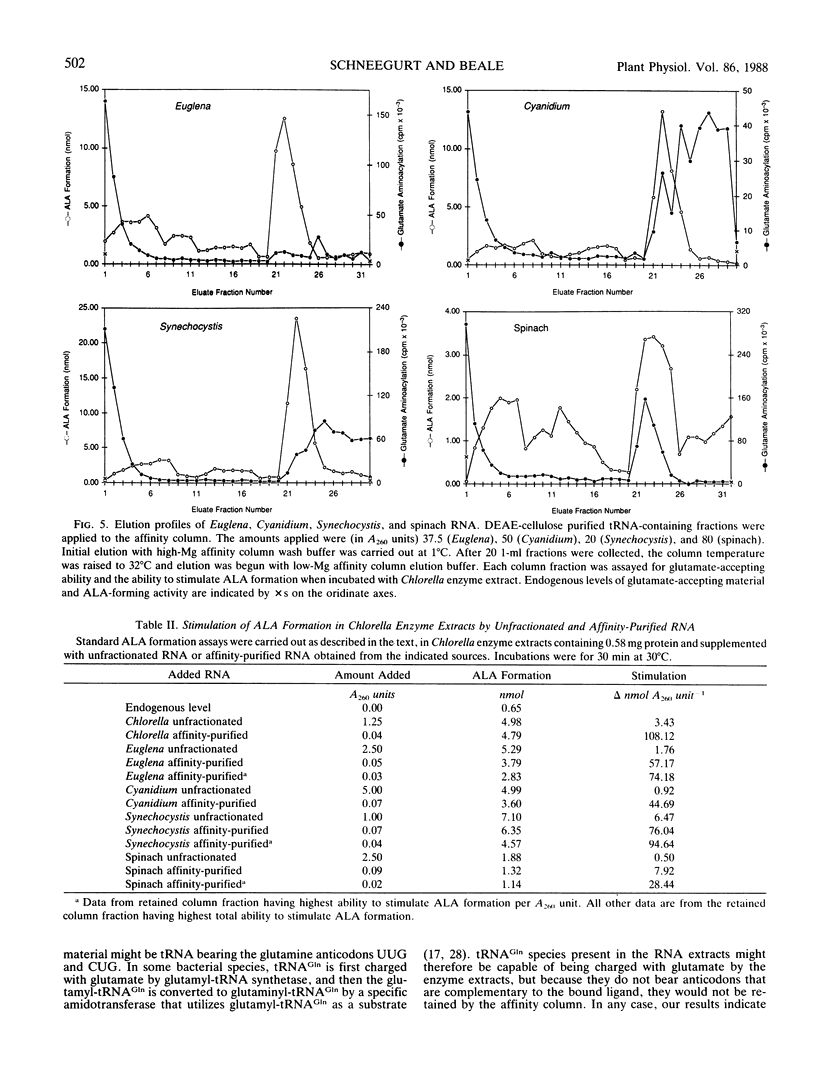
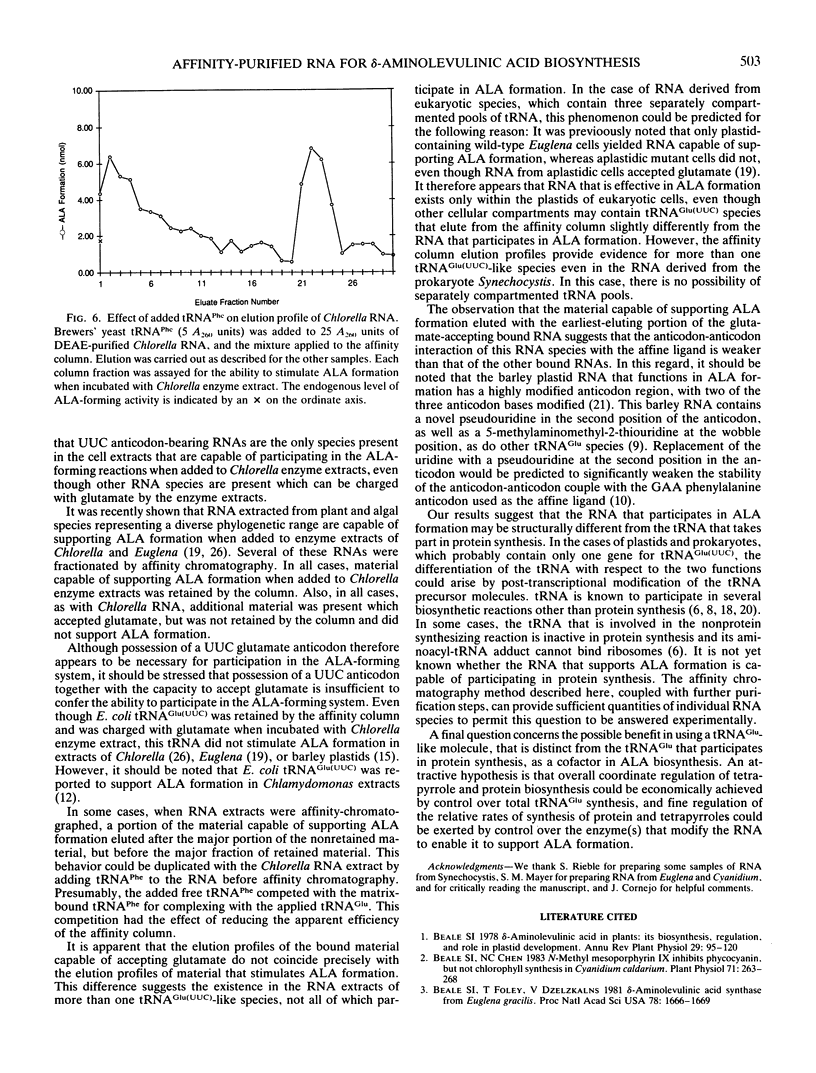
The heme and chlorophyll precursor δ-aminolevulinic acid acid (ALA) is formed in plants and algae from glutamate in a process that requires at least three enzyme components plus a low molecular weight RNA which co-purifies with the tRNA fraction during DEAE-cellulose column chromatography. RNA that is effective in the in vitro ALA biosynthetic system was extracted from several plant and algal species that form ALA via this route. In all cases, the effective RNA contained the UUC glutamate anticodon, as determined by its specific retention on an affinity resin containing an affine ligand directed against this anticodon. Construction of the affinity resin was based on the fact that the UUC glutamate anticodon is complementary to the GAA phenylalanine anticodon. By covalently linking the 3′ terminus of yeast tRNAPhe(GAA) to hydrazine-activated polyacrylamide gel beads, a resin carrying an affine ligand specific for the anticodon of tRNAGlu(UUC) was obtained. Column chromatography of plant and algal RNA extracts over this resin yielded a fraction that was highly enriched in the ability to stimulate ALA formation from glutamate when added to enzyme extracts of the unicellular green alga Chlorella vulgaris. Enhancement of ALA formation per A260 unit added was as much as 50 times greater with the affinity-purified RNA than with the RNA before affinity purification. The affinity column selectively retained RNA which supported ALA formation upon chromatography of RNA extracts from species of the diverse algal groups Chlorophyta (Chlorella Vulgaris), Euglenophyta (Euglena gracilis), Rhodophyta (Cyanidium caldarium), and Cyanophyta (Synechocystis sp. PCC 6803), and a higher plant (spinach). Other glutamate-accepting tRNAs that were not retained by the affinity column were ineffective in supporting ALA formation. These results indicate that possession of the UUC glutamate anticodon is a general requirement for RNA to participate in the conversion of glutamate to ALA in plants and algae.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale S. I., Chen N. C. N-Methyl Mesoporphyrin IX Inhibits Phycocyanin, but Not Chlorophyll Synthesis in Cyanidium caldarium. Plant Physiol. 1983 Feb;71(2):263–268. doi: 10.1104/pp.71.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Foley T., Dzelzkalns V. delta-Aminolevulinic acid synthase from Euglena gracilis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1666–1669. doi: 10.1073/pnas.78.3.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bumsted R. M., Dahl J. L., Söll D., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. X. Further study of the glycyl transfer ribonucleic acids active in peptidoglycan synthesis in Staphylococcus aureus. J Biol Chem. 1968 Feb 25;243(4):779–782. [PubMed] [Google Scholar]
- Ferber S., Ciechanover A. Role of arginine-tRNA in protein degradation by the ubiquitin pathway. Nature. 1987 Apr 23;326(6115):808–811. doi: 10.1038/326808a0. [DOI] [PubMed] [Google Scholar]
- Grosjean H. J., de Henau S., Crothers D. M. On the physical basis for ambiguity in genetic coding interactions. Proc Natl Acad Sci U S A. 1978 Feb;75(2):610–614. doi: 10.1073/pnas.75.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Takada C., Petre J. Complex formation between transfer RNAs with complementary anticodons: use of matrix bound tRNA. Biochem Biophys Res Commun. 1973 Aug 6;53(3):882–893. doi: 10.1016/0006-291x(73)90175-7. [DOI] [PubMed] [Google Scholar]
- Huang D. D., Wang W. Y. Chlorophyll biosynthesis in Chlamydomonas starts with the formation of glutamyl-tRNA. J Biol Chem. 1986 Oct 15;261(29):13451–13455. [PubMed] [Google Scholar]
- Huang D. D., Wang W. Y., Gough S. P., Kannangara C. G. delta-Aminolevulinic acid-synthesizing enzymes need an RNA moiety for activity. Science. 1984 Sep 28;225(4669):1482–1484. doi: 10.1126/science.6206568. [DOI] [PubMed] [Google Scholar]
- Inman J. K., Dintzis H. M. The derivatization of cross-linked polyacrylamide beads. Controlled introduction of functional groups for the preparation of special-purpose, biochemical adsorbents. Biochemistry. 1969 Oct;8(10):4074–4082. doi: 10.1021/bi00838a026. [DOI] [PubMed] [Google Scholar]
- Kern D., Potier S., Boulanger Y., Lapointe J. The monomeric glutamyl-tRNA synthetase of Escherichia coli. Purification and relation between its structural and catalytic properties. J Biol Chem. 1979 Jan 25;254(2):518–524. [PubMed] [Google Scholar]
- Lapointe J., Duplain L., Proulx M. A single glutamyl-tRNA synthetase aminoacylates tRNAGlu and tRNAGln in Bacillus subtilis and efficiently misacylates Escherichia coli tRNAGln1 in vitro. J Bacteriol. 1986 Jan;165(1):88–93. doi: 10.1128/jb.165.1.88-93.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz M. J., Soffer R. L. A soluble enzyme from Escherichia coli which catalyzes the transfer of leucine and phenylalanine from tRNA to acceptor proteins. Biochem Biophys Res Commun. 1969 Jul 7;36(1):47–53. doi: 10.1016/0006-291x(69)90647-0. [DOI] [PubMed] [Google Scholar]
- Mayer S. M., Beale S. I., Weinstein J. D. Enzymatic conversion of glutamate to delta-aminolevulinic acid in soluble extracts of Euglena gracilis. J Biol Chem. 1987 Sep 15;262(26):12541–12549. [PubMed] [Google Scholar]
- Nesbitt J. A., 3rd, Lennarz W. J. Participation of aminoacyl transfer ribonucleic acid in aminoacyl phosphatidylglycerol synthesis. I. Specificity of lysyl phosphatidylglycerol synthetase. J Biol Chem. 1968 Jun 10;243(11):3088–3095. [PubMed] [Google Scholar]
- Schön A., Krupp G., Gough S., Berry-Lowe S., Kannangara C. G., Söll D. The RNA required in the first step of chlorophyll biosynthesis is a chloroplast glutamate tRNA. Nature. 1986 Jul 17;322(6076):281–284. doi: 10.1038/322281a0. [DOI] [PubMed] [Google Scholar]
- Wang W. Y., Huang D. D., Stachon D., Gough S. P., Kannangara C. G. Purification, Characterization, and Fractionation of the delta-Aminolevulinic Acid Synthesizing Enzymes from Light-Grown Chlamydomonas reinhardtii Cells. Plant Physiol. 1984 Mar;74(3):569–575. doi: 10.1104/pp.74.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Enzymatic conversion of glutamate to delta-aminolevulinate in soluble extracts of the unicellular green alga, Chlorella vulgaris. Arch Biochem Biophys. 1985 Mar;237(2):454–464. doi: 10.1016/0003-9861(85)90299-1. [DOI] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. RNA is required for enzymatic conversion of glutamate to delta-aminolevulinate by extracts of Chlorella vulgaris. Arch Biochem Biophys. 1985 May 15;239(1):87–93. doi: 10.1016/0003-9861(85)90814-8. [DOI] [PubMed] [Google Scholar]
- Weinstein J. D., Mayer S. M., Beale S. I. Formation of delta-Aminolevulinic Acid from Glutamic Acid in Algal Extracts : Separation into an RNA and Three Required Enzyme Components by Serial Affinity Chromatography. Plant Physiol. 1987 Jun;84(2):244–250. doi: 10.1104/pp.84.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. D., Mayer S. M., Beale S. I. Stimulation of delta-Aminolevulinic Acid Formation in Algal Extracts by Heterologous RNA. Plant Physiol. 1986 Dec;82(4):1096–1101. doi: 10.1104/pp.82.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox M. Gamma-phosphoryl ester of glu-tRNA-GLN as an intermediate in Bacillus subtilis glutaminyl-tRNA synthesis. Cold Spring Harb Symp Quant Biol. 1969;34:521–528. doi: 10.1101/sqb.1969.034.01.059. [DOI] [PubMed] [Google Scholar]


